Growth Characteristics of Fibroblasts Isolated From the Trunk and Distal Aspect of the Limb of Horses and Ponies
Supported by the Committees on Research, Department of Veterinary Medicine and Surgery, and the College of Veterinary Medicine, University of Missouri, Columbia, MO.
Address reprint requests to David A. Wilson, DVM, A366 Clydesdale Hall, University of Missouri, College of Veterinary Medicine, Columbia, MO, 65211.
Abstract
Objective— To determine if there is a difference in in vitro growth of fibroblasts isolated from the trunk and distal aspect of the limb of horses and ponies. To determine the effects of a corticosteroid and monokine on in vitro growth of fibroblasts isolated from the trunk and distal aspect of the limb of horses and ponies.
Study Design— Growth of fibroblasts from tissues harvested from the trunk and limb were compared from horse and pony samples grown in control media and control media with triamcinolone or monokine added.
Animals or Sample Population— Dermal and subcutaneous tissue from 22 horses and 17 ponies of various ages and breeds.
Methods— Fibroblast growth was assessed by tritiated thymidine uptake using standard cell culture techniques. The effect of a monokine or triamcinolone plus control media were compared with control media for fibroblast growth.
Results— Fibroblast growth from tissues isolated from the horse limb was significantly less than growth from the horse trunk and the limb and trunk of ponies. Monokine was more effective than triamcinolone in suppressing fibroblast growth from tissues isolated from the trunk and limb in both horses and ponies.
Conclusions— There are growth differences in fibroblasts isolated from the limb of horses compared with those isolated from the trunk and from the limb and trunk of ponies.
Clinical Relevance— The difference in fibroblast growth from tissues isolated from the trunk and limb of horses and ponies may provide evidence for the difference reported in the healing characteristics of limb wounds in horses and ponies. Influencing fibroblast growth may provide a key to controlling the development of exuberant granulation tissue in horses and ponies.
Xuberant Granulation Tissue is a common complication associated with healing of limb wounds in horses. Granulation tissue consists primarily of developing blood vessels, fibroblasts, and their protein products that form the surrounding matrix.1 Exuberant granulation tissue is unsightly, prone to abrasion and secondary infection, and may produce a mechanical restriction to normal movement. Exuberant granulation tissue retards wound healing by interfering with epithelialization and wound contraction. Effective control of exuberant granulation tissue remains problematic. Several studies have reported differences in the amount of exuberant granulation tissue produced by horses and ponies.2,3 There is also a difference in the quantity and rate of formation of exuberant granulation tissue produced between trunk and limb wounds.2,3 In horses, wounds on the distal aspect of the limb produce more exuberant granulation tissue than trunk wounds. In comparison with limb wounds, trunk wounds heal more quickly with a faster rate of epithelialization, fewer complications, and rarely produce exuberant granulation tissue.2,4–6
A specific cause for development of exuberant granulation tissue is unknown. Reported factors associated with its production in limb wounds include motion, infection, poor blood supply, tension of the skin surrounding the wound, inflammation after application of topical irritants, imbalance of collagen metabolism, and lack of underlying muscle or other soft tissue.5,7 Various treatments can alter the amount of granulation tissue that develops in wounds.8 Treatments used to reduce the amount of exuberant granulation tissue include topical corticosteroids,9,10 collagen gels and membranes,3,11 equine amnion,12 live yeast cell derivative,13 various pharmacological inhibitors,7 bandaging in association with topical antibiotics and hydrotherapy,8 tissue adhesives,14 and occlusive and semiocclusive dressings.15
Corticosteroids have been reported to inhibit fibroblast proliferation in vitro and in vivo.7,9,10,16 Corticosteroids also affect wound healing by inhibiting the inflammatory phase through stabilization of lysosomal membranes. Corticosteroids suppress angiogenesis, inhibit epithelialization, retard wound contraction, reduce mucopolysaccharide and collagen synthesis, decrease wound tensile strength, produce less exudate, and result in smoother surfaced wounds.1,7,10
A specific monokine of rabbit macrophage origin (activated macrophage supernatant or complete supernatant from activated macrophages in cell culture [AMS]) has been identified that inhibits the proliferation of fibroblasts both in vitro and in vivo.17 Monokines are cytokines derived from activated monocytes, many having diverse affects on the inflammatory process.17 In a preliminary investigation using AMS, an in vitro comparison was made between fibroblast growth from tissues harvested from limb and trunk sites of horses.18 In the presence of AMS, fibroblast growth was inhibited from the tissues of the limb and trunk of 2 horses (88% and 82%, respectively). In that same study, an in vivo comparison was made between surgically created wounds of ponies treated with and without AMS. No significant differences in healing characteristics were observed between the treatment groups.18
The primary purpose of our study was to compare the in vitro growth of fibroblasts isolated from dermal and subcutaneous tissues harvested from the trunk and distal aspect of the limb of horses and ponies. An inherent difference in the growth characteristics of fibroblasts between the horse or pony or between trunk or limb fibroblasts may be a contributing factor in the development of exuberant granulation tissue. Because equine limb wounds are more prone to excess granulation tissue production than trunk wounds, we hypothesized that dermal and subcutaneous fibroblasts of limb origin would exhibit more rapid growth in vitro than those of trunk origin. A secondary purpose of our study was to determine the inhibitory effects of a corticosteroid and a monokine on fibroblast growth from the same tissues. Because fibroblasts make up a significant portion of exuberant granulation tissue, controlling fibroblast growth may provide a means to regulate the production of exuberant granulation tissue. We also hypothesized that AMS is more effective than corticosteroids in limiting in vitro fibroblast growth from the equine trunk and limb.
Materials and methods
Full-thickness 4-cm2 skin specimens were excised using aseptic technique from the most proximal, lateral aspect of the flank and dorsal aspect of the third metacarpus from 16 horses and 7 ponies, killed for reasons unrelated to skin disorders. Additional samples were obtained from live horses (6) and ponies (10) with no evidence of skin disease. After sedation with detomidine hydrochloride (10–20 μg/kg, intravenously) and butorphanol tartrate (0.02 mg/kg intravenously), aseptic preparation of the collection sites and local skin anesthesia with 2% lidocaine hydrochloride, 6-mm full-thickness punch biopsies were taken from the proximal lateral flank and dorsal aspect of the third metacarpus from the live animals. Samples were chosen randomly from the left or right flank and left or right forelimb.
After initial tissue harvest, dermal and subcutaneous tissues were minced and placed in ventilated flasks containing Dulbecco's modified Eagle medium (DME). Fibrogenesis was assessed by measuring radiolabeled 3H-thymidine uptake.17 Fibroblasts were grown to confluence in DME supplemented with 10% fetal bovine serum (FBS), antimicrobial agents (gentamicin [8 mg/dL], amphotericin B [50 μg/dL]), insulin (20 units/dL), l-glutamine (200 mM/dL), sodium pyruvate (100 mM/dL), hydrocortisone (0.1 mg/dL), vitamins (1 mL/dL), (MEM vitamin solution 100×; Fisher Scientific, Catalog # MT-25-020-CI, St. Louis, MO), and non-essential amino acid solution (2 mM/dL) (MEM Non-essential amino acid solution 100×; Fisher Scientific, Catalog # MT-25-025-CI, St. Louis, MO). After removal by trypsin treatment (approximately 1–2 minutes), fibroblasts were suspended in DME to achieve a concentration of 2 × 105 cells/mL, and 100-μL aliquots were placed into each well of a 96-well microtiter plate. One hundred microliters of monokine media (AMS), triamcinolone (Kenalog; Apothecon, a Bristol-Myers Squibb Co, Princeton, NJ) (200 μg/mL), or control media (DME) was then added to each well. The monokine, having a molecular weight <1,000 d, was prepared from a macrophage supernatant and its activity validated as previously described.17 The molecular-weight fraction of the monokine was obtained by filtering the macrophage supernatant through a 1,000-d millipore filter. Fifty microliters [3H]-thymidine (0.4 μCi) was then added and the cells incubated for 42 hours at 37°C in 7% humidified CO2. Cells were harvested with a Mash II automated cell harvester onto glass fiber filters and placed in scintillation vials filled with 3 mL of scintillation cocktail (ScintiVerse BD; Fisher Scientific, St. Louis, MO). Vials were placed in a beta-scintillation counter (Beckman LS9000) and counted for 1 minute. The radiation counts per minute (CPM) of the control and experimental media (triamcinolone or AMS) were then compared. Percent inhibition was calculated by the difference between the average cell CPM of samples incubated in control media and control media plus either triamcinolone or AMS and dividing by the mean cell CPM of the control media samples. The product was then multiplied by 100.
Mean cell counts (CPM) and % inhibition of fibroblast growth were evaluated using a mixed-model ANOVA with treatment and site as fixed effects and horse or pony as random effects. Post-hoc comparisons between groups were evaluated with Fisher's LSD and α= .05. Results were considered significant at P < .05.
Results
Tissue samples were collected from 22 horses and 17 ponies. Of the 22 horses, 11 had generalized systemic disease (laminitis, gastrointestinal disease, or Cushing's disease) and 11 had localized disease (abscess, guttural pouch mycosis, sinusitis, fracture, or lameness). Of the 17 ponies, 10 were healthy and 7 had generalized systemic or localized disease. Gross examination of full-thickness skin samples during harvesting revealed that the trunk specimens were always thicker than those from the limb. Fibroblast growth was sufficient from both the trunk and limb specimens in 7 of the 22 horses and 12 of the 17 ponies. Fibroblasts were cultured from both sites in only 2 of 11 horses with systemic disease and in 5 of 11 horses with localized disease. Fibroblasts failed to grow from one or both sites of all animals that were killed following an acute fracture. Fibroblasts were cultured from both sites in all 10 healthy ponies but in only 2 of 7 with signs of localized or systemic disease.
Effect of Site
Mean control fibroblast growth for the horse limb (3,952 ± 2,255 [CPM ± SD]) was less than horse trunk (15,023 ± 5,147; P= .002; Fig 1A) and pony limb (10,856 ± 7,310; P= .028; Fig 1B, Table 1). There was no significant difference between the growth of fibroblasts isolated from the trunk of horses and ponies. There was also no significant difference between the growth of fibroblasts isolated from the trunk and limb of ponies.
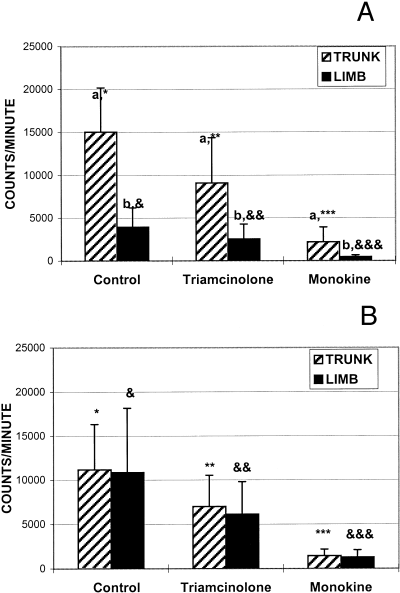
Mean (±SD) fibroblast growth expressed in radiation counts per minute from cells isolated from either the trunk or limb of (A) horses or (B) ponies and cultured in control media, and control media with triamcinolone, or monokine added. Different letters indicate significant differences between mean fibroblast growth for trunk and limb specimens for each of control, triamcinolone and monokine media. Different number of asterisks (*) indicate significant differences between mean trunk fibroblast growth for control, triamcinolone or monokine media. Different numbers of ampersands (&) indicate significant differences between mean limb fibroblast growth for control, triamcinolone or monokine media.
| Control Media | Triamcinolone | Monokine | % Inhibition Triamcinolone | % Inhibition Monokine | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trunk | Distal | Trunk | Distal | Trunk | Distal | Trunk | Distal | Trunk | Distal | |
| Horse | ||||||||||
| 6 | 9589 | 3772 | 1385 | 1878 | 1148 | 357 | 86% | 50% | 88% | 91% |
| 17 | 10542 | 1045 | 6290 | 785 | 1633 | 274 | 40% | 25% | 85% | 74% |
| 18 | 14841 | 2063 | 9426 | 1181 | 1005 | 228 | 36% | 43% | 93% | 89% |
| 19 | 17678 | 6781 | 11614 | 5732 | 2820 | 730 | 34% | 15% | 84% | 89% |
| 20 | 10096 | 6480 | 7177 | 3539 | 1367 | 643 | 29% | 45% | 86% | 90% |
| 21 | 20344 | 5172 | 9332 | 3178 | 1460 | 605 | 54% | 39% | 93% | 88% |
| 22 | 22069 | 2348 | 18481 | 1513 | 5868 | 305 | 16% | 36% | 73% | 87% |
| Horse Mean | 15023 | 3952 | 9101 | 2544 | 2186 | 449 | 42% | 36% | 86% | 87% |
| SD | 5147 | 2255 | 5254 | 1733 | 1729 | 204 | 22% | 12% | 7% | 6% |
| P= | .002 | .018 | .04 | .455 | .807 | |||||
| Pony | ||||||||||
| 1 | 12659 | 4231 | 8718 | 2193 | 1403 | 255 | 31% | 48% | 89% | 94% |
| 2 | 11375 | 1892 | 6526 | 1297 | 650 | 272 | 43% | 31% | 94% | 86% |
| 3 | 3492 | 16547 | 2273 | 9593 | 344 | 1853 | 35% | 42% | 90% | 89% |
| 4 | 14544 | 16605 | 10876 | 11558 | 1505 | 1367 | 25% | 30% | 90% | 92% |
| 5 | 3887 | 13505 | 1060 | 8152 | 367 | 1160 | 73% | 40% | 91% | 91% |
| 6 | 6283 | 11928 | 4123 | 8127 | 1103 | 1765 | 34% | 32% | 82% | 85% |
| 7 | 15256 | 15234 | 12414 | 7196 | 1930 | 2254 | 19% | 53% | 87% | 85% |
| 8 | 13500 | 4493 | 9843 | 2986 | 1348 | 1633 | 27% | 34% | 90% | 64% |
| 9 | 11372 | 15190 | 4925 | 8923 | 1636 | 1468 | 57% | 41% | 86% | 90% |
| 10 | 6312 | 1894 | 5266 | 1355 | 2556 | 159 | 17% | 28% | 60% | 92% |
| 11 | 15062 | 4215 | 8580 | 3072 | 1990 | 529 | 43% | 27% | 87% | 87% |
| 12 | 20285 | 24535 | 9427 | 9104 | 2387 | 2632 | 54% | 63% | 88% | 89% |
| Pony Mean | 11169 | 10856 | 7003 | 6130 | 1435 | 1279 | 38% | 39% | 86% | 87% |
| SD | 5166 | 7310 | 3532 | 3671 | 728 | 821 | 17% | 11% | 9% | 8% |
| P= | .895 | .585 | .625 | .837 | .815 | |||||
- NOTE. Percent inhibition was calculated as described in Materials and Methods.
Effect of Treatment
Compared with control media (10,450 ± 6,411), both triamcinolone (1,342 ± 1,067) and AMS (6,292 ± 4,123) suppressed fibroblast growth; however, AMS was more effective than triamcinolone (P < .0001). Similar results were evident in both horses (9,487 ± 6,897 v 5,822 ± 5,069 vs. 1,317 ± 1,487; P < .007; Fig 1A) and ponies (11,012 ± 6,192 vs. 6,566 ± 3,551 vs. 1,357 ± 763; P < .0001, Fig 1B) for control, triamcinolone, and AMS, respectively.
Percent inhibition of fibroblast growth was greater for those samples incubated with control media plus AMS than control media plus triamcinolone for horse trunk (86%± 7%v 42%± 22%; P < .0001) and limb (87%± 6%v 36%± 12%; P < .0001). Percent inhibition of fibroblast growth was greater for samples incubated in AMS versus triamcinolone for pony trunk (86%± 9%v 38%± 17%; P < .0001) and pony limb (87%± 8%v 39%± 11%; P < .0001).
Discussion
Several differences between fibroblast growth from the trunk and limb sites of horses and ponies were observed in this in vitro study. Mean fibroblast growth from the trunk of horses was significantly greater than that for the limb. Mean fibroblast growth from the limb of ponies was significantly greater than from the limb of horses. There was no significant difference in the growth rates between trunk and limb sites in ponies. There was also no significant difference between horses and ponies in growth of fibroblasts isolated from the trunk. Fibroblasts from the limbs of horses grew less than from any other site yielding in vitro results in contrast to that anticipated from clinical experience with respect to granulation tissue production in the horse. The findings of this study provide some evidence of a difference between the wound-healing processes of horses and ponies, as well as between limb and trunk sites.
If the growth of horse dermal and subcutaneous tissue fibroblasts of limb origin is truly less than those of trunk origin, this may be a significant contribution to the poor wound-healing characteristics of wounds of the distal aspect of the limbs of horses. Because fibroblast growth and the production of granulation tissue of limb origin can become excessive, there may be a problem in the control of matrix component production from limb fibroblasts. Less fibroblast growth may lead to a reduction in myofibroblast numbers, poor wound contraction, and reduced collagen matrix formation during limb wound healing. Endothelial cells may contribute more than previously considered in the development of exuberant granulation tissue. Greater fibroblast and myofibroblast activity may inhibit exuberant granulation tissue and stimulate normal wound healing.
The fibroblast growth observed in this in vitro study was influenced by the artificial environment created with standard tissue culture. In vitro and in vivo fibroblast growth characteristics may differ considerably. The in vivo environment is one of constant change with the influence of a variety of cellular growth mediators and cellular interactions guided by a living system. An in vitro environment is restricted to the influences of the artificial environment created for cell culture. Although an in vitro system attempts to provide an environment similar to in vivo, there will always be variations in nutrients and cell mediators provided by cell culture media, as well as differences in local O2 tensions, humidity, and temperature.
Several factors likely influenced the poor in vitro fibroblast growth that occurred from some skin specimens. As was previously stated in the results, only 7 of 22 horses and 12 of 17 ponies achieved sufficient fibroblast growth from both anatomic sites for comparison of in vitro growth. Some of these animals had either no or insufficient growth from one or both anatomic sites. Many of the horses and ponies were sampled immediately following or just before killing. Although these animals were free of any gross evidence of dermatologic disease, many were killed for systemic illness that may likely have influenced dermatologic cellular metabolism and growth potential. Some animals had samples harvested while anesthetized, before killing. Anesthetic drugs, including the lethal solutions used, may have a detrimental affect on tissues harvested for studies. Some samples developed microbial contamination during processing despite attempts at strict aseptic preparation for surgical procedures. Errors in tissue culture harvesting and preparation could also have contributed to poor growth.
It is difficult to explain the random variation in cell counts among the majority of pony samples, especially because most of the ponies sampled were alive and apparently free of systemic disease. Fibroblasts were cultured from both sites of all 10 healthy ponies. None of these ponies were anesthetized, but all were sedated and samples were collected after local anesthesia. Some of these ponies had gross hoof characteristics typical of chronic laminitis. The relationship between laminitis and fibroblast growth from the trunk or limb of ponies is unknown. Based on our results of poor growth from horses and ponies with systemic disease, an affect on fibroblast growth during the acute phase of laminitis could be expected; however, an affect of chronic laminitis on fibroblast growth was apparently less evident.
There was consistent inhibition of fibroblast growth from the trunk and limb by both corticosteroid and monokine. The monokine resulted in significantly greater inhibition of growth than corticosteroid for both sites of horses and ponies. In a previous study, no difference was detected in the healing characteristics of wounds in ponies treated topically with or without AMS.18 Factors that may contribute to the differences observed between in vitro and in vivo activity include lack of sufficient contact or absorption of the AMS with the wound surface, the possible short half-life of AMS activity, the possible inhibitory effects on AMS activity by other growth factors present in the wound environment, and the exudative nature of granulating wounds that may displace the AMS from the wound surface. Other studies have also noted the differences between in vitro and in vivo studies.19–21 Use of growth factors for the treatment of human chronic wounds in clinical trials have been disappointing when compared with in vitro results.21 Wounds exist in a relatively unstable in vivo environment. It has been proposed that leakage of macromolecules such as albumin may trap growth factors, making them unavailable to the wound tissues.
Adequate fibroblast inhibition may require more frequent application or the use of a repository vehicle to provide sustained exposure to the monokine. Under the appropriate wound conditions, AMS and other growth factors may be very beneficial in controlling exuberant granulation tissue. What constitutes appropriate wound conditions remains unclear and will require further study to identify the appropriate stage of wound healing for application and knowledge of the complex interaction of growth factors and cellular response in the wound environment. Several studies have demonstrated that a combination of growth factors is superior to single factors in wound-healing models.19,22
The exact identity of the monokine used in this study is unknown. This monokine has been used in two previous wound-healing studies.17,18 The compound may be one whose chemical structure has been previously identified and has been used for treatment of other conditions. It could also be an active fragment of another growth factor or cytokine. In an earlier study,17 the authors speculated that this monokine could be mediated by a metabolite of arachidonic acid such as prostaglandin E2 (PGE2) because of its small molecular weight (<3,000 d). The macrophage supernatant remained effective for fibroblast suppression in vitro after the addition of an anti-PGE2 antibody. The macrophage supernatant collected for use in the present study was filtered through a filtration membrane of 1,000 d and the supernatant effectively inhibited in vitro fibroblast growth. Further studies are needed to identify the monokine's chemical composition. Many growth factors and cytokines are released from macrophages.21,23–25 The molecular weights of these previously identified products were all greater than 3,000 d.
Several macrophage-derived factors have been demonstrated to both stimulate and inhibit fibroblast proliferation such as interferon-γ (IFN-γ), interleukin-1 (IL-1), and transforming growth factor β (TGF-β).25 Many macrophage-derived factors have stimulatory affects on wound healing such as epidermal growth factor, fibroblast growth factor, platelet-derived growth factor, insulin growth factors, TGF-β1, and TGF-β2 by encouraging fibroblast and collagen proliferation either directly or by stimulating other growth factors.19,21,25,26 For example, TGF-β is a potent stimulator for fibroblast synthesis of extracellular matrix, but its proliferative effects on these cells is observed only after prolonged exposure in vitro, probably through stimulation of PDGF synthesis.19 Other growth factors noted to stimulate fibroblast proliferation include heparin binding EGF, IL-1, and tumor necrosis factor.27 Those growth factors that have been demonstrated to inhibit fibroblast growth include TGF-β, IL-1, and INF-γ.27
The ability of trunk tissues to heal more quickly with fewer complications than the limb tissues most likely results from a combination of many factors, of which the fibroblast and its controlling elements play a vital role. The differences are undoubtedly affected by the wound microenvironment, growth factors, collagen content, fibroblasts, myofibroblasts, endothelial cells, and their inherent biochemical and structural characteristics.




