High risk for venous thromboembolism in diabetics with hyperosmolar state: comparison with other acute medical illnesses
Abstract
Summary. Background: Diabetes mellitus is generally not recognized as an important risk factor for venous thromboembolism (VTE). However, clinical observations and case reports have suggested that patients with diabetes and hyperosmolarity may be at increased risk for VTE. Objectives: To determine the risk of VTE in patients hospitalized for diabetes with hyperosmolar state compared to patients with other acute medical illnesses. Patients/methods: The California Patient Discharge Data Set was used to determine the incidence of first-time VTE in all patients admitted between 1995 and 2000 for diabetes with hyperosmolarity and 11 other acute medical conditions. Proportional hazard modeling was used to adjust for age, race, gender, and prior hospitalization within 3 months. Results: Among 2859 patients with diabetes and hyperosmolarity, 34 (1.2%) developed VTE during the hospitalization and 14 (0.5%) developed VTE within 91 days after discharge. In an adjusted multivariate model comparing the risk of VTE to cases with depression, patients with hyperosmolarity had a significantly higher risk of VTE [hazard ratio (HR) = 16.3; 95% confidence interval (CI): 10–25] comparable to the risk associated with sepsis (HR = 19.3; 95% CI: 13–29) or acute connective tissue disease (HR = 21; 95% CI: 15–31). Compared to uncomplicated diabetes, patients with hyperosmolarity had a significantly higher risk of VTE (HR = 3.0; 95% CI: 2.1–4.5) whereas patients with ketoacidosis were not at higher risk (HR = 1.2; 95% CI: 0.8–1.7). Conclusions: Patients hospitalized for diabetes with hyperosmolarity are at increased risk for developing VTE both during their inpatient stay and in the 3 months after discharge. Thromboprophylaxis in these patients appears warranted, and extended prophylaxis for after hospital discharge should be studied.
Introduction
Venous thromboembolism (VTE) is a significant problem in hospitalized medical patients, as they have an approximately 8-fold higher risk of VTE compared to non-hospitalized individuals [1]. Moreover, after excluding cases with cancer, 26% of all first-time cases of VTE are diagnosed either during a medical hospitalization or within 8 weeks after hospital discharge [2]. In an effort to reduce the incidence of VTE, recent initiatives sponsored by the Joint Commission for Accreditation of Healthcare Organizations will require that all hospitalized patients be assessed for VTE risk and have appropriate VTE prophylaxis administered [3]. Medical prophylaxis, using either low-dose unfractionated heparin or low-molecular weight heparin, has been shown to reduce the incidence of VTE in higher risk medical inpatients [4].
Although a number of conditions are known to be associated with an increased risk of VTE during a medical hospitalization, patients with medical illnesses have been studied less than surgical patients, and no study has comprehensively determined the magnitude of risk associated with hospitalization for a spectrum of medical conditions. Some of the medical illnesses that have been associated with an increased risk of VTE include acute respiratory failure, acute stroke or paralysis, congestive heart failure (CHF), acute infection, chronic obstructive pulmonary disease (COPD), acute inflammatory bowel disease, rheumatologic disease, nephrotic syndrome, prior VTE, and active cancer [5,6].
Clinical observation of VTE developing in several patients with diabetes and hyperosmolarity prompted us to study whether this condition is associated with a higher risk of VTE. A literature review revealed several published case reports from the late 1960s and early 1970s that described this association [7–10]. However, prior epidemiologic studies have not consistently found diabetes to be strong risk factor for VTE, and so this association is controversial [11,12]. We hypothesized that patients hospitalized for diabetes with hyperosmolarity are at significantly higher risk for VTE compared with patients hospitalized for uncomplicated diabetes and other common medical conditions. To test this hypothesis, we determined the incidence of VTE both during the index hospitalization and in the 3-month period immediately after discharge in a large cohort of patients admitted specifically for diabetes with hyperosmolarity. For comparison, we determined the VTE incidence in patients admitted with a variety of medical disorders. Patients hospitalized for depression were used as a referent group, as we assumed these patients would have a low risk of having a concomitant acute medical illness.
Materials and methods
Discharge data set
The California Patient Discharge Data Set has been previously described [13]. Briefly, all non-federal hospitals in California must provide demographic data, a principal diagnosis (‘the condition occasioning admission’), up to 24 other diagnoses, a principal procedure, and up to 20 other procedures. Cases admitted with a specific medical condition can be readily identified. Since 1990, serial hospitalizations can be linked together and to the master death registry [14]. All procedures and diagnoses are coded using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). The study was approved by the California Health and Welfare Agency Committee for the Protection of Human Subjects, and the University of California, Davis Human Subjects Committee. Informed consent was obtained from all participants.
Study cohort
We analyzed hospital discharge records of all cases who were 18 years or older on the day of admission and who were hospitalized for two or more days between January 1, 1995 and December 30, 2000, and who had one of 11 specific principal medical diagnoses (defined by ICD-9-CM codes): diabetes with hyperosmolar state (250.2 = marked hyperglycemia/hyperosmolarity, dehydration, no acidosis; coma not required), diabetes without complication (250.0), diabetes with acidosis (250.1), COPD (492.8, 492.22), myocardial infarction (410.0), CHF – unspecified (428), Crohn’s disease (555.x), connective tissue diseases (710.x, 714.0), depression (300.4, 309.1, 311), acute renal failure (584.5–584.9), and sepsis (38.x, 775.59, 790.7). In order to exclude cases that underwent surgery and in order to confirm the medical diagnosis, we required that there be a medical DRG code that matched the principal diagnosis for each condition (see Appendix A). Cases were also excluded if there was a diagnosis of cancer (ICD-9-CM codes = 141–208, except 173, non-melanoma skin cancers) at the time of admission or in the prior 6-month period.
Definition of outcomes
VTE during hospitalization A diagnosis of venous throm- bosis during the initial hospitalization was based on the presence of an ICD-9-CM for pulmonary embolism (ICD-9-CM code = 415.1x) or deep vein thrombosis (ICD-9-CM codes = 451.1x; 451.2; 451.81; 451.9; 453.1; 453.2; 453.8; 453.9). Cases with upper extremity thrombosis, superficial vein thrombosis, or arterial thromboses were not included in this analysis. The date of the VTE event was assumed to be the date of a procedure used to diagnose or treat VTE (arterio-graphy = 88.43 or 88.44; venogram = 88.66; ventilation/per-fusion scan = 92.15; venous ultrasound = 88.77; chest computed tomography scan = 87.41; plethysmography = 89.58; vena cava filter = 38.7; injection of anticoagulant = 99.19). In the absence of a procedure date, the median hospital day was assumed to be the date of the VTE. To improve the specificity of the diagnosis codes for incident VTE events and to exclude the powerful effect of a history of prior VTE, all cases with a diagnosis of VTE during any hospitalization between July 1990 and the index hospitalization or cases that were coded as having a history of VTE (ICD-9-CM codes = V12.51 and V12.52) were excluded.
VTE after hospital discharge Cases that did not develop VTE during the index hospitalization but that were rehospitalized for 2 or more days with a principal diagnosis of VTE within 91 days of the index hospitalization were classified as ‘late’ VTE.
Statistical analysis
Categorical data were analyzed using chi-square testing. Differences in person-time rates were compared using a mid-P exact test. Linear regression was used to determine the correlation between the incidence of VTE during the index hospitalization and the subsequent 91-day follow-up period. Proportional hazard modeling was used to analyze the risk of developing VTE, adjusting for age, race, gender, and presence or absence of one or more hospitalizations in the 91-day period immediately preceding the index hospitalization. Models were adjusted for sex, gender, and ethnicity in order to fairly compare the findings between groups, which frequently differed substantially in these demographic characteristics. Each linked record was censored at the time of a diagnosis of VTE or at the time of death. We did not include the hospital length of stay in the model because diagnosis of VTE likely leads to an increase in the length of hospitalization. The proportionality assumptions were assessed and were met. Analyses were performed using sas and a two-sided P-value of <0.05 was considered statistically significant.
Results
A total of 426 831 cases had one of the 11 specified principal diagnoses, and 3770 (0.8%) of these cases were diagnosed with VTE. This included 2341 in-hospital VTE cases (62%), and 1429 late VTE cases (38%), which were diagnosed within 91 days after discharge. Table 1 shows the total number of cases for each of the specified illnesses and the corresponding number of cases diagnosed with acute VTE. The four illnesses associated with the highest overall incidence of acute VTE were a connective tissue disease (2.1%), sepsis (2%), acute renal failure (1.8%), and diabetes with hyperosmolarity (1.7%). Figure 1 shows the incidence of VTE after hospital discharge. For the 11 illnesses, the incidence of VTE during the index hospitalization correlated strongly with the incidence of VTE during the 91-day follow-up period (r = 0.81, P < 0.002).
| Medical condition leading to hospitalization | N | Median LOS* | VTE 3 months (N) | Hospital VTE (%) | 91-day postdischarge VTE (%) | Total VTE (%) |
|---|---|---|---|---|---|---|
| Diabetes, no complication | 10 050 | 3 (4–6) | 53 | 0.3 | 0.2 | 0.5 |
| Diabetes with hyperosmolarity | 2859 | 4 (5–7) | 48 | 1.2 | 0.5 | 1.7 |
| Diabetes with ketoacidosis | 10 599 | 3 (4–6) | 69 | 0.4 | 0.2 | 0.7 |
| Acute renal failure | 24 838 | 4 (6–9) | 431 | 1.2 | 0.6 | 1.8 |
| Acute myocardial infarction | 109 881 | 4 (5–6) | 901 | 0.5 | 0.3 | 0.8 |
| Sepsis | 3227 | 4 (6–9) | 66 | 1.5 | 0.6 | 2.0 |
| Congestive heart failure | 136 665 | 3 (4–7) | 1393 | 0.7 | 0.3 | 1.0 |
| Connective tissue disease | 6755 | 4 (6–9) | 143 | 1.2 | 0.9 | 2.1 |
| Chronic obstructive pulmonary disease | 79 046 | 3 (5–6) | 579 | 0.3 | 0.4 | 0.7 |
| Crohn’s disease | 5214 | 4 (5–7) | 52 | 0.5 | 0.5 | 1.0 |
| Depression | 37 707 | 3 (5–9) | 35 | 0.0 | 0.1 | 0.1 |
| Total | 426 831 | 3770 | 0.5 | 0.3 | 0.8 |
- *LOS = length of stay and 25%–75% interquartile range.
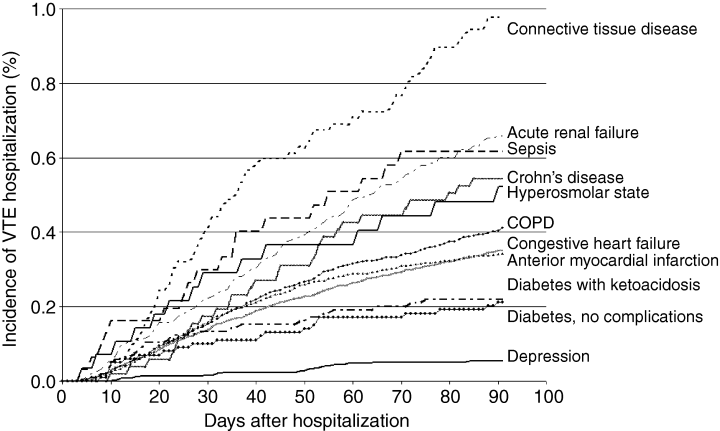
Kaplan-Meier plot showing the 91 day incidence of venous thromboembolism (VTE) after hospital discharge. COPD, chronic obstructive pulmonary disease.
Table 2 shows the results of the proportional hazard modeling. Cases hospitalized for uncomplicated diabetes had significantly higher risk of developing VTE compared to patients with depression [hazard ratio (HR) = 5.2; 95% confidence interval (CI): 3.4–8.1], as did cases with diabetic ketoacidosis (DKA; HR = 6.8; 95% CI: 4.5–10.3), but there was no significant difference in the risk of VTE when cases with DKA were compared to cases with uncomplicated diabetes (HR = 1.2; 95% CI: 0.8–1.7; P = 0.4). Cases admitted for diabetes with hyperosmolarity had a significantly higher risk of developing VTE compared to patients with depression (HR = 16.3; 95% CI: 10–25; P < 0.001), and they had significantly higher risk compared to cases admitted for DKA (HR = 2.3; 95% CI: 1.6–3.3; P < 0.01).
| Variable | Hazard ratio (95% confidence interval) | P-value |
|---|---|---|
| Principal diagnosis | ||
| Depression | 1.0 (referent) | – |
| Diabetes without complication | 5.2 (3.4–8.1) | <0.0001 |
| Diabetes with ketoacidosis | 6.8 (4.5–10.3) | <0.0001 |
| Diabetes with hyperosmolarity | 16.3 (10.4–25.4) | <0.0001 |
| Acute renal failure | 16.0 (11.2–22.8) | <0.0001 |
| Myocardial infarction | 8.1 (5.7–11.5) | <0.0001 |
| Sepsis | 19.3 (12.7–29.3) | <0.0001 |
| Congestive heart failure | 9.1 (6.4–12.9) | <0.0001 |
| Connective tissue disease | 21.3 (14.7–31.0) | <0.0001 |
| Chronic obstructive pulmonary disease | 6.7 (4.7–9.6) | <0.0001 |
| Crohn’s disease | 10.3 (6.7–15.9) | <0.0001 |
| Gender | ||
| Male | 1.0 (referent) | – |
| Female | 1.15 (1.1–1.2) | <0.0001 |
| Age (years) | ||
| 18–44 | 1.0 | – |
| 45–64 | 1.1 (0.9–1.3) | 0.3 |
| 65–74 | 1.3 (1.1–1.9) | 0.002 |
| ≥75 | 1.3 (1.1–1.5) | <0.001 |
| Racial/ethnic group | ||
| Caucasian | 1.0 | – |
| Black | 1.2 (1.0–1.3) | 0.009 |
| Hispanic | 0.9 (0.7–1.0) | 0.01 |
| Asian/Pacific Islander | 0.5 (0.5–0.6) | <0.0001 |
| Prior hospitalization (<3 months) | 1.3 (1.2–1.4) | <0.001 |
Table 2 also shows the effects of age, gender, race/ethnicity, and a preceding hospitalization within 3 months of the index admission on the relative risk of developing VTE. Women were more likely than men to develop VTE (HR = 1.15; 95% CI: 1.1–1.2), and the risk of VTE was significantly higher among cases age 65–74, and age 75 or older, when compared to cases between 18 and 44 years in age. African–American cases had a modestly higher risk of developing VTE (HR = 1.2; 95% CI: 1.0–1.3; P = 0.009), whereas Hispanic (HR = 0.9; 95% CI: 0.7–0.1; P = 0.01) and Asian/Pacific Islanders (HR = 0.5; 95% CI: 0.5–0.6; P < 0.001) had significantly lower risk of developing VTE compared to Caucasian cases. One or more prior hospitalizations within 3 months was associated with significantly higher risk of developing VTE (HR = 1.3; 95% CI: 1.2–1.4; P < 0.001).
Discussion
The findings of this study support our hypothesis that patients with diabetes with hyperosmolarity have an increased risk of developing VTE. The overall incidence of VTE among patients with hyperosmolarity was 1.7%, with 71% of the cases diagnosed during the index hospitalization, and 29% diagnosed during the 3 months after hospital discharge. In fact, the incidence of VTE in these patients with hyperosmolarity was very similar to the incidence among patients admitted for sepsis or acute connective tissue disease, medical conditions known to be strongly associated with a higher incidence of VTE [5]. The VTE incidence of 1.7% compares to a 91-day incidence of VTE of 2.8% among patients undergoing total hip arthroplasty (THA) determined using the same administrative database [15,16] and an incidence of clinically symptomatic VTE in total hip arthroplasty patients of 3.7% reported by Colwell et al. [17] in a large clinical trial of extended thromboprophylaxis. Thus, the 1.7% incidence of clinically diagnosed VTE in patients with diabetes with hyperosmolarity was only modestly lower than the incidence in hip arthroplasty patients, a group that is generally accepted as being at ‘very high risk’ for VTE.
Compared to the patients with depression, patients with hyperosmolarity had a sixteenfold higher risk of developing VTE, patients with DKA had a 7-fold higher risk, and patients with uncomplicated diabetes had a 5-fold higher risk. The higher incidence of VTE among diabetic patients was not unexpected. A number of studies have provided evidence supporting the contention that patients with diabetes and hyperglycemia have a prothrombotic condition [18–22].
After adjusting for age, race, gender, and recent hospitalization, when patients with hyperosmolarity or DKA were compared patients with uncomplicated diabetes, the hyperosmolar group had significantly higher risk of VTE (HR = 3.0; 95% CI: 2.1–4.5) whereas cases with DKA did not have increased risk (HR = 1.2; 95% CI: 0.8–1.7). Because patients with both DKA and hyperosmolar coma share the features of significant dehydration and some degree of hyperosmolarity, the finding of a significantly higher risk of developing VTE in the hyperosmolar group suggests that the more profound magnitude of either the hyperosmolarity or the intravascular volume depletion is the trigger for acute thrombosis. Certainly dehydration leads to vascular stasis, which may be one factor that explains the high incidence of thrombosis. Additionally, the more extreme elevation in the blood glucose level in patients with hyperosmolarity may lead to further augment a hypercoagulable state [19].
We found, as perhaps expected, that all of the patients admitted with one of the prespecified acute medical conditions had a significantly higher risk of developing VTE compared to patients admitted for depression. These findings are consistent with the findings of Heit et al. [23], who reported that patients admitted to a hospital for a wide variety of medical problems had a significantly higher risk of developing VTE compared to patients in the community. The observed incidence of VTE in patients admitted for other specific medical problems was consistent with other studies that have assessed the incidence of VTE in hospitalized medical patients [24,25]. Patients with CHF, acute myocardial infarction, inflammatory bowel disease, sepsis, or a connective tissue diseases all had a moderately high to very high relative risk for developing VTE compared to the control cohort with depression [24].
The high risk of thrombosis seen in patients with acute renal failure has not been previously reported. This finding deserves a more detailed analysis, particularly because we did not analyze the exact type or nature of the underlying problem(s) associated with the development of renal failure. In addition, this high incidence of VTE might possibly reflect upper extremity deep vein thrombosis related to insertion of a dialysis catheter, because upper extremity thrombosis may be miscoded as a non-specific deep vein thrombosis. In the present analysis, patients of Asian ethnicity had a significantly lower risk of developing VTE, which has also been found in prior studies [26–28]. Overall, the consistency of the results of the current analysis with prior research provides strong external validation of our findings.
Our study adds to the literature by quantifying the risk of acute VTE both during and following an index hospitalization for a variety of medical conditions. Overall, a significant percentage (38%) of all VTE events developed in the 91-day period following hospital discharge. This finding is consistent with prior research in high-risk orthopedic patients, in whom the preponderance of symptomatic VTE events occurs after discharge [15]. The extended period of increased risk for VTE after hospital discharge suggests that extended thromboprophylaxis after hospital discharge may be warranted in very high risk medical patients. Prolonged thromboprophylaxis is currently recommended after hospital discharge in patients undergoing abdominal surgery for cancer, joint replacement surgery or hip fracture [29–32]. The EXCLAIM study, a large, randomized trial, is ongoing and is evaluating the efficacy of extended thromboprophylaxis (inpatient and/or outpatient) in high-risk medical patients [33].
Several limitations of this study should be kept in mind. Because we used an administrative database, we could not control for clinical parameters such as body mass index, duration of symptoms, presence of fever, hemoglobin level, etc., and we could not control for treatment variables such as rapidity of rehydration or the use or intensity of thromboprophylaxis. However, because hyperosmolarity is not widely recognized in the United States as being a strong risk factor for VTE, we assume that there was no significant difference in the proportion of patients in each of our diabetes groups who were treated with thromboprophylaxis in the hospital. Assuming that both the DKA patients and the hyperosmolar patients were admitted to an intensive care unit, these groups may have had a higher likelihood of receiving thromboprophylaxis [34].
Another limitation is that the late VTE events were determined by requiring rehospitalization for VTE in a non-federal California hospital. It is possible that some patients may have been diagnosed and treated as an outpatient, which would lead to an underestimation of the incidence of VTE. It is also possible that we missed some patients who were rehospitalized for VTE at a Veterans Affairs hospital or a military hospital, or were rehospitalized outside California. However, there is no reason to suspect that patients with diabetes and hyperosmolarity would be more or less likely to be treated as outpatients, or to be hospitalized at non-federal hospitals outside California compared to patients with other medical conditions. Thus, any detection bias would have led to a small reduction in the observed incidence of VTE, without a significant effect on the relative risk of developing VTE that we observed.
Some of the advantages of this study include the fact that we analyzed a large, population-based, multi-ethnic cohort of patients. In addition, because these records were linked together, we could readily identify all of the patients who required rehospitalizations for VTE irrespective of the hospital to which they were readmitted. Although there are inherent inaccuracies in administrative coding for conditions such as VTE, the VTE codes that were used have been validated [15], and we used the same codes that are currently used to define VTE by the Agency for Healthcare Research and Quality as one of their patient safety indicators [35,36].
In conclusion, patients with diabetes and marked hyperosmolarity have a high absolute and relative risk of developing VTE, particularly during their initial hospitalization, but also in the weeks that follow hospital discharge. The magnitude of the risk of VTE exceeds the risk associated with other conditions that are widely thought to predispose to VTE, such as CHF and COPD, and the risk was comparable to high-risk conditions such as sepsis and acute connective tissue disease. If future studies confirm our findings, diabetes with hyperosmolarity will need to be added to the list of acute medical illnesses associated with a high incidence of VTE that deserve more intense thromboprophylaxis.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
Appendix
Appendix A
| Principal diagnoses | Coding | ||
|---|---|---|---|
| DRG | PRIN DX (ICD-9-CM) | ||
| 1 | Congestive heart failure (acute and chronic) | 127 | 428.x |
| 2 | Inflammatory bowel disease | 179 | 555.x |
| 3 | Diabetes with hyperosmolar state | 294 | 250.2 |
| 4 | Connective tissue disease | 240, 241 | 710.x, 714.0 |
| 5 | Diabetes with ketoacidosis | 294 | 250.1 |
| 6 | Diabetes, no complications | 294 | 250.0 |
| 7 | Sepsis | 416 | 38, 785.59, 790.7 |
| 8 | Depression | 426 | 300.4, 309.1, 311 |
| 9 | Chronic obstructive pulmonary disease | 88 | 492.8, 491.21 |
| 10 | Acute renal failure | 316 | 584.5–584.9 |
| 11 | Myocardial infarction | 121–122 | 410.x1 |




