BIODEGRADABILITY OF HYDROCARBONS BY CYANOBACTERIA1
Received 17 November 2009. Accepted 19 April 2010.
Abstract
Five cyanobacterial species (Phormidium sp., Nostoc sp., Anabaena sp. Aphanothece conferta, and Synechocystis aquatilis) isolated from the Suez Canal coast at the city of Ismailia (Egypt) were tested for biodegradation of four hydrocarbon (HC) compounds: two aliphatic compounds (n-octadecane and pristine) and two aromatic compounds (phenanthrene and dibenzothiophene). High degradation efficiencies for the two aliphatic compounds were measured for A. conferta (64% for n-octadecane and 78% for pristine) and S. aquatilis (85% for n-octadecane and 90% for pristane). However, the other biodegradation percentages ranged between weak and moderate percentages.
Abbreviations:
-
- BTMA
-
- benzyl-trimethylammoniumchloride
-
- DCM
-
- dichloromethane
-
- HC
-
- hydrocarbon
-
- OCC
-
- organo-clay complexes
Pollution of marine environments with HCs has become a worldwide problem on the tide of industrialization. The sources of marine HC pollution are mainly runoff from land and municipal/industrial wastes, routine ship maintenance like bilge cleaning, air pollution from cars and industry, natural seeps, tanker accidents, and offshore oil production (NRC 1985, U.S. Coast Guard 1990). It has long been known that poly-nuclear aromatic HCs exhibit serious toxic and carcinogenic effects (Miller and Miller 1974, McCann et al. 1975). Therefore, the study of the biotransformation and biodegradability of these aromatic compounds in the environment is of basic and particular value.
The main process acting in the cleanup of HC-contaminated ecosystems is microbial biodegradation, which has been extensively studied and reviewed (Atlas 1984, Leahy and Colwell 1990, Chaillan et al. 2006). Numerous microorganisms, including bacteria, fungi, and yeasts, are known for their ability to degrade HCs (Oudot et al. 1993, Chaillan et al. 2004). In tropical crude oil production sites, cyanobacterial mats often develop on petroleum-polluted zones including surface soils and water environments. After the release of oil during the Gulf War in Kuwait, a bloom of cyanobacteria intimately associated with oil was also observed (Sorkhoh et al. 1992).
There is increasing evidence that photosynthetic microorganisms, particularly cyanobacteria, may contribute to the oxidation and degradation of HCs. However, it is important to emphasize that only in some cases were the tested cyanobacterial cultures axenic and that many studies have been carried out on nonaxenic cultures.
Among the earliest studies on the potential of photosynthetic microorganisms, including cyanobacteria, for aromatic HC oxidation is the study of Ellis (1977). This author investigated the phenol and catechol degradation potential of some microalgae and cyanobacteria. Most of the work on aliphatic HCs has focused on the potential of these phototrophs for the complete utilization of these compounds.
Previous reports have shown the ability of cyanobacteria to oxidize oil components. Al-Hasan et al. (1998) reported that nonaxenic cultures of Microcoleus chthonoplastes and Phormidium corium, isolated from oil-rich sediments of the Arabian Gulf, were able to degrade n-alkanes. Studies on Oscillatoria sp. and Agmenellum quadruplicatum demonstrated their ability to oxidize naphthalene to 1-naphthol (Cerniglia et al. 1980a). Other studies showed that Oscillatoria sp. can oxidize biphenyl to n-hydroxybiphenyl (Cerniglia et al. 1980b) and that A. quadruplicatum metabolizes phenanthrene into trans-9,10-dihydroxy-9,10-dihydrophenanthrene and 1-methoxy-phenenthrene (Narro 1985). Several other strains were reported to degrade crude oil and other complex organic compounds (Lee et al. 1974, Cerniglia et al. 1984, Yan et al. 1998, Radwan and Al-Hasan 2000, Raghukumar et al. 2001, Mansy and El-Bestway 2002). However, in most biodegradation studies with cyanobacteria, it was not clear whether the strains used were definitively axenic (Abed and Köster 2005). It is known to be very difficult to cultivate cyanobacteria in axenic culture and to clean them from naturally associated aerobic heterotrophic bacteria. Thus, the contribution of aerobic heterotrophic bacteria associated with cyanobacteria to the biodegradation process needs to be carefully evaluated. In this study, I have addressed this problem by testing the ability of five axenic unicyanobacterium strains isolated from Suez Canal, Egypt, to degrade four petroleum compounds.
Materials and Methods
Isolation, identification, and maintenance of cyanobacterial strains. Five cyanobacterial mat samples were collected from the Suez Canal coast, city of Ismailia, Egypt, and transferred to the phycological lab (Faculty of Science, Beni-Suef University, Beni-Suef, Egypt) within 24 h upon arrival; the mates were incubated in a glass container filled with aerated artificial seawater. The mats were incubated at a light regime of 12:12 light:dark (L:D) with an intensity of 80 μmol photons · m−2 · s−1. The diversity and abundance of the cyanobacteria of the mat samples were microscopically examined as described in Chaillan et al. (2006) using a Binocular Olympus microscope (serial 8H0222, Medica Instrument Mfg. Co., Mumbai, India)). Isolation and purification of cyanobacteria in a unialgal form were done according to the methods described by Rippka (1988). Briefly, the cyanobacterium was isolated after repeated light migrations on solid Z-medium prepared in seawater samples, according to Chaillan et al. (2006). These purified cyanobacterial species were identified according to Smith (1950) and Desikachary (1959). Logarithmic growth phase of each cyanobacterium was detected to obtain the healthy growth mass to be used in this experiment.
Purification of the cyanobacterial isolates from bacteria. Axenic cultures of the cyanobacterial isolates were obtained as described by Pringsheim (1949). These cultures were subjected to different trials to employ bacteria-free cultures and investigate the growth of bacteria every 20 d throughout the experimental period, according to Felfoldy and Zsuzsa (1959) and Hoshaw and Rosewski (1973).
Preparation of organo-clay complexes (OCC). n-Octadecane, pristane, phenanthrene, and dibenzothiophene KSF (Aldrich, Steinheim, Germany) were used as model compounds for petroleum constituents representing straight-chain alkanes, branched alkanes, aromatic HCs, and organo-sulfur compounds, respectively. Hydrocarboic clay was used as a carrier substance for the petroleum model compounds to overcome the low accessibility of the poorly water-soluble compounds when directly added to the medium.
A 2% aqueous suspension of montmorillonite KSF (Aldrich) was slowly mixed with a 10 mM solution of benzyl-trimethylammoniumchloride (BTMA, 0.8 mmoln · g−1 clay) and stirred for 24 h (modified after El-Nahhal et al. 2000). Subsequently, the mixture was washed three times with deionized water to remove excess BTMA and then dried. To absorb the model compounds, the hydrophobic clay (BTMA-montmorillonite) was suspended in n-hexane. The petroleum model compounds were slowly added under continuous stirring to an n-hexane suspension in total 20 mg model compounds per 100 mg of hydrophobic clay. The resulting organo-clay complex slurry (OCC) was dried in a vacuum rotary evaporator (Roteva Equitron 63 series Model 87G3.RD0.000, Medica Instrument Mfg. Co.), which yielded homogenous powder of hydrophobic clay loaded with 16.67 wt% of petroleum model compounds. To verify the amount of loaded model compounds, the OCC was reextracted with dichloromethane (DCM) and analyzed by gas chromatography according to the method described by Grötzschel et al. (2002).
Biodegradation experiment with cyanobacterial strains. The degradation experiments (three replicates) were carried out in 250 mL sterile Erlenmeyer flasks. Each Erlenmeyer flask received 99 mL of autoclaved medium, 1 mL of culture suspension, and 100 mg of OCC. Controls without culture suspensions were also determined. All flasks were incubated at 28°C with constant shaking at 100 rpm, a 12:12 L:D cycle, and 80 μmol photons · m−2 · s−1 light intensity. The experiment was run for 60 d, and samples for chemical analysis (2 mL each) were taken every 20 d.
Chemical analysis. Samples of 2 mL from the cultures were extracted ultrasonically four times with a 1:0.5:0.4 (v/v/v) mixture of methanol (MeOH), DCM, and water (modified after Bligh and Dyer 1959). DCM and water were added to the combined extract to yield a solvent mixture of MeOH:DCM:H2O at a ratio of 1:1:0.9 (v/v/v) resulting in phase separation. The DCM layer was collected, and the methanol-water phase was washed three times with DCM. The solvent of the combined DCM phase was removed in the rotary evaporator, and the extracts were diluted to concentrations appropriate for gas chromatographic analyses.
Statistical analyses. All the values or readings are the result of the mean of three replicates in addition to the standard deviation.
Results and Discussion
Data recorded in 1, 2 revealed different degradation efficiencies for the studied cyanobacteria against the tested model petroleum compounds. In the case of the control, the degradation percentages of n-alkanes throughout 60 d were 8% and 7% for n-octadecane and pristane, respectively (Fig. 1A), while they reached 7% in the case of phenanthrene (on day 20) and 6% in the case of dibenzothiophene at the end of experiment (Fig. 2A). This result might be attributed to the normal reactions between solution contents.
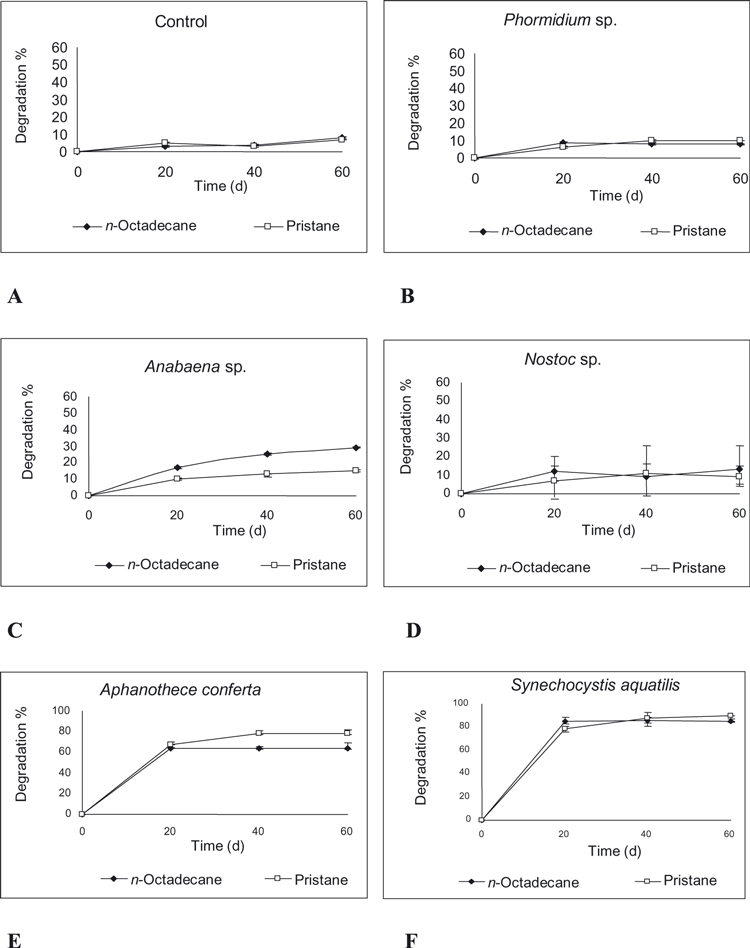
Biodegradation percentages of n-octadecane and pristane by cyanobacterial cultures. (A) Control (natural seawater), (B) Phormidium sp., (C) Anabaena sp., (D) Nostoc sp., (E) Aphanothece conferta, and (F) Synechocystis aquatilis.
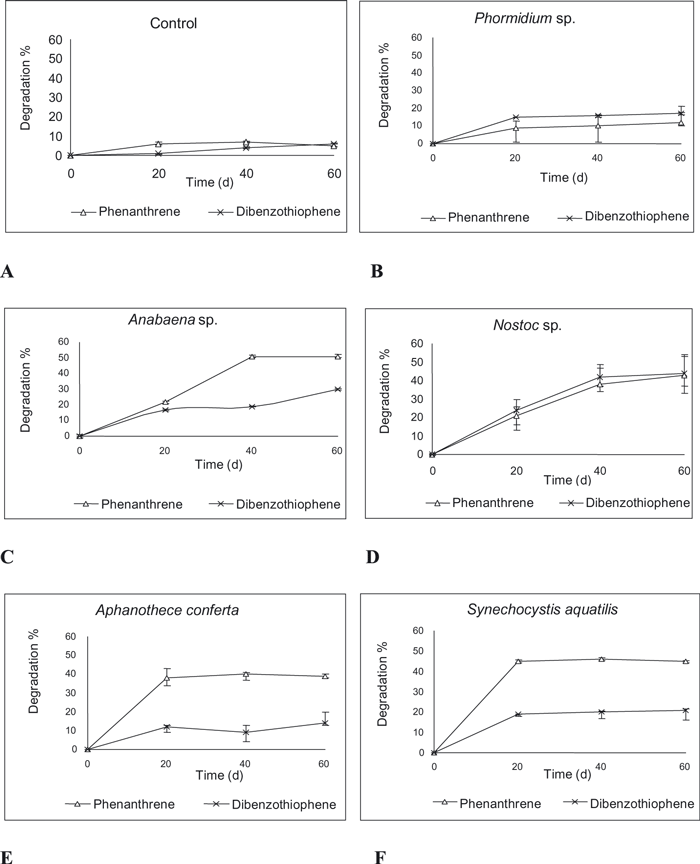
Biodegradation percentages of phenanthrene and dibenzothiophene by cyanobacterial cultures. (A) Control (natural seawater), (B) Phormidium sp., (C) Anabaena sp., (D) Nostoc sp., (E) Aphanothece conferta, and (F) Synechocystis aquatilis.
The investigated cyanobacterial isolates exhibited different degradation efficiencies, which depended on the cyanobacterial species used and the petroleum compound.
Aliphatic oxidation by cyanobacteria. While most of the work on aromatic HCs was targeted at studying the potential of cyanobacteria for oxidation of these compounds, work on aliphatic HCs was focused on studying the potential of these phototrophs for the complete utilization of these compounds. Data recorded in Figure 1 indicated that both filamentous cyanobacteria (Phormidium sp. and Nostoc sp.) exhibited weak abilities against both n-octadecane and pristane. Values were 9% (on day 20) and 10% (on day 40) for n-octadecane and pristane, respectively, by Phormidium sp., and 13% (on day 60) and 11% (on day 40) for n-octadecane and pristane, respectively, by Nostoc. However, the filamentous cyanobacterium Anabaena sp. demonstrated a moderate biodegradable activity toward the two alkanes: 29% (on day 60) for n-octadecane and weak activity (15%) for pristane on day 60 (Fig. 1C).
In contrast, both A. conferta and S. aquatilis had high efficiencies in degradation of both aliphatic compounds: 64% (on day 20) and 78% (on day 40) for n-octadecane and pristane, respectively, by A. conferta (Fig. 1E), which remained constant until the end of the experiment, and 85% (on day 20) and 90% (on day 60) for n-octadecane and pristane, respectively, by S. aquatilis (Fig. 1F). Thus, the ability of A. conferta and S. aquatilis to degrade both tested aliphatic substances was demonstrated. In this respect, Al-Hasan et al. (1998) reported important evidence for the potential of cyanobacteria to oxidize alkanes, namely, that fatty acids resulting from that oxidation were found esterified in lipid classes specified of the thylakoids, monogalactosyldiacylglycerols, digalactosyldiacylglycerols, and sulfoquinovosyldiacylglycerols. In a supporting study, Kuritz and Wolk (1995) showed that two filamentous cyanobacteria, Anabaena sp. and Nostoc ellipsosporum, have the potential to degrade the highly chlorinated pesticide lindane. The two cyanobacteria could also be genetically engineered to degrade 4-chlorobenzoate.
A number of studies reported that HCs might stimulate the process of photosynthesis and chl building, as well as the growth of cyanobacteria (Soto et al. 1975, Schroeder and Rehm 1981). This may imply that crude oil and n-alkanes do not cause any stress to cyanobacteria (Gaur and Singh 1990). In this respect, Gamila et al. (2003) evaluated the biodegradation efficiency of Oscillatoria agardhii (nonheterocystous) and Anabaena spharica (heterocystous) for petroleum HCs. They reported that both cyanobacterial strains revealed a high algal biomass (expressed as chl aμg · L−1) in comparison with that obtained by the control culture and that the n-alkanes (C10–C24) were more readily degraded than polycyclic aromatic HCs (PAHs) by both strains. On the other hand, they determined that n-alkanes were reduced to 99.5% and PAHs to 97.5% in Anabaena culture, and a significant (P < 0.05) negative correlation was observed between the microbial growth of Anabaena and the total concentration of petroleum HCs. They concluded that the biodegradation rate of saturated and aromatic fractions was time dependent, depending on the cyanobacterial strains.
Aromatic oxidation by cyanobacteria. Data recorded in Figure 2 demonstrate different biodegradable behaviors in between moderate and weak ability according to cyanobacterium species and the aromatic compound. Phormidium exhibited weak degradation for phenanthrene (12%) and dibenzothiophene (17%) on day 60 (see Fig. 2B). Both Anabaena and Nostoc had moderate degradation percentages for both aromatic compounds: 51% (on day 40) and 30% (on day 60) for phenanthrene and dibenzothiophene, respectively, by Anabaena (Fig. 2C), and 43% and 44% at the end of the experiment for phenanthrene and dibenzothiophene, respectively by Nostoc (Fig. 2D).
Although high degradation activity was exhibited by Aphanothece and Synechocystis against the two alkanes, they exhibited different sensitivities against the aromatic compounds. They exhibited moderate degradation percentages against phenanthrene, which reached 40% and 46% (on day 40) by Aphanothece and Synechocystis, respectively (Fig. 2, E and F). However, they demonstrated weak degradation percentages against dibenzothiophene, stopped at 14% and 21% by Aphanothece and Synechocystis, respectively (Fig. 2, E and F). The high efficiency of some investigated cyanobacteria to degrade some of the tested HCs might contribute to the dominance of cyanobacteria in many polluted sites including the contaminated coasts of the Arabian Gulf after the Gulf War in 1992 in Kuwait, which has given the impression that cyanobacteria possess the potential to break down oil components. In this respect, Sorkhoh et al. (1992), Radwan et al. (1999), Raghukumar et al. (2001), and De Oteyza et al. (2004) reported that the cyanobacterial mats were often reported in oil-contaminated environments, suggesting that cyanobacteria are able to tolerate oil contamination and have strong potential to degrade HCs. This finding is in agreement with previous research that reported that some aromatic HCs can be oxidized by the cyanobacterial strains (Ellis 1977). Ellis investigated the phenol and catechol degradation potential by Anabaena cylindrical and Phormidium foveolarum. He determined that both cyanobacteria degrade phenol and catechol. In this respect, Cerniglia et al. (1979, 1980a) studied the oxidation of naphthalene by Oscillatoria sp. and Agmenellum quadruplicatum, which oxidized naphthalene under photautotrophic conditions to 1-naphthol, cis-1,2-dihydroxy-12-dihydronaphthalene, and 4-hydroxy-1-tetralonen. They concluded that cyanobacteria probably possess both the monooxygenase and dioxygenase systems that catalyze the initial step of naphthalene oxidizing. However, some researchers have reported that these results were ambiguous and, consequently, that no real ability of cyanobacteria to degrade crude oil was demonstrated (Radwan and Al-Hasan 2000). Nevertheless, some cyanobacteria have been reported to metabolize simple bicyclic aromatic HCs like naphthalene (Cerniglia et al. 1980a, Narro et al. 1992, De Oteyza et al. 2004).
Studies have also been conducted on the oxidation of two other aromatic HCs, methylnaphthalene and biphenyl phenanthrene, by photosynthetic microorganisms (Smith and Rosazza 1974, Meyer and Scheline 1976, Wiebkin et al. 1976, Dodge et al. 1979, Cerniglia et al. 1980b). They demonstrated that the oxidation of methylnaphthalenes and biphenyl by cyanobacteria is probably similar to that mediated by fungal and mammalian enzyme systems and differs from that carried out by bacteria (Catelani et al. 1971, Gibson et al. 1973). In a supporting study, Cerniglia et al. (1981) reported that two autotrophically grown cyanobacteria, Agmenellum quadruplicatum and Oscillatoria sp., metabolize aniline (aromatic HCs) producing formanilide, acetanilide, and p-aminophenol. It is known that bacteria and mammals also metabolize aromatic amines by N-formylating them (Gothoskar et al. 1979, Alexander 1981).
Using hydrophobic montmorillonite clay as a carrier system in the present work is suitable to accomplish close contact of the petroleum compounds with cyanobacteria to enable biodegradation (Abed et al. 2002, Grötzschel et al. 2002). It may contribute to the ability of A. conferta and S. aquatilis to degrade alkanes and aromatic HCs. On the other hand, the pigmentation loss and subsequent death of Phormidium and Nostoc species confirm that petroleum compounds can be toxic to certain cyanobacterial strains. Many studies have demonstrated that crude oils are inhibitory to cyanobacteria even at low concentrations (Vandermeulem and Ahern 1976, Winters et al. 1976, Batterton et al. 1978). These compounds, particularly the aromatic ones, which are considered more toxic than the alkanes, might inhibit photosynthesis and growth and reduce enzyme activity and microbial biomass (Megharaj et al. 2000).
In contrast, some researchers have demonstrated that aerobic heterotrophic bacteria associated with the mucilaginous coating of cyanobacteria and macroalgae are actually the principal degraders (Sorkhoh et al. 1995, Radwan et al. 2002, Abed and Köster 2005, Chaillan et al. 2006). They reported that it is difficult to cultivate cyanobacteria in axenic culture and to clear the culture from naturally associated aerobic heterotrophs, which reflects their dependence on each other. Therefore, they suggested that the ability of axenic cyanobacteria to degrade petroleum compounds needs to be carefully examined.
Other researchers have suggested that cyanobacteria play an indirect role in mixed microbial populations like mats and biofilms (Cerniglia et al. 1984, Singer and Finnerty 1984). In this respect, Abed and Köster (2005) suggested that cyanobacteria and associated bacteria seem to form a consortium favorable for biodegradation and cleanup of polluted sites. They reported that cyanobacteria fuel the aerobic heterotrophic bacteria with oxygen and nitrogen as well as organic matter essential for their activity. In return, aerobic heterotrophs degrade petroleum compounds, thus reducing their toxic effect on cyanobacteria. Furthermore, the complete degradation of petroleum compounds leads to the regeneration of CO2, which is used by cyanobacteria for photosynthesis. Abed (2010) reported that addition of the cyanobacterial exudates to aerobic heterotrophic bacteria plays a role in stimulating their degradative activities. He concluded that the aerobic heterotrophic bacteria–cyanobacteria consortia can be very useful for bioremediating oil-polluted sites, circumventing the costly use of organic and inorganic fertilizers.
In addition, Safonova et al. (1999) have shown that the presence of alkane-utilizing bacteria in association with algae and cyanobacteria restores the growth of sensitive phototrophic strains exposed to black oil and stimulates the growth of the tolerant species. They reported that this association degraded black oil more efficiently than a pure culture of bacteria alone. Therefore, they suggested that phototrophic and heterotrophic microorganisms form a consortium that increases their potential to degrade petroleum compounds. Moreover, several studies have reported that cyanobacteria protect the associated bacteria and fungi from adverse ecological conditions like excessive light or dryness (Garcia-Pichel and Pringault 2001, Des Marais 2003).
Acknowledgments
I would like to thank all of the general practitioners who participated in this study. I also greatly appreciate the efforts of investigators regarding data collection.