A novel anti-ischemic nitric oxide donor (LA419) reduces thrombogenesis in healthy human subjects
Abstract
Summary. Background: Platelet and endothelial production of nitric oxide (NO) is known to be impaired in coronary artery disease patients. Compounds that release NO (e.g. nitrates) have antiplatelet effects, but at supratherapeutic doses with hypotensive side effects. Objectives: To investigate the antithrombotic effect on human blood of a novel NO donor (LA419) with known anti-ischemic properties but without hypotensive side effects and to compare with abciximab. Patients/methods: Healthy subjects (n = 8; 32 ± 3 years) received daily aspirin starting three days prior to the study day. Treatments (LA419 10 and 20 μm, and abciximab 4 μm) were added ex vivo to non-anticoagulated blood, and the antithrombotic properties were assessed by measuring changes in thrombus size from pretreatment baseline in the Badimon perfusion chamber at low and high shear rates. Platelet surface adhesion using a Cone and Platelet Analyzer (CPA) and platelet fibrinogen-receptor activation with flow cytometry were also evaluated. Results: At low shear rates, LA419 displayed a reduction in thrombus area of 43% ± 8% (10 μm) and 56% ± 6% (20 μm), whereas at high shear rates the reductions were 44% ± 3% (10 μm) and 62% ± 6% (20 μm). Platelet surface adhesion with the CPA was also reduced. Abciximab exhibited a strong inhibitory effect on thrombus formation, platelet surface adhesion and fibrinogen receptor activation. Conclusions: The novel NO donor, LA419, shows a strong antithrombotic effect in human blood, which is comparable to abciximab, especially under high shear rate conditions. Our observations suggest that the availability of an NO donor could prove beneficial in the prevention of thrombotic complications of cardiovascular disease. Further clinical studies are warranted.
Introduction
Nitric oxide (NO) released from platelets and endothelium under physiological conditions is involved in several vascular processes, including reduction of endotoxin- and cytokine-induced expression of tissue factor [1], monocyte adhesion to the endothelium [2], intimal hyperplasia after endothelial injury [3], low density lipoprotein (LDL) oxidation by scavenging of lipid propagatory radicals [4], vasodilation and inhibition of platelet adhesion and aggregation [5]. Many of these biological actions are mediated via activation of the intracellular receptor, soluble guanylate cyclase (sGC), which catalyzes the formation of the second messenger cyclic 3′,5′-guanosine monophosphate (cGMP). In fact, this NO–sGC–cGMP axis is fundamental in regulating blood pressure [6] and preventing platelet and leukocyte interaction with the vessel wall [7,8]. Therefore, reduced NO bioavailability plays a central role in endothelial dysfunction, initiation and progression of the atherogenic process, plaque instability and thrombus formation.
Patients with acute coronary syndrome (ACS) show not only a decrease in the production of NO [9,10], but also an increase in oxidative stress [11], which induces clearance of platelet and endothelial NO [6,7,10]. Heitzer et al. [12] helped to define the relationship between endothelial dysfunction and cardiovascular prognosis, linking both phenomena to oxidative stress. Consequently, exogenous donation of NO by nitrovasodilators or ‘NO donor drugs’ such as nitrates is a strategy being used to restore NO functions. Thus, the current standard of care for symptomatic ACS patients includes antiplatelet agents, antihypertensive drugs, statins, and nitrovasodilators.
Widespread use of antiplatelet therapy has revolutionized the contemporary care of atherothrombotic patients. The overall mortality rate from atherothrombotic disease has declined 22% mainly because of the increasing adoption of aspirin (ASA), clopidogrel and glycoprotein (GP) IIb/IIIa antagonists [13]. The incidence of recurrent events though still remains high [14]. Therefore, there is an ever-present need to develop new and more effective antithrombotic strategies.
Nitrovasodilator regimens are used for the control of ischemia primarily because of their vasodilatory activity, because their antiplatelet effects are usually detected at supra-therapeutic dosages, which can induce hypotensive side effects. Nevertheless, there is growing insight into the antiplatelet properties of new NO donors that casts nitrate therapy in a new light. It has been previously reported in the pig model that oral treatment with the new anti-ischemic NO donor LA419 (a neutral sugar organic nitrate with a protected thiol group in its molecular structure) [15] produces a strong antithrombotic effect via the cGMP pathway, which not only exceeds that observed with equipotent doses of the standard mononitrate (IS-5-MN) [16], but also enhances the effectiveness of clopidogrel [17]. Interestingly, all the antithrombotic effects were achieved without any hypotensive side effects [16,17]. Moreover, Hernandez et al. [18] have recently shown that a 5-min in vitro incubation of blood from healthy volunteers with LA419 reduces platelet deposition under low-shear-rate conditions.
The primary objective of our study was to investigate the antithrombotic effects of LA419 in ASA-treated healthy subjects under low and high shear rate flow conditions and to compare these effects with those of abciximab under the same rheologic conditions.
Methods
Subjects and study design
Healthy male and female subjects (n = 8, age = 32 ± 3 years), not taking any medications, underwent a screening process that included a detailed history and physical examination. Qualified participants were started on ASA (160 mg day–1), and on the third day each subject underwent four perfusion chamber studies: three with treatments (LA419, 10 and 20 μm; abciximab, 3 μg mL−1 blood) and one with equal volume of saline to serve as control. The order of perfusion studies for each subject was chosen randomly. Antithrombotic effects of the treatments were assessed by comparing the size of each post-treatment perfusion thrombus with the non-treatment control. The strength of this design is the ability for each subject to serve as his/her own control.
Informed consent was obtained from all subjects prior to screening, and the study was approved by the Institutional Review Board of the Mount Sinai School of Medicine.
Antithrombotic assessment
The antithrombotic effects of the drugs were assessed with the previously validated Badimon perfusion chamber under low and high shear rate conditions [19,20]. Blood mixed with LA419, abciximab or saline was exposed to a highly thrombogenic substrate and the resulting thrombi measured for size.
Non-anticoagulated blood from a 21-gauge catheter in the subjects’ antecubital vein was introduced directly into a mixing device at a constant flow rate of 10 mL min−1 (Fig. 1). Medication/saline from an infusion pump was added to this mixing device at a flow rate of 0.2 mL min−1 through a second inlet. A magnetic stirrer thoroughly mixed the drug/saline with the blood before passing the mixture to the perfusion chamber. The rheological conditions in the first chamber simulated those encountered in venous blood flow (medium caliber vessels; shear rate of 212 s−1), whereas in the second chamber, it simulated blood flow observed in stenotic or thrombotic arterial vessels (small caliber lumen; shear rate of 1690 s−1) [19,21,22].
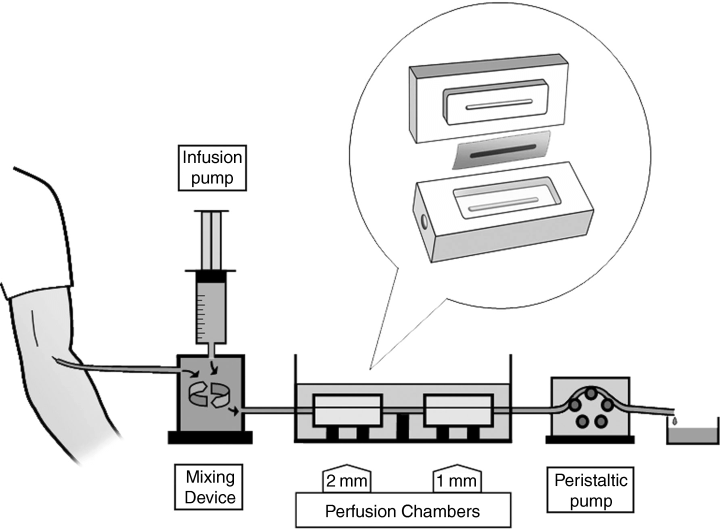
A schematic diagram of the modified Badimon perfusion system with the special mixing device for ex vivo mixing of medications with non-anticoagulated blood.
Thrombogenic substrate
Pig aorta specimens (3 cm in length and 1 cm in width) were mechanically disrupted by peeling off the intimal layer with a little subjacent media, as previously described [21]. The highly thrombogenic substrate was placed in each perfusion chamber where its medial surface was directly exposed to the flowing blood.
Morphological measurements of platelet-rich thrombus
Following each perfusion study, the substrates were fixed in 4% paraformaldehyde and sent to pathology for slide preparation and staining with Combined Masson trichrome Elastin. Evaluation of total thrombus area was performed by acquiring images of six sections from each perfusion chamber and analyzing by computer-assisted planimetry using image pro plus software (v4.5.1, Media Cybernetics, Silver Spring, MD, USA).
Measurement of platelet surface adhesion with Cone and Plate(let) Analyzer
The Cone and Plate(let) Analyzer (CPA) device allows the assessment of platelet adhesion and activation onto a polystyrene surface under controlled, high shear conditions [23]. The test and methodology have been previously described by our laboratory [24]. In brief, 200 mL of citrated blood was placed in polystyrene wells in duplicates and subjected to a shearing force (1875 s−1) with a rotating Teflon® cone for 2 min. Excess blood was drained and the wells stained with May–Grünwald stain (Sigma-Aldrich, St Louis, MO, USA) for 1 min, followed by a wash with phosphate buffered saline (PBS) to remove excess stain. Four digital images were captured from each well and the surface of the well covered with platelets measured using the nih image 1.62 software. Results were expressed as the percentage of the well surface covered by platelets. Drug-effect was calculated as the difference in surface coverage vs. control.
Measurement of platelet activation with flow cytometry
Flow cytometric determination on whole blood was performed to determine any inhibitory effect of the treatments on platelet GPIIb/IIIa receptor. Blood samples with the treatments and the saline control were collected in 3.2% sodium citrate tubes and stimulated with 10 μm of adenosine 5’-diphosphate (ADP). Platelets were labeled with the CD42b-PE antibody (Immunotech; Fullerton, CA, USA) and the fibrinogen receptors expressed on the activated platelets were identified using chicken, antihuman fibrinogen–FITC antibody (WAK-Chemie Medical GmbH, Germany). A total of 10 000 platelets were acquired and analyzed for each sample on a Becton Dickinson FACSCalibur (BD Biosciences; San Jose, CA, USA). The percentages of platelets testing positive for fibrinogen receptor were compared between treatments and control to assess the effects of LA419 and abciximab on GPIIb/IIIa receptor.
Coagulation, biochemical and hematological parameters
Determinations of complete blood count were performed before the baseline perfusion and after each treatment. Levels of prothrombin time (PT), activated partial thromboplastin time (APTT), and fibrinogen were monitored with an ST4 automated clotter (Diagnostica Stago, Asnières, France) at these same time points.
Statistical analysis
Results are expressed as mean ± SEM. Comparisons within subjects were performed using a Student’s t-test for paired data. A one-way anova test for independent experiments was applied when multiple comparisons were required. The Bonferroni test was used if statistical significance was detected. A P-value < 0.05 was considered to be statistically significant. All statistical analyses were performed using stat view (version 5.0; SAS Institute, Cary, NC, USA).
Results
Effect on thrombus formation
The NO donor LA419 demonstrated a dose-dependent reduction in platelet-rich thrombus formation at both low and high shear rate conditions (Fig. 2) when compared to control. As expected, abciximab displayed a potent antithrombotic effect in all enrolled subjects.
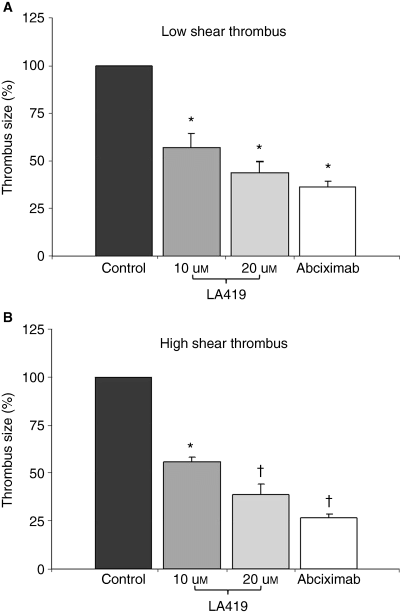
Antithrombotic effect. Bar graphs of the thrombus area under low shear rate (212 s−1) and high shear rate (1690 s−1) conditions. *P < 0.05 vs. control; †P < 0.05 vs. control and LA419 (10 μm).
Low shear rate conditions (212 s−1)
A significant, dose-dependent reduction in thrombus size was observed with LA419. Compared to the corresponding control, LA419 10 μm showed a mean reduction of 43% ± 8% in the thrombus area, whereas the mean reduction with LA419 20 μm was 56% ± 6% (P < 0.05 for both; Fig. 2A). Abciximab decreased thrombus size by a mean of 64% ± 3% (P < 0.0005). There was no statistically significant difference between the antithrombotic effects of LA419 10 μm, LA419 20 μm and abciximab.
High shear rate conditions (1690 s−1)
Under flow conditions typical of partially occluded coronary arteries, we observed a clear and significant dose-dependent antithrombotic effect of the investigational compound (Fig. 2B). LA419 10 μm produced the same degree of inhibition (44% ± 3% reduction) as observed under a low shear rate, whereas LA419 20 μm exerted a higher antithrombotic effect with a 62% ± 6% reduction vs. control. Abciximab produced a 73% ± 2% reduction in thrombus size. The antithrombotic effects of LA419 20 μm and abciximab were significantly stronger than LA419 10 μm (P < 0.05 for both); however, no statistically significant differences were observed between abciximab and the higher LA419 dose (P = 0.08), suggesting a strong antithrombotic effect of this novel NO donor at high dose.
Effect on platelet adhesion
The measurement of the platelet surface coverage using the CPA showed a reduction in platelet adhesion vs. control: 32% ± 5% with LA419 10 μm (2.8% ± 0.3% vs. 4.4% ± 0.6%, P < 0.05) and 51% ± 7% with LA419 20 μm (2.6% ± 0.4% vs. 4.4% ± 0.6%, P < 0.05). Treatment with the GPIIb/IIIa antagonist abciximab almost completely abolished platelet adhesion, resulting in a 99% ± 1% reduction in surface coverage vs. baseline (0.06% ± 0.03% vs. 4.4% ± 0.6%, P < 0.05; Fig. 3).
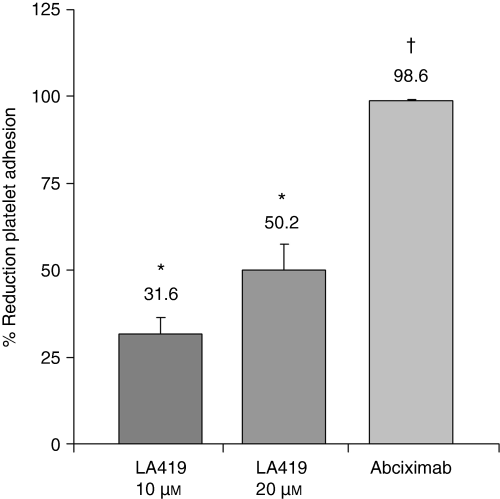
Platelet adhesion. Cone and Plate(let) Analyzer results, expressed as the percentage of reduction in surface covered by platelets compared to control. *P < 0.05 vs. control; †P < 0.05 vs. control and LA419.
Platelet fibrinogen-receptor activation
Treatment with abciximab, a GPIIb/IIIa receptor antagonist, showed a significant reduction in platelet activation vs. control (31.4% ± 8% vs. 72.5% ± 4%). The effect of LA419 on GPIIb/IIIa receptor expression was small and not statistically significant (LA419 10 μm = 70.7% ± 5% and LA419 20 μm = 70.3% ± 6%).
Effect on biochemical, hematological and coagulation parameters
No differences were observed in hematological and coagulation parameters (APTT, PT and fibrinogen) in blood samples with and without LA419 or abciximab treatment, confirming the results found in the in vivo preclinical model [16].
Discussion
We investigated the ex vivo effects on acute thrombus formation of two doses of a new platelet-specific NO donor (LA419) in ASA-treated healthy subjects, and we compared these with abciximab under identical conditions. Both doses of LA419 displayed an inhibitory effect on thrombus size at low and high shear rates with the high dose displaying an antithrombotic effect comparable to that of abciximab. The antiplatelet effects of LA419 were also observed in a separate test measuring platelet adhesion on a polystyrene surface under controlled, high shear condition.
The central role played by platelets in the pathophysiology of atherosclerosis, plaque growth, ACS, and in complications associated with percutaneous coronary intervention (PCI) is well documented [25]. Inhibition of platelet function is standard medical strategy for the prevention and treatment of adverse events in patients with, or at risk of developing, coronary syndromes and/or undergoing PCI. The meta-analysis from the Antithrombotic Trialists Collaboration [13] concluded that the use of ASA in patients at high risk of occlusive vascular disease resulted in a reduction of approximately 25% in the combined end-point of vascular death, myocardial infarction or stroke. However, platelets are activated by multiple receptors and pathways that can bypass the inhibitory effect of ASA. Therefore, especially in high-risk patients, there is a clear need for concomitant use of alternative antithrombotic therapies. The discovery of the platelet GPIIb/IIIa (integrin αIIbβ3) receptor antagonist abciximab has been a major advance in the pharmacotherapy of unstable angina/myocardial infarction patients undergoing PCI. By binding to about 60% of the GPIIb/IIIa receptor, abciximab inhibits the final common pathway of platelet aggregation [26] and thus has demonstrated robust benefit in several large PCI trials [27–30] and meta-analysis. Using flow cytometry, we observed that fibrinogen binding to the GPIIb/IIIa receptor was reduced by 55% when platelets were activated by high dose of ADP [31]. However, spontaneous or mechanically induced plaque rupture also creates an unstable coronary environment, which contributes not only to coronary thrombus but also to spasm and acute vessel closure, leading to ischemia and potential clinical events. Furthermore, coronary vessel disruption results in concomitant impairment of endothelial function. Therefore, NO donation strategies such as nitrates are currently used to increase the availability of NO, leading to improvement in endothelial function and symptoms of ischemia (vasodilation). However, nitrate compounds have weak antiplatelet activity, induce tolerance, and may induce other side effects such as hypotension. Thus, an intensive search for new compounds capable of generating NO is being conducted [32]. Oral administration of this new nitrate derivative LA419 has demonstrated powerful antithrombotic properties via cGMP in the pig species, exceeding those observed with the standard mononitrate IS-5-MN [16]. With the present human study, we have demonstrated that ex vivo administration of this new nitrate derivative in healthy ASA-pretreated volunteers exerts a powerful effect in reducing thrombus size triggered by disrupted vessels under high shear rate conditions comparable to that of abciximab.
NO, via cGMP, is also known to be a potent inhibitor of smooth muscle cell proliferation (VSMC), both in vitro [33] and in vivo [34,35]. As VSMC proliferation and their overproduction of extracellular matrix is one of the key events in vascular remodeling [36], adjunct treatment with LA419 may also help to reduce restenosis, which often occurs following vascular procedures [34]. Moreover, NO also synergizes with thrombolytic agents improving outcomes [37]. Thus, because ASA has demonstrated inhibition of vessel wall-derived NO and prostacyclin synthesis, resulting in vasoconstriction, platelet aggregation and a reduction on thrombolysis [38], supplementation with an exogenous source of NO could abrogate these adverse effects, improving the environmental milieu of the atherothrombotic process.
In conclusion, we have evidence that ex vivo administration of the novel NO donor LA419 exerts a strong antiplatelet effect, comparable to that of abciximab under rheologic conditions typically encountered in stenotic coronary arteries. Because the bioavailability of NO represents a central feature in maintaining homeostasis, regulating platelet activity, and preventing the onset of thrombosis and clinical vascular events, the strong antiplatelet effects of LA419 have the potential to offer additional, unique benefits to the current antithrombotic treatments for high-risk patients.
Acknowledgements
This work has been supported in part by a grant from Laboratorios Lacer S.A. and Juan de la Cierva – ICCC (Spanish Ministry of Science and Education) to G. Vilahur. The authors thank Dr Jose Rodriguez and Anthony Lopez for their technical help.
Disclosure of Conflict of Interests
E. Salas is one of the inventors/patent applicants for the study compound. The other authors state that they have no conflict of interest.




