Occult Subchondral Osseous Cyst-like Lesions of the Equine Tarsocrural Joint
Presented at the 35th Annual Scientific Meeting of the American College of Veterinary Surgeons, October 2000, Arlington, VA.
Abstract
Objective— To report the clinical signs, diagnosis, management, and outcome of horses with occult sub-chondral osseous cyst-like lesions of the tarsocrural joint.
Design— Retrospective study.
Animals— Twelve horses with subchondral osseous cyst-like lesions (SOCLs) in the tarsocrural joint.
Methods— Information about history, examination findings, diagnostic techniques, and surgical procedures as obtained from medical records. Outcome was determined by patient re-evaluation and telephone communication with the referring veterinarian, owner, or trainer.
Results— Horses were aged from 3 to 29 years. Lameness (2 weeks to 1 year) varied from moderate to severe. Synovial fluid analyses (9 horses) yielded changes consistent with suppurative inflammation and positive bacterial culture was obtained in 4 horses. Eight of 9 horses that had intra-articular analgesia had a dramatic reduction in lameness. No abnormalities were detected on tarsal radiographs in 10 horses. Scintigraphy identified foci of increased radiopharmaceutical uptake in the distal tibia or talus of all horses, and the lesion was further characterized by computed tomography in 7 horses. SOCLs were identified in the medial malleolus (5), intertrochlear groove of the talus (4), lateral malleolus (2), and distal intermediate ridge of the tibia (1). One horse was euthanatized, 6 horses had surgical debridement, and 5 horses were managed conservatively. Four horses treated surgically (67%) returned to soundness. Conservatively treated horses continued to exhibit lameness but 2 were sufficiently sound for light pleasure riding.
Conclusions— Occult lesions of the tarsus not visible on radiographs can be detected by computed tomography and scintigraphy and may be a source of lameness.
Clinical Relevance— SOCL, possibly of septic origin, should be a differential diagnosis for persistent lameness localized to the tarsocrural joint without radiographic abnormalities. Surgical debridement of the lesions may offer the best prognosis for a return to athletic soundness.
Introduction
SUBCHONDRAL OSSEOUS cyst-like lesions (SOCLs) have been reported in horses.1–5 Their etiopathogenesis is incompletely understood, although developmental factors (osteochondrosis), sepsis, trauma, and ischemia have been proposed.6 The medial condyle of the distal femur is the most common location in horses but other sites include the phalanges, carpal bones, tibia, third metacarpal/tarsal bones, radius, talus, sesamoid bones, humerus, patella, scapula, tarsal bones, and hemimandible.1–5 Lameness associated with SOCL ranges from mild to severe 2,3,6 and management recommendations vary from conservative to surgical.2,3,6–8
Bone mineral density must change by approximately 30–50% before osseous lesions become radiographically apparent,9 so early or small SOCL may go unrecognized as a cause of lameness. Use of more sensitive diagnostic imaging modalities, such as scintigraphy, computed tomography (CT), and magnetic resonance imaging may be beneficial for early diagnosis of SOCL.9,10
SOCL of the tarsocrural joint have been described as rare with a poor prognosis for return to soundness.1,4,11 We hypothesized that diagnosis may be improved with scintigraphy and CT, and that surgical debridement of SOCL may result in a more favorable prognosis than previously reported. We report our experience with 12 horses with SOCL.
Materials and Methods
Medical records of horses admitted between 1986 and 2003 with confirmed SOCL in the tarsus were reviewed. Signalment, history, initial lameness evaluation, and imaging techniques were used, and surgical or medical treatments were recorded. The outcome was determined by lameness examination by the authors or was obtained by telephone interview of referring veterinarians, owners, and trainers.
Initial Evaluation
All horses had physical and lameness examinations, several had a complete blood count, arthrocentesis for joint fluid analysis, and intra-articular anesthesia (Table 1). Synovial samples were obtained after aseptic preparation of the joint, avoiding areas with evidence of soft tissue damage or cellulitis to avoid introducing potential contaminants into the joint. When samples were submitted for microbial culture they were placed in sterile culture swabs (BBL CultureSwab; Becton, Dickinson and Company, Sparks, MD) and immediately delivered to the laboratory for analysis. Lameness was graded using a 0–5 scale12 (0=sound; 5=non-weight bearing lameness) with the horse at a straight trot on a firm level surface.
| Horse | Signalment* | History | Lameness Evaluation† | Lesion‡ | Synovial Fluid§ | Bacterial Culture | Surgery | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 8 STD G | Soft-tissue trauma, 8 weeks duration | 4/5 LH, marked improvement with IA anesthesia of TCJ | MM | TNC, 1000 cells/μL TS, 2.3 g/dL SG, 1.018 | Negative | Yes | Persistent severe lameness |
| 2 | 29 TB G | Acute lameness 14 days post-injection with corticosteroids | 3/5 RH, moderate improvement with IA anesthesia of TCJ | MM | TNC, 590 cells/μL TS, 4.7 g/dL SG, 1.030 | Negative | No | Mild lameness; pleasure riding |
| 3 | 12 AP M | Laceration over tarsus, 3 weeks before admission | 3–5/5 RH, marked cellulitis over tarsus; significant improvement with IA anesthesia of TCJ | MM | TNC, 1800 cells/μL TS, 3.2 g/dL SG, 1.022 | Negative | No | Mild lameness, pleasure riding |
| 4 | 8 STD G | Trauma and cellulitis; 4 weeks duration | 2/5 RH, 4/5 after upper limb flexion; marked improvement after IA anesthesia of TCJ | MM | Not done | Not done | No | Persistent severe lameness |
| 5 | 3 MG M | Soft-tissue swelling after trauma during shipping | 2–5/5 intermittent LH, sound after IA anesthesia of TCJ | MM | TNC 2800 cells/μL TS, 3.2 g/dL SG, 1.022 | Negative | Yes | Sound |
| 6 | 5 STD S | Soft-tissue trauma with cellulitis 8 weeks duration | 5/5 RH, moderate improvement after IA anesthesia TCJ, sound after tibial and peroneal nerve blocks | ITG | TNC 900 cells/μL TS 2.0 g/dL SG 1.011 | Positive (ref. veterinarian) | No | Persistent severe lameness |
| 7 | 5 STD S | Injury to tarsus TCJ treated with corticosteroids | 3/5 LH, IA blocks not performed | ITG | TNC 6700 cells/μL (day 1) TS 2.0 g/dL SG 1.016 | Enterobacter agglomerans | Yes | Racing sound |
| 8 | 4 STD G | Cellulitis after trauma 6 weeks before treatment with antibiotics, NSAID | 4/5 left hind limb, 80% better after IA anesthesia TCJ | ITG | TNC – not available, TS, <2.0 SG 1.012 | Negative | Yes | Racing sound |
| 9 | 3 STD M | Soft-tissue swelling 6 weeks duration | 3/5 RH, IA blocks not performed | ITG | Not done | Not done | No | Persistent severe lameness |
| 10 | 14 WB G | Traumatic injury 12 weeks prior | 3/5 LH, unresponsive to IA anesthesia of TCJ | LM | Within normal limits (ref. veterinarian) | Not done | Yes | Persistent severe lameness |
| 11 | 7 QH G | Lameness, 1 year. Multiple TCJ treatments with corticosteroids | 4/5 LH, IA blocks not performed | LM | TNC 1900 cells/μL TS 3.3 g/dL SG 1.023 | Staphylococcus intermedius | No | Euthanasia |
| 12 | 3 TB G | Acute swelling after fall 2 weeks duration | 4–5/5 RH, moderate improvement after IA anesthesia of TCJ | DIT | TNC 39,000 cells/μL TS 7.3 g/dL SG 1.041 | Coagulase positive Staphylococcus | Yes | Sound |
- * Signalment (age in years, breed (STB, Standardbred; TB, Thoroughbred; WB, Warmblood; MG, Morgan; QH, Quarter Horse; AP, Appaloosa), sex (G, gelding; S, stallion; M, mare).
- † IA, intra-articular; TCJ, tarsocrural joint.
- ‡ MM, medial malleolus; LM, lateral malleolus; ITG, intertrochlear groove of the talus; DIT, distal intermediate ridge of the tibia.
- § TNC, total nucleated cell count; TS, total solids; SG, specific gravity.
- NSAID, non steroidal anti-inflammatory drugs.
Diagnostic Imaging
Each horse had radiographs of the affected tarsus (OG-1 film, Lanex Regular screens; Eastman Kodak, Rochester, NY or OM-1 film, Lanex Fine screens; Eastman Kodak). Projections were flexed lateral and off-angle dorsoplantar views, and standard projections (dorsoplantar, lateromedial, dorsolateral plantaromedial oblique, and dorsomedial plantarolateral oblique). All had scintigraphy after intravenous (IV) injection of approximately 200 mCi technetium Tc99m-labeled diphosphonate compound (Mallinckrodt, North Attleboro, MA). Static images of the axial skeleton were obtained 3–4 hours later using a large field of view gamma camera (Siemens Gammasonics Inc., Des Plaines, IL). Seven horses (1, 5–8, 10, 11), were anesthetized and had CT examination (Delta-Scan 2010, Ohio-Nuclear Inc./Technicare, Solon, OH, or SCT 300 T, Shimadzu, Kyoto, Japan; 400 mAs, 120 kVp, 7-mm axial slices) of the distal tibia and tarsus of affected and contralateral joints.
Surgical Procedures
Tarsocrural joint arthroscopy13 was performed in 6 horses (Table 1) positioned in dorsal recumbency. For lesions of the medial malleolus and talus (horses 1, 5, 7, 8), a dorsomedial approach was used. After joint distension, an arthroscopic sleeve and trocar were inserted through a 1-cm skin incision located close to the tendons of the cranial tibial and peroneus tertius muscles, just axial to the saphenous vein. For lesions of the lateral malleolus and distal intermediate ridge of the tibia (horses 10, 12), either a dorsomedial or dorsolateral approach (1-cm incision next to the long digital extensor tendon) was used. The instrument portal site was located by use of 20 g 1 1/2′′ needles to ensure adequate triangulation of the lesion. Cysts were debrided arthroscopically in 5 horses (1, 7, 8, 10, 12) using curved elevators (round and sharp end), curettes, and large- and medium-sized Ferris–Smith rongeurs (Sontec Instruments, Englewood, CO).
In horse 1, the cyst was filled with autogenous bone graft collected from the sternum whereas in horse 5, the cyst was filled with polymethylmethacrylate (PMMA) bone cement impregnated with 1-g-amikacin sulfate. This was accomplished in horse 5 using radio-opaque markers positioned over the distal tibia and tarsus during CT. Under fluoroscopic guidance, the markers were identified during surgery, allowing us to triangulate the location of the cyst and access it using an extra-articular trans-osseous approach. Several access channels into the cyst were made using a 2-mm drill overdrilled with a 3.5-mm drill bit. Once the cyst was entered, synovial-like fluid was observed coming out from the drill holes. After curettage, the cyst and drill channels were filled with the antibiotic-impregnated bone cement.
Postoperative Care
Potassium penicillin (22,000 U/kg IV, every 6 hours), ampicillin (10 mg/kg IV every 8 hours), or ceftiofur (2.2 mg/kg IV every 12 hours), in combination with gentamicin sulfate (2.2 mg/kg IV every 8 hours or 6.6 mg/kg IV once daily) was administered to all horses that had surgery and was continued for 24–120 hours, or longer depending on clinician preference. Phenylbutazone (4.4 mg/kg IV or orally every 12 hours) or flunixin meglumine (1.1 mg/kg IV every 12 hours) was administered pre-operatively and maintained (2.2 mg/kg orally every 12 hours) for up to 7 days. In some horses, anti-inflammatory drugs were administered for an extended period. The surgical site was bandaged until the sutures were removed at 2 weeks. Horses were box stall confined for 2 weeks followed by 4–6 weeks of stall rest with hand-walking exercise before providing turn out in a small paddock for a further 4–8 weeks.
Results
There were 6 Standardbred (SDB), 2 Thoroughbred (TB), 1 Quarter Horse (QH), 1 Warmblood (WB), 1 Morgan (MGN), and 1 Appaloosa (AP; Table 1). Ages ranged from 3 to 29 years (median, 6 years). Seven horses were geldings, 3 were females, and 2 were intact males. All horses were admitted with a history of chronic hindlimb lameness (range, 2–52 weeks; mean [±SD], 9.5±13.7 weeks; median, 5.5 weeks) associated with trauma/laceration (horses 1, 3–10, 12) or after intra-articular medication of the tarsocrural joint by the referring veterinarian (horses 2, 11; Table 1).
Lameness Examination
Lameness observed at admission was graded from 2 to 5 (mean, 3.5±0.8; median, 3.8). Seven of 8 eight horses that had intra-articular anesthesia (mepivacaine hydrochloride; Carbocaine-V, Pharmacia & Upjohn Co, Kalamazoo, MI) of the tarsocrural joint (Table 1) had a dramatic improvement in lameness. Horse 6 required anesthesia of the tibial and peroneal nerves to achieve complete soundness.
Synovial Analysis
Cytologic analysis and culture of the synovial fluid was performed in 9 horses. Nucleated cell count was considered above the normal reference range (≤500 cells/μL) in 8 horses (not available for horse 8) and ranged from 590–39,000 cells/μL (mean, 6836±13,141 cells/μL; median, 1850 cells/μL). Total solids were above the normal reference range (≤2.0 g/dL) in 6 horses, ranging from 2.3–7.3 g/dL (mean, 4±1.8 g/dL; median, 3.25 g/dL). Cytologic evaluation was consistent with suppurative inflammation in 4 horses with a positive bacterial culture (horses 6, 7, 11, 12). Bacteria isolated included Enterobacter agglomerans (horse 7), Staphylococcus intermedius (horse 11), and coagulase-positive Staphylococcus (horse 12). In horse 6, the referring veterinarian obtained a positive culture but the organism isolated was not documented.
Imaging
Abnormalities were not initially identified on tarsal radiographs in 10 (83%) horses (Fig 1), although in horses 3 and 12, SOCL were identified after scintigraphy using high-detail radiography. On scintigraphy, focal areas of intense radioisotope uptake were identified in all horses at the level of the distal aspect of the tibia or the talus (Fig 2). In 7 horses, lesions were further characterized as a hypodense focus surrounded by a sclerotic rim using CT (3, 4). In these horses, the lesions ranged in diameter from 5 to 8 mm (mean [±SD], 6.57±0.98 mm). Horses 1–5 had SOCL of the medial malleolus, horses 6–9 had SOCL of the talus, horses 10 and 11 had SOCL of the lateral malleolus, and horse 12 had SOCL of the distal intermediate ridge of the tibia. Horse 11 was euthanatized after SOCL was diagnosed. Gross dissection of the limb confirmed the presence of the cyst.
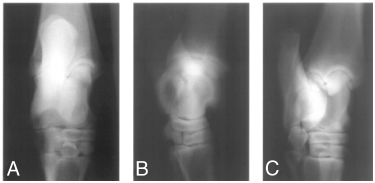
Horse 5. Dorsoplantar (A), lateromedial (B) and dorsolateral plantaromedial oblique (C) radiographic projections of the tarsus at admission. Note the lack of radiographic abnormalities .
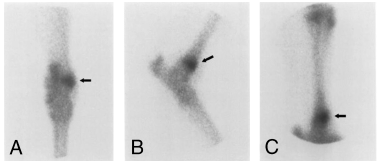
Horse 5. Scintigraphic evaluation of the tarsus with projections similar to 1. Note the focal area of radioisotope uptake at the level of the medial malleolus (dark arrow) .
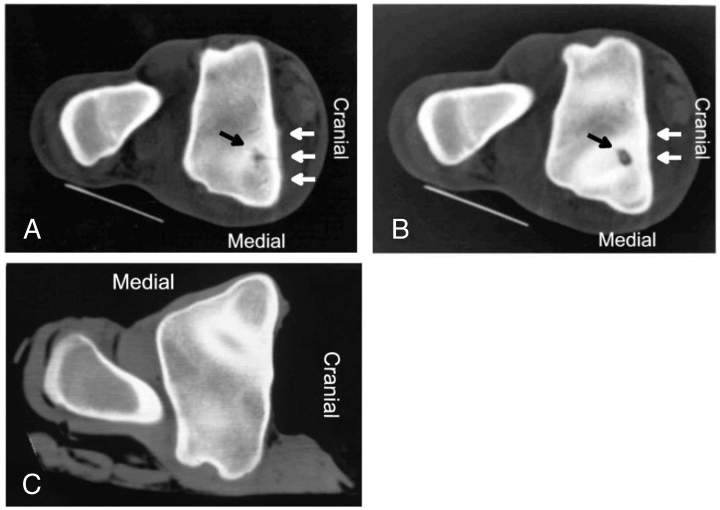
Horse 5. (A, B) Cross-sectional computed tomography (CT) images (proximal to distal) of the distal aspect of the tibia. A hypodense focus with a sclerotic rim is apparent at the level of the medial trochlear groove/medial malleolus (dark arrow). Note the mild periosteal reaction on the cranial aspect of the tibia (white arrows). (C) Cross-sectional CT scan of the same area in the contralateral normal limb .
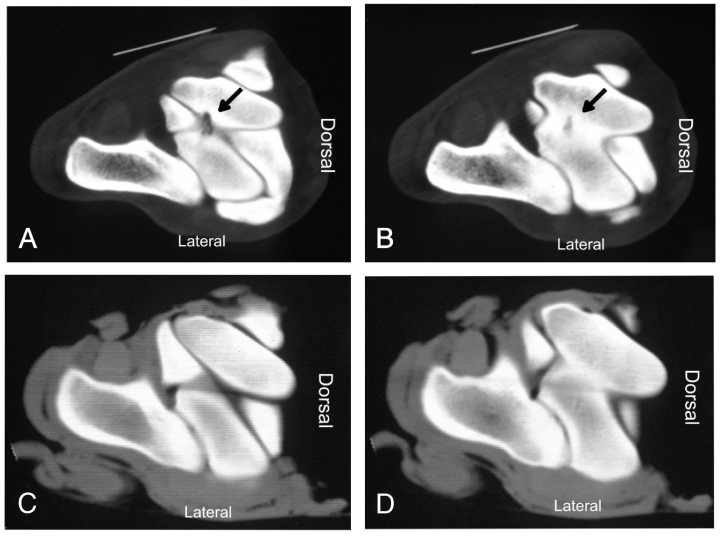
Horse 8. (A, B) Cross-sectional computed tomography (CT) images (proximal to distal) of the talus. A hypodense focus with a sclerotic rim is apparent at the level of the intertrochlear groove of the talus (dark arrow). (C, D) Cross-sectional CT image of the same area in the contralateral normal limb .
Surgical Treatment
Six horses had surgical debridement of the SOCL. Four (67%) returned to their previous level of soundness, whereas the other 2 remained lame (Table 1). Horse 1, which had the cyst packed with an autogenous bone graft, had persistent severe lameness after surgery. Horse 5, which had the cyst foraged through an extra-articular approach and packed with antibiotic-impregnated PMMA, returned to complete soundness. Horse 12 had regional limb perfusion with either amikacin sulfate or ceftiofur at surgery, and every other day for 3 treatments, and was sound under saddle 6 months later.
Medical Treatment
Five horses were treated medically after the owners declined surgical intervention. Treatment was stall rest with hand-walking exercise or limited turnout in a small paddock for a period of 2–8 weeks (mean, 4.4±2.2; median, 4), administration of phenylbutazone (2.2–4.4 mg/kg orally twice daily for 7–14 days, and then 2.2 mg/kg orally once daily for 7–14 days), before resuming controlled exercise. Owners of horses 2 and 3 continued to administer phenylbutazone as needed. All 5 horses had variable lameness after treatment, although horses 2 and 3 were used for light pleasure riding.
Discussion
SOCL at locations other than the medial condyle of the femur have a relatively low incidence.3,6,8,14–16 The development of SOCL is not completely understood but several causes have been postulated including failure of endochondral ossification, local trauma, focal sepsis, and ischemia.3,7,15–18 Failure of endochondral ossification could potentially explain the cysts in our horses, but the late onset of clinical signs (horses with a median age of 7 years) makes this unlikely. Trauma, although possible, would likewise not easily explain lesions located in the relatively non-weight bearing lateral and medial malleoli. We consider infection a likely cause of SOCL in our horses because of the severity of lameness, sudden onset, history of previous intra-articular injection, soft-tissue trauma involving the joint, or positive bacterial culture from synovial fluid. However, because of the retrospective nature of our study and with only 4 horses yielding positive synovial fluid culture, infection cannot be considered the unequivocal cause in all of these horses.
Inflammatory mediators such as eicosanoids and matrix metalloproteinases may have a role in formation of unicameral bone cysts in human patients.18 Although such cysts do not correlate well with SOCL in horses, the role of these inflammatory mediators could be similar. Von Rechenberg and colleagues19,20 reported production of neutral metalloproteinases, nitric oxide, and prostaglandin E2 by fibrous tissue found within SOCL that appear to be involved in progression and expansion of these lesions by osteolysis and bone resorption. It is possible that during septic arthritis, bacteria and these enzymes could cause significant degradation of the articular cartilage. Once this happens, a fissure on the articular surface is created, which will allow the intrusion of synovial fluid into the underlying bone. This, together with the increase intra-articular pressure created by the synovitis (hydrodynamic theory),21 the presence of degradative enzymes and potentially, secondary necrosis caused by a septic process, could lead to the formation of SOCL. The role of the synovial fluid in the formation of SOCL has been explained by Schmalzried et al, 21 who proposed a defect in the articular cartilage and an increase in synovial pressure as fundamental factors in the development of SOCL in 4 mature human patients.21
Our data suggests that SOCL are frequently unaccompanied by radiographic abnormalities and hence their incidence as a cause of tarsal lameness may be higher than previously reported. Considering the comparatively small size of SOCL, the need for a 30–50% change in bone density for a lesion to become radiographically apparent, the irregular contours of the tarsal skeleton, and the summation of bone densities reflected in conventional radiographs, it is not surprising that SOCL are often not detected on radiographs. By contrast, scintigraphy has the necessary sensitivity to localize lesions, but lacks the specificity to characterize features such as size, degree of mineralization, and communication with adjacent articulations. This lack of sensitivity is not the case for CT whereby multiple images of a particular area can be obtained resulting in an increased level of detail as well as a 3-dimensional representation for recognition and characterization of a specific lesion. To accurately identify SOCL and avoid confusion with normal anatomical structures (e.g., synovial fossae), the contralateral limb was used as a control (3, 4), as well as published guidelines, which describe the CT anatomy of the equine tarsus.22
Use of local anesthesia can aid diagnosis of SOCL. Nine horses had intra-articular tarsocrural anesthesia using mepivacaine hydrochloride and 8 horses had substantial reduction in lameness (≥50%). This response may have been related to the presence of a communicating channel, or cloacae, between the cyst and joint. However, it is unclear why horse 10 whose cyst communicated directly with the joint and was debrided arthroscopically within the joint, did not respond to local anesthesia. Possible reasons include use of a small volume of local anesthetic and insufficient time for an effect to be observed. Because of this study's retrospective nature, it was not possible to discern the cause further. Three horses did not have diagnostic anesthesia because this had previously been performed by the referring veterinarian, or because of the presence of joint effusion, or the considered potential for joint infection secondary to periarticular cellulitis.
Six horses were managed surgically by arthroscopic or extra-articular debridement of the SOCL; 4 (67%) horses returned to their previous level of soundness and resumed training or racing. Two horses remained lame. Horse 1 had an autogenous bone graft, which did not improve the lameness. A similar outcome was reported by Jackson et al, 23 who grafted medial femoral condyle cysts without observing an improved outcome. Grafted cysts developed secondary cysts, which were not apparent in ungrafted lesions.23 Their proposed cause for this phenomenon includes microanatomic or microvascular differences between the graft and parent bone, as well as a failure of neovascularization and graft incorporation because of synovial fluid around the spaces created during placement of the cancellous bone graft.23 Based on their results, we question the potential benefit of grafting SOCL.
Pluripotential mesenchymal stem-cell transplantation has yielded disappointing results.24 Chondrocyte and insulin-like growth factor-1 grafting of SOCL in the equine stifle has shown some promise although more research is needed.25 In the horse with the SOCL packed with antibiotic-impregnated PMMA, the successful outcome might reflect local delivery of high antibiotic concentration, adequate decompression of the cyst, or obliteration of the cyst with cement thereby preventing re-entry of synovial fluid which might otherwise have perpetuated the cyst. More novel products that can deliver high concentration of antibiotics to a specific site, as well as establish a scaffold for new bone growth, such as collagen sponges, calcium-phosphate cement, and hydroxyapatites, which do not have the shortcomings of PMMA (non-biodegradable, exothermic curing reaction, poor long-term material properties), are available for veterinary use,26–28 which would make the material we used in horse 5 sub-optimal.
All 5 horses treated conservatively had variable lameness. Only 2 (40%) horses were used for light pleasure riding with the aid of anti-inflammatory drugs 4–6 months after hospital discharge. None of these horses were managed with systemic long-term antibiotics. We recommend that more aggressive management, including surgical debridement of SOCL and local infusion of high-concentration antibiotics by intra-articular injection,29 regional limb perfusion,30 and/or biodegradable carrier systems,27,28 should be considered when managing tarsocrural SOCL when a septic process is highly likely or cannot be ruled out. Regional limb perfusion was used both during and after surgery in horse 12, where culture yielded a coagulase positive Staphylococcus organism. This horse responded favorably to treatment and returned to its previous soundness. Scheuch et al.,30 reported a 61-fold increase over the previously published minimum inhibitory concentration (MIC) of amikacin sulfate for common synovial pathogens of the tarsocrural joint, after regional limb perfusion using the saphenous vein.30 Werner et al,31 reported bone concentrations of gentamicin above the MIC for 8 hours after regional limb perfusion of the metacarpophalangeal joint using the lateral palmar vein.31 Regional limb perfusion has the advantage of being relatively easy to perform, delivering a high concentration of antibiotics to a specific area more effectively than routine systemic administration, and maintaining systemic serum concentrations to a minimum, minimizing negative side-effects of the particular drug, such as nephrotoxicity, etc.30 In addition, because of the smaller volume of the drug that is needed to deliver the desired antibiotic concentration, the cost of treatment can be substantially reduced and allow clinicians to use drugs which otherwise would have been prohibitively expensive.
Outcome for horses with tarsocrural SOCL appears to be variable regardless of treatment and hence the prognosis should be guarded. However, the likelihood of affected horses returning to their previous level of soundness is apparently enhanced by arthroscopic or extra-articular curettage of the SOCL. Surgical management of tarsocrural SOCL is consistent with recommendations for treating SOCLs at other locations4,8,10,11,16 although conservative management has resulted in satisfactory resolution in some horses.6,11
SOCL of the tarsocrural joint are best diagnosed by enhanced imaging modalities including CT and scintigraphy. SOCL can cause marked and chronic lameness. Whereas aggressive surgical debridement of SOCL provides the greatest likelihood for future soundness, a guarded prognosis should be offered.




