Collagenolytic Protease Expression in Cranial Cruciate Ligament and Stifle Synovial Fluid in Dogs with Cranial Cruciate Ligament Rupture
Supported by a grant from the American Kennel Club Canine Health Foundation, a Hohn-Johnson Research Award from the Veterinary Orthapaedic Society, and an American College of Veterinary Surgeons Surgeon-in-training grant awarded to Dr. Danova. The contents of this publication are the sole responsibility of the authors and do not necessarily represent the views of the American Kennel Club Canine Health Foundation.
This work was presented at the 14th Annual American College of Veterinary Surgeons Symposium, Denver, CO, October 2004.
Abstract
Objective— To determine expression of collagenolytic genes and collagen degradation in stifle tissues of dogs with ruptured cranial cruciate ligament (CCL).
Animals— Six dogs with CCL rupture and 11 dogs with intact CCL.
Procedures— Gene expression in CCL tissue and synovial fluid cells was studied using reverse transcriptase-polymerase chain reaction (RT-PCR). Collagen degradation was studied using CCL explant cultures and a synovial fluid bioassay.
Results— Expression of matrix metalloproteases (MMP) was not found in young Beagles with intact CCL; however, increased expression of MMP-3 was found in CCL tissue from older hounds with intact CCL, when compared with young Beagles. In dogs with ruptured CCL, expression of MMP-2 and -9 was increased in stifle tissues, when compared with dogs with intact CCL. Similar to MMP-9, expression of tartrate-resistant acid phospatase (TRAP) and cathepsin S was only found in stifle tissues from dogs with ruptured CCL; in contrast, expression of cathepsin K was found in all ruptured and intact CCL. Collagen degradation was increased in ruptured CCL, when compared with intact CCL.
Conclusion— Rupture of the CCL is associated with up-regulation of expression of MMP-2 and -9 (gelatinase A and B), TRAP, and cathepsin S, and increased degradation of collagen.
Clinical Relevance— These findings suggest that MMP-2, -9, cathepsin S, and TRAP may be important mediators of progressive joint destruction in dogs with CCL rupture. These genes are markers for macrophages and dendritic cells. MMP and cathepsin S pathways may offer novel targets for anti-inflammatory medical therapy aimed at ameliorating joint degradation associated with inflammatory arthritis.
INTRODUCTION
RUPTURE OF the cranial cruciate ligament (CCL) in most dogs is a degenerative condition associated with development of inflammatory stifle arthritis.1–3 Stifle arthritis and associated mid-substance CCL rupture is an important cause of lameness in the dog with poor long-term prognosis; progressive lameness and stifle osteoarthritis usually develop, irrespective of surgical treatment.4–6 Dogs with CCL rupture typically have inflammatory changes in the synovial intima, the epiligament of the CCL, the core region of the CCL, and alterations to the cell population of the stifle synovial fluid.1,2,7 These inflammatory changes and the reduction in the structural properties of the CCL associated with chondroid degeneration of ligament fibroblasts that develops with aging in the dog8,9 may be important factors that mediate progressive tearing and then complete rupture of the CCL during normal activity. Synovitis is an important factor that can promote structural degradation of the CCL experimentally in rabbits.10
Although the cause of the synovial inflammation is unknown, recent work has shown that the synovial intima contains large numbers of dendritic cells and macrophage-like cells which express tartrate-resistant acid phosphatase (TRAP), together with increased immunoglobulin deposition.2,3,11,12 TRAP+ mononuclear cells within the synovium typically express many degradative collagenolytic enzymes including cathepsin K, cathepsin S, and matrix metalloproteases (MMPs), such as MMP-9; the presence of TRAP+ mononuclear cells within the synovium is a characteristic feature of inflammatory arthritis, and is considered a key factor promoting progressive and irreversible articular cartilage and joint destruction.13–17 Production of MMPs, cathepsins, and TRAP at sites of inflammation potentially all contribute to matrix degradation.14,16–18 Collagen is the major biological substrate of cathepsin K; fragmentation of collagen occurs both outside and within cells during pathologic matrix turnover.19,20 Although the specific function of TRAP in macrophages is not fully understood, TRAP is thought to have an important role in macrophage and dendritic cell-induced inflammatory responses, including phagocytosis.21,22 TRAP may also have a specific functional role in antigen presentation during intracellular processing of MHC class II-containing vesicles.21 TRAP can also fragment triple helical collagen by generation of reactive oxygen species.18 Dogs with CCL rupture typically have osteophyte formation at the time of surgery; this suggests that synovitis has been established for some time, as the synovial macrophage is the key mediator of osteophyte development in arthritic joints; degenerative changes are also commonly found in the contralateral stifle of affected dogs.23,24 Increased immunoglobulin deposition and expression of MHC class II in the synovium of dogs with CCL rupture3,11 suggests that the associated inflammatory arthritis has an immune-mediated component. However, the antigen which acts as the trigger for persistent stifle synovitis is not known; it is unlikely to be exposure of neo-epitopes of collagen.25
To further understanding of how inflammatory stifle arthritis may contribute to progressive CCL degradation and eventual rupture, we wished to determine the pattern of collagenolytic gene expression in the CCL and the stifle synovial fluid. We also wished to determine whether collagenolytic activity within the stifle joint was primarily cell associated. We hypothesized that the expression of MMPs, cathepsins, and TRAP would be associated with CCL rupture and that this degradative activity would be primarily cell associated.
MATERIALS AND METHODS
Dogs
Specimens of ruptured CCL and stifle synovial fluid were collected from 6 dogs with cruciate disease during surgical treatment. In addition, CCL specimens were collected from 11 normal dogs with intact CCL that were humanely euthanatized by intravenous administration of barbiturates for reasons unrelated to our study. This group of both small young (5 beagles) and large dogs (6 hounds) was selected as a baseline for comparison with the CCL rupture group. Age, weight, and gender were recorded for each dog.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Immediately after collection, a portion of each CCL tissue and stifle synovial fluid specimen was processed for RT-PCR. For RNA extraction, ruptured CCL were dissected and homogenized in 1 mL Trizol reagent (Invitrogen, Carlsbad, CA). Cell pellets from each stifle synovial fluid specimen were also mixed with 1 mL Trizol reagent. The cell-free fluid component of the stifle synovial fluid was stored at −80°C for use in an explant culture experiment (see below). After incubation at approximately 25°C (room temperature [RT]) for 5 minutes, 200 μL chloroform (Sigma Chemical Co., St Louis, MO) was added and the mixture was shaken vigorously by hand for 15 seconds and then incubated at RT for a further 10 minutes. The aqueous phase was separated by centrifugation at 4°C and 12,000 rpm for 15 minutes. The aqueous phase was then mixed with 500 μL of isopropyl alcohol (Fisher Scientific, Hannover Park, IL) in a new microtube. The mixture was incubated at RT for 10 minutes and then centrifuged at 4°C and 12,000 r.p.m. for 10 minutes.The pellet was washed with 1 mL 75% isopropyl alcohol (Fisher Scientific) and centrifuged at 4°C, 8600 r.p.m. for 5 minutes. The pellet was then air dried for 10 minutes at RT and dissolved in 100 μL of RNase-free water. Total RNA was further purified using a RNA clean-up kit (Qiagen, Valencia, CA). cDNA was generated from 0.2 to 1 μg of total RNA by using the superscript III first-strand synthesis system for RT-PCR (Invitrogen).
PCR was performed using standard methods. A panel of oligonucleotide primers was designed for the following collagenolytic enzymes: MMP-1 (collagenase 1), MMP-2 (gelatinase A), MMP-3 (stromelysin 1), MMP-9 (Gelatinase B), MMP-13 (collagenase 3), TRAP, cathepsin K, and cathepsin S (Table 1). Primers were designed from known canine gene sequences or regions of homology between the specific genes of other higher mammals. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene. All PCR reactions were carried out in a final volume of 50 μL, which contained 5 μL 10 × magnesium-free PCR buffer (Invitrogen), 1.5 μL 50 mM MgCl2 (Invitrogen), 1 μL 10 mM dNTPs (Invitrogen), 1 μL 25 μM forward primer, 1 μL 25 μM reverse primer, 1 μL cDNA and 0.25 μL platinum Taq polymerase enzyme (Invitrogen). cDNA synthesis was performed by denaturing the reaction mixture for 2 minutes at 94°C. Thirty cycles of amplification (denaturizing at 94°C for 30 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 90 seconds) were then performed. A final extension was then performed for 4 minutes at 72°C with an end step at 4°C. PCR fragments were separated on 1.5% agarose gels, stained with ethidium bromide staining and observed using UV light. PCR products were subsequently sequenced to confirm production of the correct amplicon.
| mRNA Targets | Primer Type | Olignonucleotides (5’–3′) | Amplicon Size (bp) | Sequence References |
|---|---|---|---|---|
| MMP-1 | Forward | TTCGGGGAGAAGTGATGTTC | 530 | NM-002421 (human), NM-174112 (bovine) |
| Reverse | GCAGTTGAACCAGCTATTAGC | |||
| MMP-2 | Forward | ATGGCAAATACGGCTTCTGC | 288 | AF177217 (canine) |
| Reverse | TGCAGCTCTCATGCTTGTTG | |||
| MMP-3 | Forward | ACAGTGGTCCTGTCGTTGAA | 269 | AY183143 (canine) |
| Reverse | AGTCACCTCCTTCCAGACAT | |||
| MMP-9 | Forward | CGCTATGGCTACACTCAAGT | 217 | AB006421 (canine) |
| Reverse | AAGTGATGTCGTTGTGGTGC | |||
| MMP-13 | Forward | CTGAGGAAGACTTCCAGCTT | 250 | AF201729 (canine) |
| Reverse | TTGGACCACTTGAGAGTTCG | |||
| TRAP | Forward | CAGCTGTCCTGGCTCAA | 288 | NM001611 (human), NM019144 (murine) |
| Reverse | TAGCCGTTGGGGACCTT | |||
| Cathepsin K | Forward | CAGTGTGGTTCCTGTTGGGCTTT | 578 | AY738221 (canine) |
| Reverse | CACATCTTGGGGAAGCTGG | |||
| Cathepsin S | Forward | CGTCTCATCTGGGAAAAGAA | 482 | AY156692 (canine) |
| Reverse | GCTTTGTAGGGATAGGAAGC | |||
| GAPDH | Forward | ACCACAGTCCATGCCATCAC | 450 | NM002046 (human), NM017008 (murine) |
| Reverse | TCCACCACCCTGTTGCTGTA |
- MMP, matrix metalloproteinase; TRAP, tartrate-resistant acid phosphatase; GAPDH, glyeraldehyde-3-phosphate dehydrogenase.
CCL Explant Culture and Synovial Fluid Bioassay
To assess collagen fragmentation in CCL tissue specimens, CCL explants were cultured in 12-well plates, each containing 1 mL of culture medium. Serum-free Dulbecco's modified Eagle's medium, supplemented with 20 mM HEPES (DMEM containing HEPES, Invitrogen) and antibiotic–antimycotic (streptomycin 100 μg/mL, benzylpenicillin 100 μg/mL, amphotericin 0.25 μg/mL; antibiotic–antimycotic (100 ×) liquid, Invitrogen), was used and explants were cultured for 2 days at 37°C in an atmosphere of 5% CO2. Collagenolytic activity in the extracellular component of stifle synovial fluid was determined by using a piece of previously frozen patella tendon from a normal dog as a collagen substrate in a bioassay. Patella tendon was incubated with a mixture of 50% synovial fluid and 50% culture medium for 48 hours. This assay was not performed for small dogs with intact CCL because the available volume of synovial fluid was insufficient for the assay to be performed. Supernatants were stored at −80°C for further analyses.
Generation of collagen fragments in explant culture and bioassay supernatants was studied using immunoassays for epitopes from the triple helical region (∝1(I) 620–633α helical peptide [HEL]) and the telopeptide regions (cross-linked carboxyterminal telopeptide of type I collagen [ICTP], and the hydroxypyridinium collagen cross-link pyridinoline [PYD]) of the collagen molecule) (Metra Helical Peptide and Metra PYD ELISA, Quidel Corporation; ICTP RIA, Diasorin).
Statistical Analysis
Differences between groups (dogs with CCL rupture, young dogs with intact CCL, large dogs with intact CCL) in weight and age were analyzed with an ANOVA and the Kruskal–Wallis ANOVA test respectively; data were not normally distributed for age. The Kruskal–Wallis ANOVA test was used to determine whether differences in collagenolytic gene expression existed between groups. The Kruskal–Wallis ANOVA test was also used to determine whether there were differences in the generation of HEL, PYD, and ICTP fragments from CCL explants between groups; data were not normally distributed. Student's t-test for unpaired data was used to determine whether there were differences in the generation of collagen fragments from the stifle synovial fluid bioassays. Differences were considered significant at P<.05.
RESULTS
Dogs with ruptured CCL were Golden retriever (n=2), Labrador retriever (n=1), Pit Bull Terrier (n=1), Dobermann Pinscher (n=1), and Bulldog (n=1); mean (±SD) weight 36.0±10.4 kg and age, 4.5±2.7 years. Three dogs were ovariohysterectomized females, 2 dogs were castrated males, and 1 dog was male. Lameness duration was 16±9 weeks (range, 4–28 weeks). In 1 dog, CCL rupture was associated with medial patella luxation. All dogs with CCL rupture had palpable instability of the stifle on physical examination.
All large dogs with intact CCL were female Hounds (weight, 23.4±2.6 kg; age, 3.4±0.9 years). All small dogs with intact CCL were female Beagles (weight, 9.3±2.1 kg; age, 0.8±0.1 years). Body weight was significantly different between all 3 groups (P<.01). Beagles were significantly younger than the other 2 groups (P<.05).
Expression of MMPs was variable among groups of dogs. In the young Beagles with intact CCL, expression of MMPs was not detected in either CCL tissue or stifle synovial fluid cells. In contrast, expression of MMP-3 in CCL tissue was significantly increased in large dogs with intact CCL (P<.05), compared with young Beagles with intact CCL; expression of MMP-1 in CCL tissue was also found. Expression of MMP-1 and -2 in stifle synovial fluid cells was also significantly increased in large dogs with intact CCL, when compared with small dogs with intact CCL (P<.05). In dogs with ruptured CCL, expression of all MMP genes studied was detected in CCL tissue. In particular, expression of MMPs-2 and -9 was significantly increased in CCL tissue, when compared with both small and large dogs with intact CCL (P<.05); this change in gene expression was also seen in stifle synovial fluid cells from dogs with ruptured CCL. Expression of MMP-9 was only found with tissues from dogs with ruptured CCL. Similarly, expression of MMP-13 was only found in ligament tissue from dogs with CCL rupture (Fig 1).
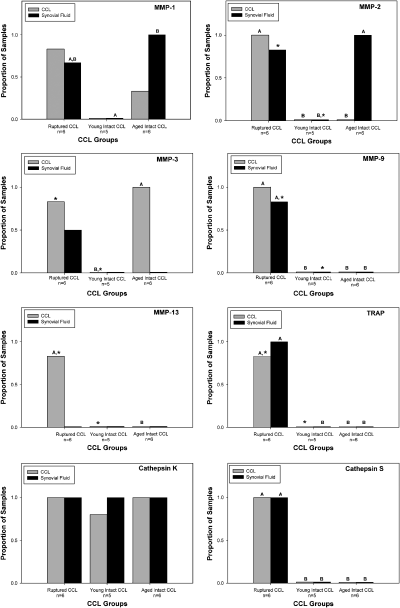
Effect of cranial cruciate ligament rupture (CCL) on expression of a panel of collagenolytic genes in CCL tissue and stifle synovial fluid cells. Gene expression was determined using reverse transcriptase-polymerase chain reaction. Within a tissue type, columns with differing letters are significantly different; columns without letters are not significantly different. For comparison of pairs of columns within a tissue type which are marked with an *, P=.06.
Similar to MMP-9, expression of TRAP and cathepsin S was only found in CCL tissue and stifle synovial fluid from dogs with ruptured CCL; expression of TRAP and cathepsin S was significantly increased in dogs with ruptured CCL (P<.05). In contrast, expression of cathepsin K in CCL tissue and stifle synovial fluid cells was similar in all groups of dogs (Fig 1).
The generation of fragments from both the triple helical HEL and telopeptide regions (ICTP, PYD) of the collagen molecule was increased in ruptured CCL explants, when compared with explants prepared from intact CCL (P<.05). Although the concentrations of HEL, ICTP, and PYD in the supernatants from the stifle synovial fluid bioassay experiment were much higher than for the CCL explant experiment, only ICTP concentrations were increased in the synovial fluid bioassay supernatants from dogs with ruptured CCL, when compared with large dogs with intact CCL (P<.05; Fig 2).
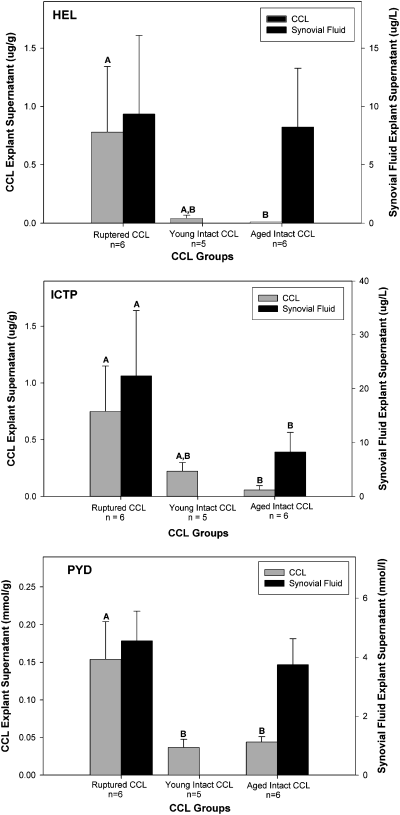
Effect of cranial cruciate ligament rupture disease status (CCL) and collagenolytic activity of the extracellular component of stifle synovial fluid on production of collagen fragments. Production of collagen fragments by CCL explants and a stifle synovial fluid bioassay was determined using ELISA for epitopes from the triple helical region (∝1(I) 620–633α helical peptide [HEL]) and the telopeptide regions (cross-linked carboxyterminal telopeptide of type I collagen [ICTP], and the hydroxypyridinium collagen cross-link pyridinoline [PYD]) of the collagen molecule. Within a tissue type, columns with differing letters are significantly different; columns without letters are not significantly different. For comparison of pairs of columns within a tissue type which are marked with an *, P=.06.
DISCUSSION
Although it has been recognized for some time that inflammation within the synovium of the stifle is typically found in dogs with CCL rupture, the importance of this pathologic feature in the mechanism that leads to progressive rupture of the CCL and the development of severe stifle arthritis remains controversial. Key features of the synovial inflammation include the presence of large numbers of dendritic cells expressing MHC Class II,3 and the presence of TRAP+ macrophage-like cells that express cathepsin K in both the synovium and the CCL epiligament.1,2,12 In the present study, we detected cathepsin K, cathepsin S, and TRAP mRNA within the CCL and associated synovial intima; expression of these genes is consistent with an inflammatory arthritis with an immune-mediated component.14–16,21,22
We only found expression of TRAP and cathepsin S mRNA in the stifles of dogs with CCL rupture. In contrast, cathepsin K mRNA appeared constitutively expressed in stifle tissues from both dogs with ruptured and intact CCL. Detection of cathepsin K in all stifle tissues is not surprising, as the main biological substrate for cathepsin K is triple helical collagen, and cathepsin K is known to have an important role in normal connective tissue remodeling,19,26 as well as in pathologic degradation of connective tissue.14,15,20,27
The critical role of cathepsin S in antigen presentation has only recently been recognized. In activated macrophages and dendritic cells, cathepsin S mediates the degradation of the MHC class II invariant chain Ii during antigen presentation.28,29 Therefore, it is not surprising that both expression of cathepsin S mRNA, TRAP mRNA,21,22 and MHC class II protein3 is found in stifles from dogs with ruptured CCL. Detection of cathepsin S expression in macrophage-like synovial cells suggests that cathepsin S may have dual functions of antigen presentation and matrix degradation in inflamed synovium.15 Detection of cathepsin S expression in stifles of dogs with CCL rupture supports the hypothesis that the synovial inflammation is, at least in part, immune-mediated. This is an important finding, as inhibition of cathepsin S in vivo can ameliorate immune-mediated inflammation.28,30 The antigens within the stifle joint that may stimulate expression of cathepsin S and contribute to the initiation and progression of the inflammatory stifle arthritis are unknown. This is a key gap in knowledge, given our general working hypothesis that inflammation of the stifle joint precedes and eventually precipitates rupture of the CCL in many dogs.
Although the role of TRAP in the development of joint inflammation is not fully understood, expression of TRAP is associated with the production of many other degradative enzymes in synovial macrophage-like cells.16 Therefore, it is not surprising that we found that expression of TRAP in stifle tissues from dogs with ruptured CCL is also associated with MMP-2 and -9; 2 metalloproteases that are known to be expressed in joints affected with inflammatory arthritis, and in synovial macrophage-like cells.16,17,31 Although MMP-1 and -3 are considered important mediators of joint destruction in rheumatoid arthritis,32 expression of these metalloproteases was found in both dogs with CCL rupture and dogs with intact CCL. Expression of MMP-13 was only found in ruptured CCL tissue; this MMP has also been implicated in joint degradation in rheumatoid arthritis.32 The pattern of metalloprotease expression we found in ruptured canine CCL is similar to that found in ruptured human anterior cruciate ligament.33 The finding of increased MMP expression in the intact CCL of older hounds, when compared with young Beagles is interesting; matrix turnover is known to be increased in intact Labrador Retriever CCL, a breed with increased knee laxity that is predisposed to CCL rupture, when compared with Greyhounds, a breed that is not predisposed.34 Taken together, these findings suggest that changes in the expression of collagenolytic enzymes within the CCL influence the development of subtle cranial caudal laxity over time; this may play an important role on the development of progressive CCL rupture.34
Although it is likely that upregulation of both the MMP/cathepsin and the cathepsin K/glycosaminoglycan collagenolytic pathways contribute to most of the collagen degradation within the CCL in both the intra- and extracellular compartments,19,20 the relative importance of each of these pathways remains unknown; both of these pathways are potential targets for drug therapy. Upregulation of cathepsin S and TRAP may also contribute to the milieu of degradative enzymes that fragment collagen13,15,18 and contribute to progressive destruction of the stifle. Although production of collagen fragments in the stifle synovial fluid bioassay experiment was much greater than in the CCL explant study, it is likely that much of this collagen degradation occurred within the stifle joints before the assay was set up, as the concentration of these collagen fragments was similar to that found in stifle synovial fluid from dogs with both ruptured and intact CCL.35 Differences in production of collagen fragments between dogs with ruptured CCL and dogs with intact CCL were more apparent in the CCL explant experiment, when compared with the stifle synovial fluid bioassay experiment. This finding suggests that collagenolytic activity is primarily cell- or tissue associated, and indirectly suggests that the presence of inflammatory cells expressing degradative enzymes within the synovium2 and CCL epiligament1 may be an important factor promoting weakening and rupture of the CCL,10 and that the intra-articular location of the CCL, per se, is less important.
This study has several limitations. We used relatively simple RT-PCR methods to study gene expression in CCL tissue from a small number of dogs. Use of quantitative PCR methods in a larger population of dogs would further understanding of the extent to which specific MMP and cathepsin genes are upregulated during development of inflammatory arthritis. However, we consider our methods adequate for the purpose of the present study. Quantification of collagen fragments is not a specific assay for different matrix turnover pathways. Variations in collagenolytic activity and collagen cross-linking within the patella tendon substrate may also have influenced release of collagen fragments, although the treatment of the substrate was the same for each assay performed. Further study of the relative contribution of MMPs, cathepsin K, cathepsin S, and TRAP to degradation of collagen within the CCL is warranted. All of the dogs with inflammatory arthritis we studied had complete rupture of the CCL and palpable instability of the stifle. As gene expression of cathepsin S, TRAP, and MMP-9 in stifle synovial fluid cells mirrors expression of these genes within the CCL tissue, further studies of stifle synovial fluid from dogs with mechanically stable stifles and early arthritis would help to determine whether expression of these genes of interest could be used as a specific biomarker for the early diagnosis of joint inflammation, before progressive degradation of the CCL leads to the development of stifle instability. However, in future work, it would also be important to determine whether expression of these genes is present in joints affected with less severe inflammatory changes and osteoarthritis.
Identification of a clinically relevant biomarker for inflammatory arthritis of the stifle will facilitate diagnosis of dogs with early disease for anti-inflammatory medical therapy, and allow changes in gene expression to be studied over time. As rupture of the CCL is known to be predisposed to certain breeds,36,37 identification of a biomarker for stifle joint inflammation would also help understanding whether CCL metabolism and stifle joint inflammation may be influenced by genotype or phenotype.
Surgical treatment of dogs with CCL rupture is one of the most common surgical procedures in canine orthopedics. However, despite the use of various surgical procedures, including tibial plateau leveling osteotomy, limb function after surgery is relatively poor.4 This is not surprising, given the findings in the present study and other related studies.1–3,7,9,11,31,35 The association between cathepsin S expression and CCL rupture, in particular, warrants further investigation. Progressive rupture of the CCL should be considered an idiopathic disease at the present time, the cause of which is unknown. Rupture of the CCL can occur from overt trauma, but this is not common.38 Although, the relationship between development of joint inflammation and development of stifle instability and CCL rupture remains controversial, there is now a growing body of evidence from several different research groups1–3,7,11,12,25,35 which suggests that immune-mediated inflammatory changes within the stifle are an important factor that could explain the clinical findings of chronic lameness, bilateral disease, and established osteoarthritis at the time of clinical diagnosis, that are found in most patients.4–6 Fundamental understanding of the mechanism that initiates and maintains inflammation of the stifle in dogs with CCL rupture is critical to our understanding of this important canine disease. Development of improved clinically relevant diagnostic tests for joint inflammation should help to confirm which develops first; synovial inflammation, or stifle instability and CCL rupture.
Acknowledgment
The author's would like to thank all members of the University of Wisconsin-Madison Veterinary Medical Teaching Hospital who have assisted with collection of tissue samples from dogs with CCL rupture.




