Comparison of 4 Blood Storage Methods in a Protocol for Equine Pre-operative Autologous Donation
Supported by the Center for Equine Health with funds provided by the Oak Tree Racing Association, the State of California pari-mutuel fund, and contributions by private donors. Additional support for laboratory analyses provided by IDEXX Veterinary Services, Inc., West Sacramento, CA. The John P. Hughes Memorial Endowment provided support for the pilot study performed during development of the protocol used in this study.
Presented at the 13th Annual Symposium of the American College of Veterinary Surgeons, Washington, DC, October 9–12, 2003.
Abstract
Objective— To compare viability of equine whole blood stored by 4 different methods, and to establish optimal storage protocols for an equine autologous blood donation program.
Study Design— In vitro study of stored equine whole blood.
Animals— Six healthy adult horses.
Methods— Blood from each horse was collected into 4 different containers: glass bottles containing acid–citrate–dextrose solution (ACD), plastic bags containing ACD, citrate–phosphate–dextrose (CPD), and CPD with supplemental adenine (CPDA-1). Blood was stored for 5 weeks and sampled at 2-day intervals. Standard hematologic and biochemical variables were evaluated, and adenosine-5-triphosphate (ATP) and 2,3-diphosphoglycerate (2,3-DPG) concentrations were measured and normalized to total hemoglobin content.
Results— Plasma hemoglobin, % hemolysis, lactate, potassium, ammonia, and lactate dehydrogenase (LDH) increased, whereas glucose concentration and pH decreased in all stored blood over 5 weeks. There was a temporal increase in hemolysis with all storage methods, but the increase was greatest in glass bottles. Lactate and ammonia were highest in CPD and CPDA-1 samples, indicating more active red blood cell (RBC) metabolism. 2,3-DPG concentrations decreased during storage, but were optimally preserved with CPDA-1. ATP concentrations were significantly higher for blood stored in CPDA-1, and were lowest in glass bottles.
Conclusions— Hematologic and biochemical values measured for blood stored in CPDA-1 are suggestive of improved RBC viability compared with other storage methods. With the exception of ATP, results from stored equine blood were similar to those reported for other species.
Clinical Relevance— Commercial CPDA-1 bags appear to be the optimal storage method for equine whole blood.
Introduction
INTRAOPERATIVE HEMORRHAGE is an anticipated complication of equine nasal and paranasal sinus surgery, and horses are frequently cross matched for homologous blood transfusion before elective sinus surgery.1 Traditional donor transfusions can be costly and time consuming, and are associated with numerous risks, including disease transmission and immune-mediated reactions.2,3 Eight major blood groups and over 30 alloantigens have been documented in horses, and there is a substantial potential for recognition of foreign antigens between recipient and donor.4,5 Standard erythrocyte compatibility tests of cross-matching and blood typing can fail to detect minor incompatibilities between donor and recipient and often do not accurately predict the survival of the transfused red blood cells.2,3 Even in the absence of incompatibilities or transfusion reactions, the lifespan of homologously transfused equine red blood cells is limited to 2–4 days.2,6 Autologous transfusion has potential advantages for longer lifespan of the transfused erythrocytes and minimized risk of transfusion reactions; however, therapeutic autologous transfusion has not been thoroughly investigated in horses.
As a result of the limitations of intraoperative homologous blood transfusion, pre-operative autologous donation (PAD) and subsequent transfusion during surgery has recently been used for elective sinus procedures in horses.7 PAD was developed in human medicine to minimize transfusion reactions by using the patient's own blood, and is currently used extensively in high-risk human patients.8,9 Appropriate storage is critical for this transfusion technique because the blood is drawn well in advance of surgery to allow for patient recovery. When initially developed, our equine PAD protocol involved collecting and storing blood in glass bottles containing acid–citrate–dextrose (ACD), the method traditionally used for collecting blood from donor horses.10,11
ACD was the first widely used anticoagulant and preservative for storage of human whole blood.12 Modifications to the formulation, including the addition of phosphate and an increase in pH, yielded the anticoagulant citrate–phosphate–dextrose (CPD; Table 1), which preserves 2,3-diphosphoglycerate (2,3-DPG) concentration longer in stored blood.12 Addition of adenine to CPD produced the formulation CPD with adenine (CPDA-1), which resulted in elevated ATP concentrations in stored blood as well as prolonged erythrocyte storage life.12
| Anticoagulant | ACD | CPD | CPDA-1 |
|---|---|---|---|
| Sodium citrate (g) | 1.48 | 1.66 | 1.66 |
| Citric acid (mg) | 493 | 188 | 188 |
| Sodium phosphate (mg) | –– | 140 | 140 |
| Dextrose (g) | 1.65 | 1.61 | 2.00 |
| Adenine (mg) | –– | –– | 17.3 |
| Volume (mL) | 67.5 | 63 | 63 |
- Formulations are for anticoagulants in plastic bag blood-pack units designed for collection of 450 mL of blood (Baxter Fenwal®, Deerfield, IL). ACD glass bottles contain 75 mL anticoagulant for collection of 500 mL of blood (MWI Veterinary Supply, Meridian, ID).
- ACD, acid-citrate-dextrose; CPD, citrate–phosphate–dextrose; CPDA-1, citrate–phosphate–dextrose with adenine.
Although ACD is still commonly used as an anticoagulant for equine blood collection, ACD solutions have largely been replaced by CPD and other additive solutions for human, canine, and feline whole-blood storage. Studies of the post-transfusion viability of canine and feline red blood cells have shown that whole blood can be stored with satisfactory post-transfusion viability for up to 35 days in CPDA-1 solution, compared with 21 days for blood stored in ACD solution.13
Advances in blood storage have also included modification of container types. Glass bottles originally designed for blood storage rapidly inactivate platelets, and the vacuum system can cause mechanical damage to red cells because of the resulting forceful cell impact on the glass during collection.14 By contrast, plastic bags (polyvinyl chloride) preserve platelet function, induce fewer changes in erythrocytes, and may help stabilize red cells.15
Because of the ease and convenience of rapid vacuum blood draw, glass bottles containing ACD have been recommended for equine whole-blood collection.10,11 However, glass bottles are no longer recommended for human or small animal blood storage because of poor long-term red blood cell (RBC) preservation and deleterious effects on platelets when compared with storage in plastic bags.16–18 One additional concern with glass is the risk of breakage during storage and transfusion.
Guidelines for storage of human and canine blood have been established; however, there are significant species differences in RBC metabolism, necessitating species-specific studies to design optimal protocols for equine blood storage. For example, whole-blood adenosine-5′-triphosphate (ATP) concentrations and intracellular electrolyte concentrations vary between species.19 Underlying differences in erythrocyte structure and metabolism between species may lead to altered storage responses.20,21 To our knowledge, there have not been any reports describing viability variables for erythrocytes in stored equine blood.
Evaluation of erythrocyte biochemical and hematologic variables in stored human and canine blood samples has allowed investigators to compare different blood storage media for those species. These studies have provided guidelines for storage duration of human and canine blood before transfusion. The structural, functional, and metabolic changes that occur in stored blood are reflected in part by electrolyte alterations, decreases in pH, glucose, and ATP, increases in lactic acid and plasma hemoglobin (Hb), and depletion of 2,3-DPG concentrations.12 Changes that occur during human blood storage are collectively referred to as the storage lesion, and are monitored as indirect indicators of RBC changes that ultimately lead to a loss of viability.17 The most dramatic electrolyte change is an increase in serum potassium. During storage, the sodium–potassium ATPase membrane pump progressively fails, and intracellular potassium leaks out of the cell.12 ATP and 2,3-DPG are also depleted during storage, largely because of extremely low pH levels caused by a rise in lactic acid and pyruvic acid.18 The decrease in ATP concentrations during storage has a direct deleterious effect on post-transfusion erythrocyte viability, because the energy from ATP is needed to maintain erythrocyte form, membrane pumps, and membrane phospholipid asymmetry.22 Similarly, 2,3-DPG, an organic phosphate that modulates the binding of oxygen with RBCs, has also been recognized as an important index of red cell health because of its ability to stabilize Hb in a low oxygen affinity conformation.17
Our purpose was to report biochemical changes in stored equine whole blood and to compare changes occurring in blood stored using 4 different commercially available methods: ACD glass bottles, ACD bags, CPD bags, and CPDA-1 bags. We hypothesized that viability of equine erythrocytes would be improved with storage in CPD and CPDA-1 bags compared with storage in ACD glass bottles or bags. Our ultimate goal was to improve long-term blood storage methods for use in a clinical equine PAD program.
Materials and Methods
Horses
Blood was obtained from 7 healthy adult horses (range, 4–19 years; mean, 10 years; 5 Quarter horses, 2 Thoroughbreds; 4 mares, 3 geldings.) Blood was drawn twice from 5 horses, with a 6-month recovery period between samples, whereas blood was drawn only once from 2 horses.
Blood Collection
Blood was collected from 6 horses for each section of the study. For the first set of experiments, hemolysis variables were evaluated on days 0, 3, 10, 17, 24, and 31 after blood collection. For the second part of the study a more complete biochemical analysis of samples was performed through 34 days of storage. The protocol for blood collection was identical for each section.
Blood (1850 mL) was obtained from each of 6 horses for each sampling period. Whole blood was collected directly into 4 different storage containers: ACD glass bottles (500 mL, MWI Veterinary Supply, Meridian, ID), ACD polyvinyl chloride bags (450 mL; Fenwal Products, Deerfield, IL), CPD bags (450 mL; Fenwal Products), and CPDA-1 bags (450 mL; Fenwal Products). Before sampling, the jugular catheter insertion site was shaved, prepared using standard aseptic protocols, and the skin infiltrated subcutaneously with 2 mL, 2% lidocaine. A 10 g, 3 in. (3.4 × 76 mm) intravenous catheter (Angiocath, Becton Dickinson, Sandy, UT) was inserted into the jugular vein, an injection adapter (Medex, Dublin, OH) was attached to the catheter hub, and the catheter was secured to the skin by sutures. Blood collection tubing (Blood Collection Set, Abbott Laboratories, North Chicago, IL) was attached to the injection adapter and inserted into the vacuum collection site for the glass bottles. For the 3 plastic bag storage units, collection tubing incorporated in the bags was used for blood collection under gravity flow.
Blood Storage and Sampling
Before blood storage, a sampling site coupler (Baxter Healthcare Corporation, Fenwal Division) was placed on each plastic bag to facilitate repeated sampling. Blood maintained in glass bottles was sampled through the administration port on the top of the rubber stopper. Blood was stored in a controlled-temperature blood bank refrigerator (Jewett, Ashville, NC) where temperature was consistently maintained between 1°C and 6°C. Each storage container was manually agitated for 60–90 seconds once daily and again before each sampling. For blood collected in section 1, 3 mL was aspirated aseptically from each storage container at 0, 3, 10, 17, 24, and 31 days after collection. For section 2, 1–4 mL blood was aspirated aseptically from each storage container at 0, 1, 3, 7, 8, 10, 14, 17, 21, 24, 28, 31, and 34 days after collection. All blood sampling was performed using a 22 g needle and a 3 or 6 mL syringe.
Section 1—Hemolysis Analysis
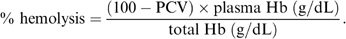
Section 2—Complete Biochemical Analysis
A commercially available enzymatic assay kit was used according to manufacturer's instructions to determine the ATP concentration of deproteinized whole-blood samples (Sigma Diagnostics, Proc. 366-UV, St Louis, MO). In brief, an aliquot of whole blood was mixed with 12% trichloroacetic acid solution (TCA) and centrifuged at 3000 rpm for 10 minutes. An aliquot of the supernatant was subsequently mixed with nicotinamide adenine dinucleotide (NADH)/3-phosphoglyceric buffered acid solution, and initial absorbance was read on a spectrophotometer (LS50B luminescence spectrophotometer, Perkin Elmer, Fremont, CA) at 340 nm. Optical density was determined again after addition of a glyceraldehyde 3-phosphate dehydrogenase/3-phosphoglyceric phosphokinase (GAPD/PGK) mixture. Blood ATP concentration (μmol/dL) was calculated from these 2 values following manufacturer's instructions. Final ATP concentrations were then normalized by total blood Hb values (μmol ATP/g Hb) for comparison between different storage methods and sampling times. Total Hb concentrations for this analysis and for 2,3-DPG analysis were measured on a Coulter Counter (T-890, Beckman). ATP assays were performed on blood sampled on days 0, 3, 7, 14, 21, 28, and 34. All values for ATP are reported as normalized values for μmol concentration of ATP/g total blood Hb.
2,3-DPG concentrations were determined using a commercially available assay kit according to manufacturer's instructions (Sigma Diagnostics, Proc. 35-UV). In brief, an aliquot of whole blood was mixed with 8% TCA and centrifuged at 3000 rpm for 10 minutes to deproteinize the sample. An aliquot of the protein-free supernatant was then mixed with NADH solution, ATP solution, and a GAPD/PGK mixture. Initial absorbance was read at 340 nm. Phosphoglycolic acid solution was then added to the mixture and final absorbance recorded after 30 minutes. Raw 2,3-DPG concentrations (μmol/mL) were determined, and final 2,3-DPG concentrations were then normalized by total blood Hb values (μmol 2,3-DPG/g Hb) for comparison between different storage methods and sampling times. 2,3-DPG assays were performed on blood sampled on days 0, 3, 7, 14, 21, 28, and 34. All values for 2,3-DPG are reported as normalized values for μmol concentration of 2,3-DPG per gram total blood Hb.
A blood gas analyzer was used to determine pH of whole-blood samples (ABL 505, Radiometer, Loveland, CO). Lactic acid concentrations were measured on an automated analyzer (Sport Lactate Analyzer; YSI 1500; Yellow Springs, OH) that measures hydrogen peroxide released from samples as lactate diffuses across a membrane. Lactate and pH were measured on days 0, 3, 8, 17, 24, and 31. Serum electrolytes (potassium, sodium, chloride) and glucose concentrations were measured on an automated chemistry analyzer (747-200, Hitachi, Mountain View). Electrolytes and glucose were evaluated on days 0, 3, 10, 17, 24, and 31. Ammonia and lactate dehydrogenase (LDH) were measured on an automated chemistry analyzer (Hitachi 717; Hitachi, Mountain View, CA). Ammonia concentrations were tested on days 0, 10, 17, 24, and 31, whereas LDH was measured on days 1 and 31.
PCV, total Hb concentration, RBC count, and mean corpuscular volume (MCV) variables were measured on an automated hematology system (Advia 120, Bayer, Tarrytown, NY). Mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) were calculated using standard formulas. Values for MCV, MCH, and MCHC were determined on days 3, 8, 17, 24, and 31.
Erythrocyte osmotic fragility was determined using a modification of the optical density procedure described by Brown.24 A 2 mL aliquot of whole blood from each storage container was centrifuged at 1400 rpm for 10 minutes. The RBC concentrate was then washed 3 times in 0.9% saline solution. A 50 μL sample of washed and resuspended blood was added to a series of 14 different concentrations of sodium chloride, ranging from 0% to 100%. Samples were then centrifuged after a 30 minute incubation period. Percent lysis was calculated based on a sigmoidal plot of the optical density of the supernatant as described.24 Osmotic fragility results were reported as median corpuscular fragility (MCF), referring to the saline concentration at which there was 50% hemolysis of the sample.25 Osmotic fragility testing was performed on days 7 and 24, and was compared with fresh blood controls drawn into heparinized tubes immediately before testing. The stored blood from each horse was compared with fresh blood samples from those same horses.
Erythrocyte morphology was evaluated on days 1, 8, and 22. Slides for evaluation were prepared using Wright–Giemsa stain. Erythrocytes were evaluated at × 100 on a high-power compound microscope.
Microbiology
At the conclusion of section 2, an aliquot of blood from each container was plated on blood agar medium and incubated at 37°C. Each sample was observed for bacterial growth at 24, 48, 72, and 96 hours.
Statistical Analysis
All data are reported as mean±SD. Differences were considered statistically significant when P<.05. Comparisons of each measured variable were performed using an analysis of variance (ANOVA) model blocked on horse, using statistical software (SAS version 8.2, SAS Institute, Cary). When appropriate, additional post hoc analysis was performed using least-square means to compare storage methods with respect to the storage method main effect. A Tukey–Kramer adjustment was used to control the Type I error rate in the post hoc analysis. A repeated measures (mixed-model) ANOVA was performed with sampling time and storage method as within-subject factors. All analyses were performed in parallel on untransformed and log-transformed data. Only the untransformed data were reported, as results of the 2 analyses were qualitatively identical.
Results
Hemolysis Analysis—Study Section 1
For the 4 storage methods tests, plasma Hb and percent hemolysis increased during storage in all containers and were significantly higher in blood stored in ACD bottles compared with the other methods (Table 2; Fig 1). However no significant temporal changes occurred in PCV or total Hb. Blood stored in ACD bottles and bags had significantly higher PCV values and total Hb content compared with blood stored in CPD and CPDA-1 (Table 2).
| ACD Bottle | ACD Bag | CPD | CPDA-1 | |
|---|---|---|---|---|
| Sodium (mEq/L) | 152±6a | 153±6a,c | 156±7b,c | 158±7b |
| Chloride (mEq/L) | 58±6a | 63±8b | 64±8b | 64±7b |
| LDH (IU/L) | 412±239a | 378±209a,b | 407±201a | 353±182b |
| MCV (fL) | 48.1±2.2a | 48.6±2.4b | 47.6±2.4c | 47.4±2.4c |
| MCH (pg) | 17.2±0.8a | 17.2±0.8a | 17.3±0.8a | 17.2±0.8a |
| MCHC (g/dL) | 35.7±0.9a | 35.5±1.0b | 36.4±1.2c | 36.4±1.1c |
| Hb (g/dL) | 12.4±1.2a | 12.2±0.9a | 11.7±0.8b | 11.5±1.3c |
| Plasma Hb (mg/dL) | 60±40a | 30±30b | 40±30b | 30±30b |
| PCV (%) | 37±4a | 37±3a | 34±2b | 33±4c |
- Values were averaged for all tests run during the 34-day storage period. Means within a row with different superscript letters are significantly (P<.05) different from one another.
- ACD, acid-citrate-dextrose; CPD, citrate–phosphate–dextrose; CPDA-1, citrate–phosphate–dextrose with adenine; LDH, lactate dehydrogenase; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, MCH concentration; Hb, hemoglobin; PCV, packed cell volume.
Mean±SD percent hemolysis values in equine whole blood obtained from 6 adult horses and kept in 4 different commercial storage containers for 31 days.
Complete Biochemical Analysis—Study Section 2
ATP and 2,3-DPG Concentrations.
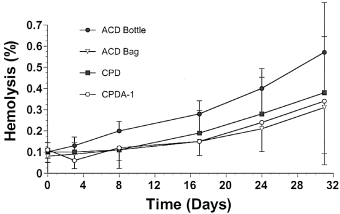
At all sampling times, including baseline, ATP concentrations for blood collected in CPDA-1 were significantly higher than for blood stored using other methods (Fig 2). Blood stored in ACD bottles had significantly lower ATP concentrations compared with all other storage methods tested, and blood stored in CPDA-1 had the highest ATP concentrations. A significant temporal decrease in ATP was detected for blood stored in ACD bottles. In contrast, although there were temporal variations in ATP concentrations for blood stored in ACD bags, no overall significant temporal reduction or increase in ATP was detected for blood stored in ACD, CPD or CPDA-1 bags. However, there was a trend towards a time-dependent increase in ATP content in CPDA-1 samples (P=.08).
Mean±SD adenosine-5′-triphosphate (ATP) values per unit hemoglobin (Hb) in equine whole blood obtained from 6 adult horses and kept in 4 commercial storage containers for 34 days.
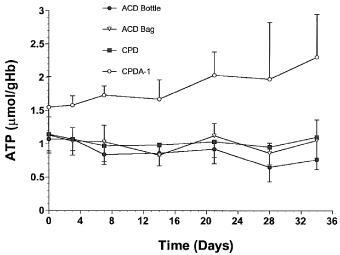
A significant temporal decrease in 2,3-DPG concentrations was detected during the 34-day storage period in blood samples from each storage method (Fig 3). Baseline 2,3-DPG concentrations were not significantly different among the storage methods. Blood stored in ACD bottles had the lowest mean 2,3-DPG concentrations, and both ACD bottles and bags had significantly lower 2,3-DPG concentrations than blood stored in CPD or CPDA-1 bags.
Mean±SD 2,3-diphosphoglycerate (2,3-DPG) values per unit hemoglobin (Hb) in equine whole blood obtained from 6 adult horses and kept in 4 commercial storage containers for 34 days.
Lactate and pH
A significant temporal increase in lactate was detected in blood drawn from all 4 storage methods over the 31-day sampling. Lactate concentrations measured from blood in ACD bottles and bags were lower than those measured from CPD and CPDA-1 samples. Lactate concentrations for blood stored in ACD bottles were significantly lower than for blood stored in ACD bags. On the day blood was collected, baseline lactate concentrations were already significantly lower in ACD bottles and bags compared with samples collected in CPD and CPDA-1 bags (Fig 4), and these differences became more substantial with prolonged storage, as lactate concentrations increased more rapidly in CPD and CPDA-1 blood samples.
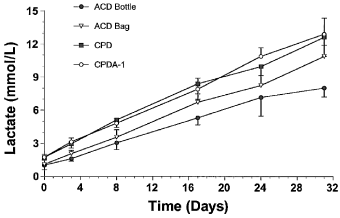
Mean±SD lactate values in equine whole blood obtained from 6 adult horses and kept in 4 commercial storage containers for 31 days.
At all time points, pH was <7.1 for all samples (Fig 5). Baseline pH, measured on the day of blood collection, differed significantly between storage methods tested, with CPD and CPDA-1 formulations having higher pH than the 2 ACD formulations. A progressive but similar decrease in pH was detected during storage for all methods. Blood stored in ACD bottles was more acidic than blood stored in ACD bags.
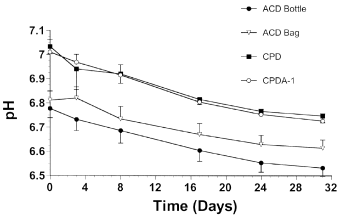
Mean±SD pH values for equine whole blood obtained from 6 adult horses and kept in 4 commercial storage containers for 31 days.
Electrolytes and Glucose Concentrations
A significant increase in serum potassium concentrations was detected for all methods during storage, with similar rates of accumulation detected for each storage type (Fig 6). Mean potassium concentrations increased from a mean of 3.9±0.4 mEq/L at baseline to a mean of 17.3±0.8 mEq/L by day 31. Potassium concentrations measured from blood stored in CPDA-1 were significantly lower than those detected in blood stored by other methods. In contrast to potassium, serum sodium and chloride concentrations were not significantly affected by storage time. Mean sodium and chloride concentrations of blood stored in ACD bottles were significantly lower than those of blood stored in CPD or CPDA-1 (Table 2).
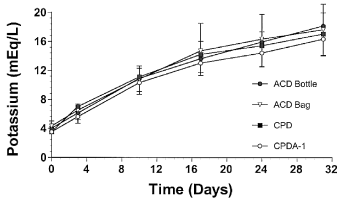
Mean±SD serum potassium concentration in equine whole blood obtained from 6 adult horses and kept in 4 commercial storage containers for 31 days.
Glucose concentration gradually declined in stored equine blood from an overall mean of 556±33 mg/dL at baseline to a mean of 410±55 mg/dL on day 31 (Fig 7), and a temporal decrease in glucose was detected with every storage type. No significant storage method differences in glucose concentration were detected from baseline measurements. However, blood stored in CPD had significantly lower glucose concentration than blood stored using other methods at all subsequent sampling times and in the overall analysis.
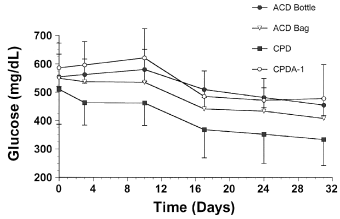
Mean±SD blood glucose concentration in equine whole blood obtained from 6 adult horses and kept in 4 commercial storage containers for 31 days.
Ammonia and LDH Concentrations
Both ammonia and LDH concentrations increased significantly during storage by all 4 storage methods (Fig 8). For baseline samples, blood collected in ACD bottles had lower ammonia concentrations than those measured from other storage methods. Overall, blood from ACD bottles had the lowest ammonia concentrations, whereas samples from CPDA-1 stored blood had the highest ammonia concentrations. Whereas there were no significant differences in baseline LDH concentrations among storage methods, on day 31, blood stored in ACD bottles had the highest measured LDH concentrations.

Mean±SD blood ammonia concentrations in equine whole blood obtained from 6 adult horses and kept in 4 commercial storage containers for 31 days.
RBC Variables
MCV, MCH, and MCHC did not vary consistently with time of storage. Red cells stored in ACD bags and bottles did have significantly higher MCV and lower MCHC values than those measured in blood stored in CPD or CPDA-1 but no significant differences in MCH were detected between storage methods (Table 2).
Osmotic Fragility and Erythrocyte Morphology
For all horses, fresh blood had lower osmotic fragility than blood stored using any method. Mean MCF of fresh blood samples was 47.49±1.66%, compared with 50.50±2.47% and 51.23±1.53% for blood stored for 7 and 24 days, respectively. No significant change in MCF was detected between days 7 and 24 of storage in ACD or CPDA-1. However there was a significant increase in MCF for blood stored in CPD. Overall, the mean MCF values measured for blood in ACD bottles and bags were higher than those measured from blood stored in CPD and CPDA-1. MCF changes identified during storage in CPD samples and the differences detected between storage methods were negligible compared with the MCF differences observed between fresh and stored blood.
Evaluation of erythrocyte morphology revealed a slight increase in acanthocyte numbers during the study for all storage methods, but no significant difference was detected among the 4 methods.
Microbiology
All cultures obtained at the end of the 34-day storage period were negative for any aerobic bacterial growth. Anaerobic cultures were not performed.
Discussion
The perceived value of autologous blood transfusions in equine surgical practice supports the need for thorough investigations to determine optimal techniques for blood collection, storage, and transfusion. Data from this study and follow-up studies will be used to define practical methods that maximize cell viability and oxygen-carrying capacity of the transfused product while minimizing adverse reactions. Our results indicate that under controlled conditions, equine whole blood can be adequately preserved for a number of weeks before surgery. The potential for prolonged storage should allow patients compromised by chronic low-grade blood loss to effectively recover from their blood donation before surgery. Optimal storage results were identified for equine whole blood stored in CPDA-1 polyvinyl chloride bags. Nevertheless, as expected, equine blood did deteriorate during storage in all tested storage media, with significant alterations detected for 2,3-DPG, plasma Hb, pH, lactate, ammonia, and potassium concentrations. Most of the identified alterations in stored equine whole blood directly parallel those reported as storage lesions in stored human and canine blood, with the most notable exception being ATP concentrations.
Erythrocytes enter the circulation without a nucleus or mitochondria to support oxidative metabolism. Nevertheless, functional cells remain metabolically active and continue to carry out energy-requiring processes.17 Consequently, a number of variables, including adenine nucleotide concentrations have traditionally been examined in stored blood as surrogate in vitro measures of red cell status. ATP concentrations are routinely monitored in stored blood and, during prolonged storage of human blood in conventional storage media, including ACD, CPD, and CPDA-1, the ATP content of cells generally declines.17 Although there is some correlation with subsequent cell viability in human transfusions, this relationship is not linear until ATP concentrations have dropped below 50% of normal.26
We measured the highest ATP concentrations in CPDA-1 stored equine blood. The addition of adenine to commercial CPD storage medium was specifically designed to preserve ATP concentrations in stored blood by restoring the adenine nucleotide pool. Although erythrocytes cannot synthesize adenine, they can incorporate preformed adenine into the nucleotide pool.17 In fact, the effect of adenine in preserving ATP concentrations and prolonging the storage life of blood has been confirmed in numerous human blood storage investigations.17,25
We observed a temporal decrease in ATP similar to that measured in human blood banks for equine blood stored in ACD bottles. However, ATP concentrations in stored equine blood did not consistently fall in ACD or CPD bags and there was actually a trend toward an increase during prolonged storage with CPDA-1. Although an increase in ATP has been reported in banked blood from other species during the first 2 weeks of storage,27,28 an increase after 4–5 weeks of storage in CPDA-1 has not been reported. The ready supply of glucose and adenine in CPDA-1 may be responsible for this increase in ATP. It has been reported that as red cells age in vivo, ATP concentrations increase because of reduced ATP-deaminase activity,17 and a similar mechanism could be responsible for trend toward increasing ATP concentrations observed in this in vitro study.
Overall, the ATP values we report were higher than those reported for fresh equine blood.29 Those ATP concentrations were measured in fresh heparinized blood using a bioluminescence assay whereas we used an enzymatic assay. Our assay technique or the storage media used could be responsible for the elevated ATP concentrations we detected. Regardless of differences from previously reported fresh blood ATP values, the comparisons of values within our study revealed significant differences among blood storage methods.
In equine whole blood, ATP was optimally preserved with CPDA-1 storage, suggesting that blood stored in CPDA-1 may have better post-transfusion viability in the horse compared with other storage methods. However, because the correlation between ATP concentrations and post-transfusion viability has not been confirmed with in vivo studies in the horse, viability determinations with labeled equine blood cells would be required to fully support this conclusion.
In addition to ATP, other variables, including 2,3-DPG, have been routinely monitored to indirectly assess cell viability during storage. Red cell 2,3-DPG concentrations are critically important for tissue oxygenation because this molecule modulates the oxygen affinity of Hb; therefore, lower 2,3-DPG concentrations can reduce peripheral oxygen delivery.30 Based on human and canine blood storage literature, we had anticipated the gradual depletion of 2,3-DPG that was observed during storage of equine blood.12,18 Various factors affect the concentration of 2,3-DPG in RBCs, influencing the balance between its rate of synthesis and its rate of degradation. Specifically, a pH>7 favors synthesis, and a major factor causing a decrease in 2,3-DPG concentrations during blood storage is the fall in pH that takes place with continued glycolysis.31 In our study of equine blood, pH dropped below 7.0 for all methods by day 3 of storage.
Storage in CPD and CPDA-1 resulted in better preservation of 2,3-DPG concentrations when compared with storage in ACD. These differences in preservation of 2,3-DPG are probably predominantly because of differences in pH, because the higher pH of CPD and CPDA-1 helps to maintain 2,3-DPG synthesis and prevent hydrolysis. There was no significant difference between samples stored in CPD and CPDA-1, despite the fact that adenine has been shown to have a deleterious effect on the concentration of 2,3-DPG in human red blood cells.32 The baseline 2,3-DPG values we obtained are similar to those previously reported for fresh equine whole blood.33–35
While not an accurate predictor of erythrocyte viability, depletion of 2,3-DPG may significantly influence patient morbidity and mortality. Transfusion of blood with reduced 2,3-DPG concentration has been shown experimentally to cause hypoxia.36 Concentrations of 2,3-DPG are replenished within 24 hours post-transfusion;37 however, the need for adequate oxygen delivery to tissues is often urgent.38 Tissue oxygenation is particularly important in hypovolemic, anesthetized equine patients, and an increase in tissue hypoxia can be associated with post-anesthetic myopathy and poor recovery.39 Blood stored in CPD and CPDA-1 had higher concentrations of 2,3-DPG in our study, and these storage solutions should be considered when stored blood is transfused with the intent of improving oxygen delivery during anesthesia.
Glucose, a six-carbon monosaccharide sugar, is the physiologic substrate for energy metabolism in RBCs, serving as an intermediate molecule in a variety of metabolic processes. In mammalian RBCs, the main catabolic pathway for glucose utilization is the glycolytic pathway converting glucose to pyruvic and lactic acid, and generating ATP as well as 2,3-DPG.17 In the present study, glucose concentrations were between 500 and 600 mg/dL for all storage methods 1 day after blood was collected. We anticipated these high glucose concentrations because of the inclusion of high concentrations of dextrose, an isomer of glucose, in commercial storage media to support erythrocyte metabolism.38 Although CPDA-1 does contain a higher concentration of glucose, there were no significant differences detected between storage types at the outset of the study. As a result of cell activity in stored blood, glucose concentrations gradually decline,17 and a mean decrease in glucose concentration of 26% was detected during the 31 day storage period. This decrease was substantially less than the 70% and 89% reductions reported previously for human and canine blood stored for 35 days in CPDA-1.25,40 This difference in glucose utilization rates suggests that stored equine blood may be less metabolically active than stored human or canine blood.
The lower glucose concentrations detected in CPD-stored equine blood compared with ACD and CPDA-1 storage methods tested here has not been reported for other species, but this difference may reflect the lower glucose concentrations in the CPD anticoagulant formulation. There was no statistically significant difference in glucose concentrations at baseline, so another reason for the lower glucose concentrations in CPD may be increased glucose consumption in that storage medium; however, blood stored in CPD did not have higher lactate concentrations than CPDA-1-stored samples. Despite the statistical significance of the difference between CPD and the other 3 storage types, none of the samples had a glucose level below that required to support continued RBC viability, and as a result, these differences are unlikely to have clinical relevance.
Changes in the acid base status of stored blood should be anticipated in any species. The final acid products of glucose catabolism, lactic acid and pyruvic acid, are transported through the erythrocyte membrane, and in a closed storage system these acids accumulate and produce a corresponding decline in pH.11 In addition, the anticoagulants themselves are acidic, and immediately lower the pH in the collection container.32 In this study, mean pH values of 7.03 and 7.01 were measured with CPD and CPDA-1, respectively, on the day of collection, and an even lower mean value of 6.78 was measured with ACD samples. Bacterial contamination can also contribute to a decrease in pH and increase in lactate. However, blood sample culture at the end of the storage period confirmed that this was not a factor in the acid base alterations we detected.
Changes in pH we observed were similar to those reported for stored human blood.41,42 The lower pH of the ACD stored samples in our study was consistent over the course of storage, and can be attributed to the lower pH of that anticoagulant formulation.32 The progressive decrease in pH was similar for all storage methods and can be attributed to anaerobic metabolism of the anucleated erythrocytes during storage. A sufficiently high pH is important for adequate ATP production, and at a pH value of 6.65 rates of ATP synthesis and consumption are reported to be balanced in human RBCs.43 The pH levels we measured in equine blood stored in ACD were below that level, and ATP concentrations were also depleted.
The accumulation of lactate in stored equine blood paralleled observations from human and canine studies. The higher lactate concentrations in blood stored in CPD and CPDA-1 are presumably because of higher metabolic activity compared with ACD stored samples. Higher pH concentrations in CPD and CPDA-1 may have contributed to higher lactate accumulations, because the rate of glycolysis is dependent on pH.32 Similarly, LDH concentrations increased significantly during storage with all storage methods; however, the concentrations measured at the end of storage were not as high as those reported for human blood stored in CPD bags.44 LDH concentrations were highest in blood stored in ACD glass bottles by the end of storage. In contrast to lactate, which reveals metabolic status, LDH is monitored as an indicator of cellular destruction, and the elevated LDH concentrations suggest that more erythrocyte damage occurred in blood stored in the ACD bottles. Values for all storage methods were above laboratory reference range by day 31. The analysis of LDH was limited to 2 sampling times, so the specific changes in LDH at other points during storage cannot be determined.
As ATP concentrations decline during blood storage, the RBC sodium–potassium membrane pump starts to fail, and intracellular potassium leaks out along a concentration gradient.32 The cooling of blood during storage also contributes to leakage of potassium, because the ion pumps are paralyzed.32 Increases in plasma potassium concentrations during storage in our study were dramatic and are similar to those reported for stored human blood,40,44 but significantly higher than those reported for stored canine blood.25 Intracellular potassium concentrations in human and equine erythrocytes are higher than concentrations in canine erythrocytes, and this may account for the higher extracellular potassium concentrations measured in stored equine and human blood.
Although the changes in potassium, pH, and lactate were dramatic in equine blood after only 2 weeks of storage, these changes would be unlikely to have clinical implications during small volume transfusions or transfusions to metabolically stable patients. However, there could be serious detrimental effects with larger volume transfusions in neonates, geriatric patients, or patients with renal or hepatic compromise.32,38 In these at-risk patients, potassium and pH levels in stored blood should be measured before transfusion and patients should be systemically monitored during administration of blood. Transfusion of blood <5–7 days old has been recommended for at-risk human patients, and this guideline should also be considered for high-risk equine patients.38
With prolonged storage of human blood, ammonia concentration increases as AMP is deaminated and the level of red cell sodium increases.38 We observed similar results and increases in blood ammonia concentrations detected in equine blood toward the end of the storage period were comparable to values reported for stored human blood.42,44 There were also significant differences in ammonia concentrations among storage methods, with the lowest concentrations in blood stored in ACD bottles and the highest concentrations in CPDA-1 bags. Ammonia is a waste product of RBC metabolism. Therefore, these differences may be because of higher concentrations of active RBC metabolism with storage in CPDA-1. Although comparisons of the ammonia concentrations show statistically significant differences among storage methods, these differences are of small magnitude and unlikely to be clinically significant. For all storage methods, ammonia concentrations in blood stored >10 days should be taken into consideration. By day 10, all ammonia concentrations were at least 250 μg/dL above the laboratory reference range for all storage methods. Whereas a high level of ammonia in stored blood may not have significant clinical impact for small volume transfusions, it may accumulate in patients with hepatic insufficiency.
A number of variables recorded in this study, including plasma Hb, percent hemolysis, and osmotic fragility, were measured to detect hemolysis and associated RBC fragility. Plasma Hb levels and percent hemolysis were consistently highest for blood stored in ACD glass bottles. The mechanical trauma of vacuum suction and cell damage from the glass container itself may have contributed to hemolysis with this collection method. Previous studies have documented the effect of this storage container on human blood, as plasma from glass bottles had a substantial increase in Hb after 14 days of storage.14 Plasma Hb values in ACD bottles and bags in our study were very similar to those reported for stored human blood.14,40 Nevertheless, for all storage methods, the percent hemolysis we detected after 31 days of storage was below the hemolysis limit of 1% which has been established in the United States as the safety standard for stored human blood.43
The osmotic fragility of red blood cells is known to increase during storage of human and canine whole blood12,35; however, we did not detect a significant increase in MCF by day 24 of storage. Our protocol involved washing RBCs before the osmotic fragility assay, a technique that serves to help equilibrate lactate from within the cell and thus reduces the osmotic effect of lactate.12 The osmotic fragility of stored erythrocytes has been shown to be primarily, because of the sudden unbalanced osmotic effect when lactate has accumulated within the cell, rather than to an inherent instability of the red cell membrane.45 The MCF of stored equine samples was similar to that reported for human blood,46 but higher than that reported for stored canine blood.25 Although the samples did not show a substantial increase in fragility over time, all samples had increased fragility compared with their matched fresh controls. Overall values for MCF and hemolysis suggest a mild increase in cell fragility and hemolysis during storage, with a slightly greater increase for blood stored in glass bottles. There is little evidence of clinically significant hemolysis that would limit the use of stored equine blood in any storage type. However, the elevated percent hemolysis and tendency towards increased fragility in blood stored in ACD glass bottles suggests that equine blood should not be collected into ACD glass bottles. Further investigation of RBC membrane stability could include tests of filterability and micropipette aspiration.47
The PCV of equine blood remained relatively constant throughout storage, consistent with results reported for stored human blood.42,44 Statistically higher PCV values observed for blood stored in ACD bottles and bags may be because of increased filling of these containers. Storage containers were not accurately weighed during blood collection. Therefore, the volume of blood was estimated and not standardized between containers. The higher PCV of blood in ACD containers is unlikely to have a significant impact on the hemolysis and ATP values we reported, and all PCV values were within normal laboratory range. However, alterations including increased hemolysis and more rapid decline in ATP concentrations have been reported for grossly underfilled units of human whole blood.48 Therefore, every effort should be made to adequately fill storage bags during collection.
MCV is a standard RBC measure, and it can increase in blood samples before RBC lysis. MCV did not change significantly with storage. Similar findings have been reported for other species,44 although it is known that there is an initial increase in cell volume because of the osmotic effects of the anticoagulants.22 Similar to MCV, MCH, and MCHC did not change significantly with storage, an observation that also parallels reports for stored human blood.44 There were minor but statistically significant differences in MCHC between blood stored in ACD and blood stored in CPD or CPDA-1; however, the values for blood from all storage types were within reference range for the laboratory and the minor differences are therefore unlikely to be clinically significant.
The stable erythrocyte morphology of equine stored blood in this study differed from the changes reported for stored human blood. While fairly marked echinocytic changes are characteristically reported for human red blood cells,12,22 only a minimal increase in acanthocyte numbers was observed after prolonged storage of equine RBCs. We had expected to see a larger degree of morphologic change in blood stored in bottles and ACD; however, differences in metabolic function of these cells apparently did not manifest in substantial morphologic changes.
Under storage conditions, equine RBCs underwent significant biochemical and biomechanical changes that would be expected to compromise their ability to deliver oxygen to the tissues after transfusion. Additional studies will be required to adequately document optimal autologous transfusion techniques for equine patients. Post-transfusion viability studies need to be performed to determine accurate guidelines for maximum acceptable storage length for equine whole blood. Current recommendations in human blood banks are to transfuse blood stored in ACD and CPD within 21 days, whereas CPDA-1 units can be stored for 35 days before use.49 Differences in storage responses we identified indicate that equine-specific studies will need to be performed before such guidelines can be established. In addition, modifications in commercial anticoagulant formulations have been shown to improve maintenance of 2,3-DPG in stored human blood,50 and similar modifications should be investigated for equine blood storage. Other recent advances include the use of rejuvenating solutions to improve the efficacy of outdated stored blood51 and leukoreduction to limit the negative effects of white blood cells on stored blood.43 Investigations of stored equine red blood cells will likely have implications beyond autologous donation programs, particularly for the establishment of equine blood banks.




