CONSTRUCTION OF A GENETIC LINKAGE MAP FOR PORPHYRA HAITANENSIS (BANGIALES, RHODOPHYTA) BASED ON SEQUENCE-RELATED AMPLIFIED POLYMORPHISM AND SIMPLE SEQUENCE REPEAT MARKERS1
Received 15 August 2009. Accepted 28 January 2010.
Abstract
Molecular markers and molecular genetic maps are prerequisites for molecular breeding in any plant species. A comprehensive genetic linkage map for cultivated Porphyra haitanensis T. J. Chang et B. F. Zheng has not yet been developed. In this study, 157 double haploid (DH) lines [derived from a YSIII (wildtype) × RTPM (red-type artificial pigmentation mutant) cross] were used as a mapping population in P. haitanensis. A total of 60 pairs of sequence-related amplified polymorphism (SRAP) primers and 39 pairs of simple sequence repeat (SSR) primers were used to detect polymorphisms between the two parents. Fifteen SRAP and 16 SSR polymorphic primer pairs were selected to analyze the DH population. A linkage genetic map comprising 67 SRAP markers and 20 SSR markers in five linkage groups, with a total length of 830.6 cM and an average of 10.13 cM between markers, was constructed. The markers were distributed evenly in all linkage groups without clustering. The linkage groups comprised 12–23 markers ranging in length from 134.2 to 197.3 cM. The estimated genome length of P. haitanensis was 942.4 cM, with 88.1% coverage. This is the first report of a comprehensive genetic map in P. haitanensis. The map presented here will provide a basis for the development of high-density genetic linkage maps and lay the foundation for molecular breeding work in P. haitanensis.
Abbreviations:
-
- AFLP
-
- amplification fragment length polymorphism
-
- BC
-
- backcross
-
- C oa
-
- observed genome coverage estimates, determined by Goa/Ge
-
- C of
-
- observed genome coverage estimates, determined by Gof/Ge
-
- CTAB
-
- cetyltrimethyl ammonium bromide
-
- DH
-
- double haploid
-
- G e
-
- expected length of the genome
-
- G oa
-
- total length of the framework map considering all markers
-
- G of
-
- length of the framework map
-
- MAS
-
- marker-assisted selection
-
- QTLs
-
- quantitative trait loci
-
- RAPD
-
- random amplified polymorphic DNA
-
- SRAP
-
- sequence-related amplified polymorphism
-
- SSR
-
- simple sequence repeat
Porphyra, a genus of marine red algae, is an important economic marine crop, with an annual harvest of >130,000 t (dry weight) and a value of over US$2 billion. Farming and processing of Porphyra have generated the largest seaweed industries in East Asian countries, such as China, Japan, South Korea, and North Korea (Sahoo et al. 2002). Among Porphyra species, P. haitanensis is one of the most important species. It has been widely cultivated along the coasts of South China, especially Fujian and Zhejiang provinces. In recent years, P. haitanensis has comprised 75% of the total production of cultivated Porphyra in China (Zhang et al. 2005). Although P. haitanensis sea farming has been employed since the early 1960s, most of the cultivated lines were wild varieties collected from the coasts. They had been reused over the generations without being selected. The work of germplasm purification, rejuvenation, and genetic improvements has fallen behind, resulting in the degeneration of the cultivar quality. Therefore, it is highly desirable to select or breed new lines of P. haitanensis with strong economic traits and use them for cultivation.
Recent advances in the area of plant genomics have provided many tools to assist plant breeding. Molecular markers and molecular genetic linkage maps are prerequisites for undertaking molecular breeding activities in any plant (Varshney et al. 2005). A genetic linkage map could be useful in breeding and genomic research, such as in identification and mapping of genes and quantitative trait loci (QTLs), comparative genetics, and marker-assisted selection (MAS) (Rex 2008). In crops (e.g., rice, maize, and soybean), many genetic maps using morphological or molecular markers have been obtained and have played important roles in genetic improvement (Rex 2008). However, in seaweed, few genetic linkage maps have been constructed. To study the sexual behavior of the heterothallic green alga Chlamydomonas eugametos Moewus, Michel et al. (1996) presented a partial linkage map based on three morphological markers, 15 random amplified polymorphic DNA (RAPD) markers, and one RFLP marker. Li et al. (2007) constructed two moderate-density genetic linkage maps for Laminaria based on amplification fragment length polymorphism (AFLP) markers and a “two-way pseudo-testcross” mapping strategy. To date, no genetic linkage maps of Porphyra have been reported.
The life cycle of Porphyra consists of two completely different morphological generations, a leafy gametophyte and a filamentous sporophyte. Meiosis is the breaking point of the two generations in metagenesis. The recombinational crossover of various markers in meiosis is the base of genetic linkage map construction. However, the timing of meiosis in the life cycle of Porphyra has been a highly controversial issue for algologists (Shimizu et al. 2008, Zhou et al. 2008), and the issue is also an obstacle in genetic linkage map construction. After the discovery of pigmentation mutants of Porphyra, they were used as genetic markers to study and solve the issue. Ohme et al. (1986)Ohme and Miura (1988) first deduced that meiosis of P. yezoensis occurs during the first two divisions of the germinating conchospore, based on the results of crossing experiments between pigmentation mutants and the wildtype. Although P. yezoensis seemed to be the model macroalga (Sahoo et al. 2002, Waaland et al. 2004) and its genetics and molecular biology had been extensively studied, the gametophytic blades of P. yezoensis are monoecious and can easily perform asexual reproduction. Thus, the parents of the offspring cannot be easily traced, and a genetic linkage map is still not available for P. yezoensis. Recently, Yan et al. (2005, 2008) also deduced that meiosis of P. haitanensis was the same as that in P. yezoensis, based on cytological observation and crossing experiments between artificial pigmentation mutants and wildtype P. haitanensis. They considered that the initial four cells of a developing conchosporeling constituted a linear genetic tetrad leading to the formation of a color-sectored blade of P. haitanensis; the color sector in the gametophytic blades could be separated for genetic analysis. Moreover, most of the gametophytic blades of P. haitanensis are dioecious and cannot perform asexual reproduction; the parents of the offspring can be clearly and easily traced. Thus, P. haitanensis has an advantage over P. yezoensis in genetic linkage map construction.
The main objective of this research was to construct the first genetic linkage map for the P. haitanensis genome using SSR and SRAP markers and one DH population. The map can serve as the basis for the development of high-density genetic linkage maps, which will facilitate QTL mapping of desirable traits and provide markers for MAS, map-based cloning of genes, and the introgression of beneficial genes from wild species into modern cultivars.
Materials and methods
Map population. A DH population of 157 lines was used to construct the genetic linkage map of P. haitanensis. The parental lines used in the hybridization experiment were a wildtype line (male), YSIII, and a red-type artificial pigmentation mutant line (female), RTPM. The free-living conchocelis of the wildtype line were established in 1999 from a gametophytic blade collected on the coast of Dongshan Island, Fujian Province, China, and have been maintained in the laboratory. The stock culture was maintained at 21 ± 1°C under 50–60 μmol photons · m−2 · s−1 [12:12 light:dark (L:D)] provided by cool-white fluorescent lamps (Philips, Shanghai, China, TLD 36W/54), by renewing the culture medium (MES; Wang et al. 1986) every 6 months. Free-living conchocelis of the RTPM line of P. haitanensis were obtained by treating the gametophytic blades of another wildtype with 60Co-γ rays (Chen et al. 2008).
To prepare the DH population, the mature free-living conchocelis of each parent were induced to release conchospores. The conchospores were collected into a 300 mL flask containing 200 mL culture medium and cultured with aeration in an incubator at 25 ± 1°C under 80 μmol photons · m−2 · s−1 (10:14 L:D) to develop into gametophytic blades, with renewal of culture medium every week. After ∼2 months in culture, healthy gametophytic blades were selected as parents for crossing experiments, and a male and a female blade were cocultured in a flask until carposporangia appeared. About 2 weeks later, the fertilized female blade was transferred into a new flask and cultured under the same conditions until carpospores were released. The carpospores were collected and grown individually to conchocelis colonies in a test tube. When the conchocelis colonies grew to some size, they were fragmented by a homogenizer and continued in culture until the conchospores were released. Culture conditions and methods were the same as described above. Once conchospores were released from the heterozygous conchocelis filaments, they were collected and passed gently through a 50 μm nylon mesh filter (Rui Li Da, Anping, China) and cultured in petri dishes containing the culture medium at 25 ± 1°C under 40 μmol photons · m−2 · s−1 (10:14 L:D) to obtain F1 gametophytic blades. After 40 d in culture, F1 gametophytic blades were picked out and transferred onto a slide glass to examine the types of F1 blades under a light microscope (Nikon, Tokyo, Japan, SMZ800). Each partial color phenotype F1 blade was obtained by a puncher and digested into a single vegetative cell by 2% snail enzymes dissolved in 2 mol · L−1 glucose liquor. The vegetative cells were then induced to develop into conchocelis (with double the normal amount of chromosomes) by the method of single somatic cell clone cultivation (Zeng et al. 2004), producing the DH population. After processing, 166 color sectors were gained from 50 F1 blades, and only 157 color sectors were developed into conchocelis.
DNA extraction and marker analysis. DNA was isolated from free-living conchocelis of each parental line and 157 DH lines. The collected free-living conchocelis were ground into powder with a high-speed homogenizer (IKA, Staufen, Germany, T8 basic), and DNA was extracted and purified by the cetyltrimethyl ammonium bromide (CTAB) method (Joseph and David 2001). DNA concentrations were determined with a DU-600 spectrophotometer (Beckman Coulter, Fullerton, CA, USA) and adjusted to 5 ng · μL−1 for PCR amplification.
SRAP analysis was conducted according to previously established protocols (Li and Quiros 2001). In this assay, 60 different primer combinations were employed using six forward primers and 10 reverse primers combined randomly (Table S1 in the supplementary material).
SSR analysis was conducted according to previously established protocols (Xie et al. 2009). In this assay, 39 different primer combinations, which were developed from the genome data or expressed sequence tag (EST) data of P. haitanensis, were used (Table S2 in the supplementary material) (Zuo et al. 2006, Xie et al. 2009).
The PCR amplifications for SRAP and SSR analysis were performed in a 25 μL PCR reaction mixture containing 2.5 μL of 10 × PCR buffer, 5 ng of genomic DNA, 1.0 U of Taq polymerase (Takara Biotechnology Co. Ltd., Dalian, China), 0.2 μM of forward primer (Takara), 0.2 μM of reverse primer (Takara), and 200 μM of dNTP (Takara). The amplifications were performed in an MT programmable thermal controller PTC-200 (Bio-Rad, Hercules, CA, USA). The PCR conditions were as follows:
- 1
For SRAP primers: 5 min of denaturing at 94°C and five cycles of three steps: 1 min of denaturing at 94°C, 1 min of annealing at 35°C, and 2 min of elongation at 72°C. In the following 35 cycles, the annealing temperature was increased to 50°C with a final elongation step of 10 min at 72°C.
- 2
For SSR primers: 5 min of denaturing at 94°C and 35 cycles of three steps: 45 s of denaturing at 94°C, 45 s of annealing at given temperature (depending on the different primer pairs, Table S2), and 1 min of elongation at 72°C, with a final elongation step of 10 min at 72°C.
The separation of PCR-amplified fragments of SRAP and SSR analysis was accomplished by 6% denatured PAGE at 60 W for 2 h, and the amplified products were visualized by silver staining (Joseph and David 2001). A standard molecular weight marker (mass ruler DNA ladder; MBI Fermentas, Burlington, ON, Canada) was used in each electrophoresis run.
Data analysis and linkage mapping. The polymorphic loci of SRAP and SSR were identified by comparing the band types of the parents of the mapping population with those of the 157 DH individuals. “A” represented a band type that was the same as the male parent, “B” represented a band type that was the same as the female parent, and “-” represented a band type that could not be identified or for which the data were absent. The loci were named after “primer combination + estimated fragment size” (SRAP) and “primer name + estimated fragment size” (SSR). For example, the locus name for a 290 bp fragment of SRAP produced by the primer combination of ME1 and EM7 is E1M7-290, and the locus name for a 210 bp fragment of SSR produced by the primer combination of Phes09 is Phes09-210 (see in linkage maps). A chi-squared test (P < 0.05) was performed to test the null hypothesis of 1:1 segregations on all the scored loci. Only marker loci that did not show segregation distortion (P < 0.05) from the expected 1:1 were used for the map construction, to eliminate spurious linkages.
The linkage analysis was performed using MAPMAKER/EXP (version 3.0b) (Lincoln et al. 1993). Markers were first grouped using a minimum LOD score threshold of 3.0 and at a maximum map distance of 50 cM using the “group” command. The Kosambi mapping function was used to convert recombination frequencies into map distances (cM; Kosambi 1944). The “compare” command was used for groups with less than eight loci. The “order” command was used to determine a linear order. The remaining loci in each group were placed with the “try” command and were then considered as accessory markers. The ordered marker sequences were confirmed by the “ripple” command. Linkage maps were drawn manually on an Excel spreadsheet by the software Mapdraw (Liu and Meng 2003).
Estimation of genome length and map coverage. Average marker spacing of the framework map was calculated by dividing the total length of all linkage groups by the number of intervals (the number of markers minus the number of linkage groups). The average marker spacing for each linkage group was calculated by dividing the length of the linkage group by the number of the intervals on that linkage group (the number of markers minus 1).
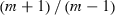 (1)
(1)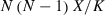 (2)
(2)Results
Analysis of markers on the mapping population. A total of 60 SRAP primer combinations were used to screen polymorphisms between the parents, and 15 primer combinations with clear polymorphisms were chosen to analyze the 157 lines of the DH population. The 15 primer pairs produced a total of 535 loci, ranging from 27 (ME4/EM6) to 45 (ME2/EM7) per primer pair (Table 1). On average, each primer pair produced 35.7 loci. Among the 535 loci produced, 123 (23.0%) were polymorphic between the two parents and segregative on the 157 DH lines. The number of polymorphic loci per primer pair ranged from five (ME6/EM7) to 12 (ME3/EM7); the level of polymorphism varied among primer pairs, ranging from 14.7% to 31.6% (Table 1).
| Marker type | Primer combination | No. of total loci | No. of polymorphic loci | Percentage of polymorphic loci |
|---|---|---|---|---|
| SRAP | ME1/EM5 | 34 | 8 | 23.5 |
| ME1/EM7 | 37 | 7 | 18.9 | |
| ME1/EM9 | 29 | 9 | 31.0 | |
| ME2/EM1 | 41 | 8 | 19.5 | |
| ME2/EM5 | 37 | 10 | 27.0 | |
| ME2/EM7 | 45 | 11 | 24.4 | |
| ME2/EM9 | 30 | 9 | 30.0 | |
| ME3/EM7 | 38 | 12 | 31.6 | |
| ME3/EM9 | 42 | 7 | 16.7 | |
| ME4/EM6 | 27 | 6 | 22.2 | |
| ME5/EM4 | 33 | 7 | 21.2 | |
| ME5/EM6 | 36 | 8 | 22.2 | |
| ME5/EM7 | 40 | 9 | 22.5 | |
| ME6/EM7 | 34 | 5 | 14.7 | |
| ME6/EM9 | 32 | 7 | 21.9 | |
| Total | 535 | 123 | 23.0 | |
| SSR | Phes02 | 5 | 1 | 20.0 |
| Phes03 | 4 | 1 | 25.0 | |
| Phes06 | 11 | 4 | 36.4 | |
| Phes07 | 5 | 2 | 40.0 | |
| Phes09 | 9 | 3 | 33.3 | |
| Phes11 | 8 | 3 | 37.5 | |
| Phes12 | 9 | 2 | 22.2 | |
| Phes14 | 7 | 1 | 14.3 | |
| Phes15 | 13 | 3 | 23.1 | |
| Phes17 | 8 | 1 | 12.5 | |
| Phes20 | 9 | 2 | 22.2 | |
| Phes24 | 10 | 3 | 30.0 | |
| Phes28 | 7 | 2 | 28.6 | |
| PH14 | 4 | 1 | 25.0 | |
| PH47 | 8 | 3 | 37.5 | |
| PH49 | 5 | 2 | 40.0 | |
| Total | 122 | 34 | 27.9 |
- DH, double haploid; SRAP, sequence-related amplified polymorphism; SSR, simple sequence repeat.
A total of 39 SSR primer pairs were used to screen polymorphisms between the parents, and 16 primer pairs with clear polymorphisms were chosen to analyze the 157 DH lines. A single primer pair could generate four (Phes03, PH14) to 13 (Phes15) loci, and 122 loci were generated in total with an average 7.6 loci per primer pair (Table 1). Among the 122 loci produced, 34 (27.9%) loci were polymorphic between the two parents and segregative on the 157 DH lines. The number of polymorphic loci per primer pair ranged from one (Phes02, Phes03, Phes14, Phes17, PH14) to four (Phes06). The level of polymorphism also varied among primer pairs, ranging from 12.5% to 40.0% (Table 1).
Genetic linkage map construction. Using the “one locus as one marker” assumption, the segregations of the 123 SRAP markers and 34 SSR markers were first tested against the 1:1 Mendelian ratios using a chi-squared test. There were 37 (30.1%) SRAP markers and nine (26.5%) SSR markers that did not give the expected 1:1 ratio (P < 0.05) (Table 2). The remaining 86 SRAP and 25 SSR undistorted markers were used for linkage analysis and construction of a genetic linkage map for P. haitanensis with MAPMAKER 3.0. Among all the markers used, 67 SRAP markers and 20 SSR markers were assigned to the framework map, and 19 SRAP markers (22.1%) and five SSR markers (20%) were unlinked. The unlinked markers included one triplet, three doublets, and 15 single markers (Table 2). The framework map (Fig. 1, Table 3) consisted of five linkage groups, with a total length of 830.6 cM (Table 3). The size of the linkage groups ranged from 134.2 to 197.3 cM, and the number of markers per linkage group varied from 12 to 23. The average marker spacing was 10.13 cM. Linkage group 5 had the largest average interval, 12.2 cM, and group 3 had the smallest average interval, 8.79 cM. The maximum marker spacing was in group 2 (37.1 cM), and the minimum marker spacing was in group 1 (0.4 cM) (Table 3).
| Molecular marker type | No. of polymorphic markers | No. of distorted markers | Rate of distorted markers | No. of linked markers | No. of unlinked markers in triplets | No. of unlinked markers in doublets | No. of unlinked singles | Rate of unlinked markers |
|---|---|---|---|---|---|---|---|---|
| SRAP | 123 | 37 | 30.1% | 67 | 2 | 5 | 12 | 22.1% |
| SSR | 34 | 9 | 26.5% | 20 | 1 | 1 | 3 | 20.0% |
| Total | 157 | 46 | 29.3% | 87 | 3 | 6 | 15 | 21.6% |
- SRAP, sequence-related amplified polymorphism; SSR, simple sequence repeat.
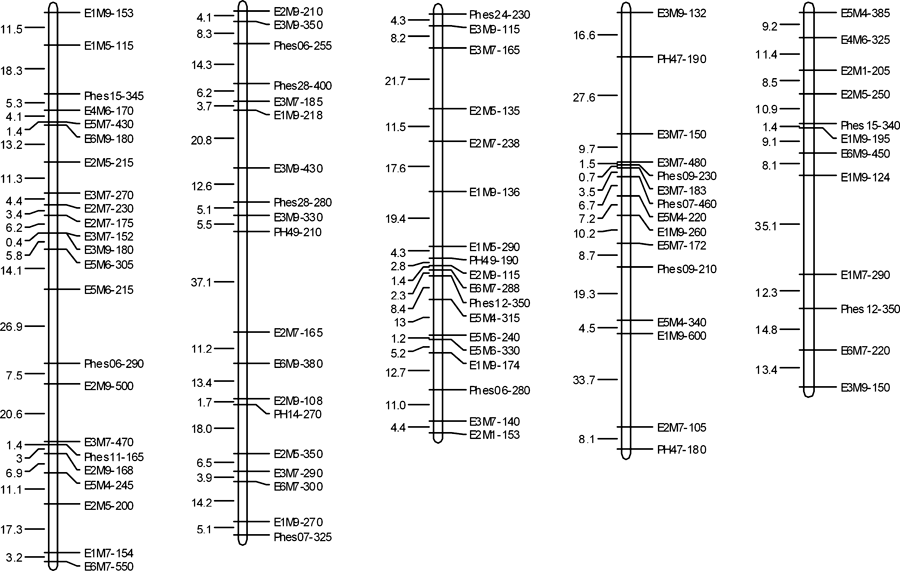
Genetic linkage maps of Porphyra haitanensis. Size of the linkage group (before slashes) and the marker number (after slashes) of each group are at the top, in parenthesis. Marker names are shown on the right of each group, and the adjacent marker spacing is displayed on the left in cM Kosambi.
| Linkage group | Length of group (cM) | No. of markers | Average marker spacing (cM) | Maximum marker spacing (cM) | Minimum marker spacing (cM) |
|---|---|---|---|---|---|
| 1 | 197.3 | 23 | 8.97 | 26.9 | 0.4 |
| 2 | 191.7 | 19 | 10.65 | 37.1 | 1.7 |
| 3 | 149.4 | 18 | 8.79 | 19.4 | 1.2 |
| 4 | 158.0 | 15 | 11.29 | 33.7 | 0.7 |
| 5 | 134.2 | 12 | 12.2 | 35.1 | 1.4 |
| Total | 830.6 | 87 | 10.13 | 37.1 | 0.4 |
Genome length and map coverage. The observed framework map length was 830.6 cM for the genetic linkage map of P. haitanensis. When unlinked doublets and triplets were considered along with the framework markers, the observed genome length increased to 884.7 cM. Three different methods gave similar estimates for the expected genome size of the P. haitanensis (Table 4). The estimated genome length was 942.4 cM (ranging from 934.4 to 958.3 cM). The observed genome coverage (Cof) was 88.1% for the framework maps. When unlinked doublets and triplets were considered, genome coverage (Coa) became 93.9% for the map (Table 4).
| Parameter | Observed genome length (cM) | Estimated genome length (cM) | Genome coverage (%) | |||||
|---|---|---|---|---|---|---|---|---|
| G of | G oa | G e1 | G e2 | G e3 | G e | C of | C oa | |
| Value | 830.6 | 884.7 | 934.4 | 934.4 | 958.3 | 942.4 | 88.1 | 93.9 |
- G of, observed total length of the framework map; Goa, the map length based on framework map plus triples and doublets; Ge, the average of the estimated genome length Ge1, Ge2, and Ge3 (see Materials and Methods); Cof and Coa, the genome coverage of Gof and Goa, respectively.
Discussion
Molecular markers. A fine linkage map requires a good molecular system to produce large numbers of molecular markers. Potential marker abundance in a genome, marker availability, and high throughput detection at a reasonable cost are major factors that determine the usefulness of a molecular marker system. Although many molecular marker methods (e.g., RFLP, RAPD, AFLP, SSR, and SRAP) have been used to construct genetic linkage maps of many crops, each molecular marker method has unique advantages and disadvantages (Peters et al. 2003). RFLP has the advantage of producing mostly codominant markers, good repetition, and consistence, but the operation is too complicated to automate. Moreover, a large amount of high-quality DNA is required. It is thus very expensive to analyze a big population. RAPD is a simple method to fingerprint genomic DNA, but its poor consistency and low output limit its application. AFLP is now widely used for a variety of applications due to its high multiplex ratio, but the operation is very complicated. SSR is a good marker system for its simplicity and mostly codominant information; however, the development of SSR primers is expensive and time consuming. SRAP is a simple marker-detection method that was recently developed (Li and Quiros 2001). It has the advantages of simplicity, reliability, moderate throughput ratio, and facile sequencing of selected bands. It has been widely used in map construction (Li and Quiros 2001, Sun et al. 2007), comparative genetics analysis (Li et al. 2003, Budak et al. 2004a), and genetic diversity analysis (Ferriol et al. 2003, Budak et al. 2004b). However, the method is less efficient for centromeres and telomeres because it mainly targets coding sequences in the genome. Some scientists think that SRAP combined with SSR could amplify repeat regions to construct a genetic linkage map that may cover the whole genome (Ahmad et al. 2004, Lin et al. 2005, Levi et al. 2006). Fortunately, Zuo et al. (2006) and Xie et al. (2009) have developed some SSR primers from genome or EST data of P. haitanensis. Thus, in the present study, SSR and SRAP markers were used to construct the linkage map of P. haitanensis. The results indicated that the two marker systems were effective. In this assay, each primer combination of SRAP and SSR could independently generate an average of 8.2 and 2.1 polymorphic loci, with a maximum of 12 and four polymorphic loci produced by each method, respectively. The SRAP and SSR markers distributed evenly on the map, and genome coverage reached 88.1% (if unlinked doublets and triplets were considered, the genome coverage reached 93.9%). Thus, the combined use of SRAP and SSR markers is very useful for constructing a high-resolution linkage map of P. haitanensis.
Linkage map. Linkage maps, especially high-density ones, facilitate a number of important biological investigations. They are necessary for efficient mapping of QTLs, MAS, and comparative genome mapping (Rex 2008). In this work, based on SSR and SRAP marker segregation data, the first comprehensive linkage map of P. haitanensis was constructed.
In this map, the linkage group number was equal to the haploid chromosome number of P. haitanensis; however, considering the few markers (87) used and the absence of cytology markers, we cannot judge whether the each linkage group corresponded to each chromosome. The observed framework map length is 830.6 cM (if unlinked doublets and triplets were considered, the map length was 884.7 cM) (Table 4), and the 87 markers were evenly and randomly distributed in the five linkage groups, with no clusters or gaps as in other species. Although the marker density was low (average marker spacing 10.13 cM), the maps provided reasonably good coverage of the P. haitanensis genome (93.9%) when all linked markers were considered (Table 4). Therefore, the maps will provide a basic framework for the development of high-density genetic linkage maps, which will facilitate QTL mapping of desirable traits, and provide markers for MAS, map-based cloning of genes, and the introgression of beneficial genes from wild species into modern cultivars.
Map populations and marker segregation distortion. Numerous populations, including F2 populations, backcross (BC) populations, recombinant inbred line (RIL) populations, and DH populations, have been used to construct genetic linkage maps of various plants. F2 populations and BC populations are highly heterozygous and cannot be propagated indefinitely through seeds. Moreover, the experimental data based on the F2 and BC populations cannot be correlated across laboratories, so these two populations have been gradually phased out for linkage map construction and QTL identification. DH populations and RIL populations are two types of permanent populations. Linkage maps based on DH or RIL populations are permanent and suitable for sustained genetic studies, especially for QTL identification. Furthermore, DHs are usually obtained in a single generation, whereas the development of recombinant inbred lines requires many generations. DH populations have become standard resources in genetic mapping for species in which DHs are readily available (Forster and Thomas 2005). Thus, the DH population was selected to construct the linkage map of P. haitanensis in this study.
The analysis of markers in the DH population showed a high segregation distortion ratio. In this study, when considering all polymorphic loci, the segregation distortion ratios of SRAP and SSR markers were 30.1% and 26.5%, respectively. Segregation distortion can be found with varying degrees in each population and for each type of marker (Foisset and Delourme 1996). Many genetic explanations for distortion of segregation ratios in plants have been put forth, including chromosome loss (Kasha and Kao 1970), genetic isolation mechanisms (Zamir and Tadmor 1986), and the presence of viability genes (Bradshaw and Stettler 1994). Nonbiological factors, such as scoring errors (Nikaido et al. 1999) and sampling errors (Echt and Nelson 1997), can also lead to distortion of segregation ratios. Furthermore, the map population also is a key factor (Haitham et al. 2002). Xu et al. (1997) compared segregation distortion in 56 populations of different species and showed that RIL populations had significantly higher frequencies of distorted markers than DH and BC populations. F2 populations tended to have lower frequencies of distorted markers and higher variability between individual crosses. Haitham et al. (2002) compared the segregation ratios in a DH population and an F2 population of the same cross and showed that segregation distortion in the DH population was 44.2% of the observed loci, much higher than in the F2 population (16.3%). The high segregation distortion ratio of DH populations is related to the higher selection pressures induced by many recessive lethal or sterility genes when they become homozygous and to sampling errors in DH line production (Haitham et al. 2002). In this study, sampling errors were inescapable when picking out the F1 gametophytic blades and each color sector of the F1 blades in DH line production. First, the number of F1 gametophytic blades was very large, and only part of the F1 gametophytic blades could be picked out for further analysis. Second, the color phenotype in the gametophytic blades of Porphyra was due to two (or more) recessive mutations (Yan et al. 2005); similar colors of genetically different sectors border one another and could not be distinguished. Third, some of the vegetative cells that were obtained from the color sectors could not be developed into conchocelis, which could also have induced the segregation distortion of markers. Thus, the level of segregation distortion observed in this study is higher than in other studies.
In linkage analyses, the distorted markers can affect not only the estimation of genetic distance between two markers, but also the order of markers on the same linkage map; however, if they are ignored, the distorted markers can result in the loss of some important information (Zhu et al. 2007). Some methods have been developed to solve the distorted markers in linkage map construction, including appending the distorted markers into the linkage group by higher LOD score threshold (Murigneux et al. 1993), comparing maps between different populations (Cloutier et al. 1997), and adjusting the map population (Wang et al. 2004). Lorieux et al. (1995a,b) developed two maximum-likelihood models for distorted markers in BC and F2 populations. However, the models also raised some other problems (Zhu et al. 2007); therefore, Zhu et al. (2007) further developed a multipoint approach via a hidden Markov chain model to solve the distorted markers in F2 populations. However, no similar models were developed in the DH populations. In this study, we tried to include the distorted markers in the linkage map by enhancing the LOD score threshold to 4.0, and only seven SRAP markers and two SSR markers were appended into the five linkage groups. The new appended distorted markers were distributed in the five linkage groups randomly (they were not clustered in some regions of the linkage map) and did affect the genetic distance and order of the undistorted markers on the same linkage group (data not shown). Thus, to ensure the veracity of the linkage map of P. haitanensis, all the distorted markers were discarded, despite reducing the genome coverage of the map.
Acknowledgments
This research was supported in part by the National High Technology Research & Development Program of China (“863” Program) (Grant no. 2006AA10A413); the National Natural Science Foundation of China (Grant no. 40676077 and 40806065); the National Natural Science Foundation of Fujian Province, China (Grant no. 2007J0064); the Construction Program of Science and Technological Platform of Fujian Province, China (Grant no. 2007N2011); the Foundation for Young Professors of Jimei University, China (Grant no. 2007C002); and the Foundation of Key Laboratory of Science and Technology for Aquaculture and Food Safety, Fujian Province University (Grant no. 2008J202).