MORPHOLOGICAL FLEXIBILITY OF COCCONEIS PLACENTULA (BACILLARIOPHYCEAE) NANOSTRUCTURE TO CHANGING SALINITY LEVELS1
Received 21 August 2009. Accepted 24 March 2010.
Abstract
Diatoms possess a silica frustule decorated with unique patterns of nanosize features. Here, we show for the first time from in situ samples that the size of the nanopores present at the surface of the diatom Cocconeis placentula Ehrenb. varies with fluctuating salinity levels. The observed reduction in nanopore size with decreasing salinity agrees with previous laboratory experiments. We also uniquely combined our observations with theoretical considerations to demonstrate that the decrease in the diffusive layer thickness is compensated for by the changes in pore size, which maintain a steady diffusive flux toward the diatom’s cell at different salinities. This process allows diatoms to absorb similar amount of nutrients whatever the salinity and as such to increase their ecological competitiveness in fluctuating environments. These results further suggest that the overall ecological success of diatoms, and their ability to react to environmental changes, may be controlled by the flexibility of the morphological characteristics of their frustules.
Abbreviations:
-
- FESEM
-
- field-emission scanning electron microscopy
-
- PSU
-
- practical salinity units
-
- SDV
-
- silica deposition vesicle
Diatoms (Bacillariophyceae) are among the most abundant phototrophs on earth (Falkowski et al. 1998) and account for 40% of the total primary production in the ocean (Nelson et al. 1995, Smetacek 1999, Tréguer and Pondaven 2000). They are seen as the main players in biogeochemical cycles (Buesseler et al. 1998) and as keystone organisms significantly affecting zooplankton grazing and recruitment (Irigoien et al. 2002, Ianora et al. 2004, Jones and Flynn 2005). In addition, they are increasingly recognized as useful biological indicators of water quality (Gell et al. 2002).
Each of the estimated 105 diatom species has a specific frustule shape (or cell wall) similar to a petri dish with overlapping upper and lower halves, respectively referred to as epitheca and hypotheca (Hildebrand 2008). Capping the thecae are the valves, which are decorated with a unique pattern of nanosize features (i.e., pores, channels, ridges, spikes, spines; Round et al. 1990). The two valves are called the epivalve, which originates from the parent cell, and the hypovalve, which is formed after cell division. The nanosize features present at the valve surface, such as the pores, are species-specific and provide precise features for the identification of diatoms (Gell et al. 2005). In particular, the architecture of the valves of some diatom species (e.g., Coscinodiscus sp. and Thalassiosira eccentrica) shows fundamental morphological differences including the number of layers, size, shape, density, and geometric arrangement of pores (Losic et al. 2006). These pores allow the exchange between the internal cell and the external environment and are thus important features for diatom growth. Recent experimental work has shown that the architecture and distribution of the pores appear to be influenced by environmental factors, such as pH (Vrieling et al. 1999) and salinity (Gordon and Brodland 1990, Vrieling et al. 2000, 2007). However, to our knowledge, no study has addressed the issue of the impact of salinity on pore architecture, based on naturally occurring diatoms. The only reference to salinity in an investigation of diatom pores is the identification of salt crystals at the surface of Biddulphia sinensis taken from the North Sea (Vrieling et al. 2000).
In this framework, our study aimed (i) to assess the potential variability in the size of pores present at the surface of diatoms in contrasted salinity conditions and (ii) to infer their potential contribution to the diffusive fluxes through the frustule. This has specifically been addressed along the natural salinity gradient (from estuarine, S = 25 PSU, to hypersaline, S > 150 PSU; Schapira et al. 2009) occurring in a coastal lagoon extending over a distance of 100 km along the coastline of South Australia (Fig. 1), the Coorong wetlands.
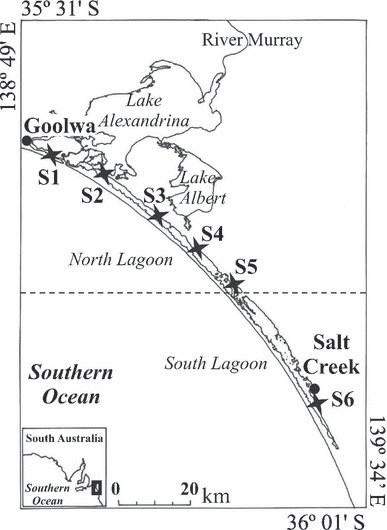
Wetlands of the Coorong National Park in South Australia. The different regions (north and south lagoons) as well as the sampling sites (sites S1 to S6; 27–154 PSU) are indicated.
Along the 100 km distance of the Coorong wetlands, six sites were monitored and showed a salinity gradient ranging from 27 PSU in the north lagoon (S1; Fig. 1) to 154 PSU in the south lagoon (S6; Fig. 1). At each site, diatoms were collected using a plankton net (60 μm mesh size). Hydrological parameters were determined in situ using a YSI-85 oxygen, salinity, and temperature meter (TPS WP Series, TPS Brisbane, Qld, Australia). Water samples were collected and frozen prior to assessing nutrient concentrations (i.e., nitrate, nitrite, ammonium, and phosphate) in the laboratory using an Aquaspex photometer (Aquaspex Water Testing Products, Blackwood, South Australia, Australia).
The only diatom species that was present at several sampling sites (S3 to S5) was Cocconeis placentula. However, the abundance of C. placentula at S5 was too small to be able to undertake further analysis on the population of that site. We then specifically focused on two sites, S3 and S4, with a salinity of 67 and 100 PSU, respectively. The organic material present on the surface of C. placentula was removed using sulfuric acid (50% concentration) following Losic et al. (2006). The architecture and distribution of the pores at the surface of the C. placentula frustules were determined using field-emission scanning electron microscopy (FESEM). FESEM images were acquired using a Philips XL30 field-emission scanning electron microscope (Philips Electronics, Andover, MA, USA) operated at 2–10 kV (Fig. 2). We then measured the nanopores of 20 diatoms from each of the sites (sites S3 and S4; Fig. 1). These 20 diatoms corresponded to the measurement of a total of ∼400 pores per site. For each individual diatom, the size (i.e., length and width) of the frustules and pores was measured from the FESEM images using the MOTIC Images plus 2.0 software (MOTIC, http://www.motic.com).
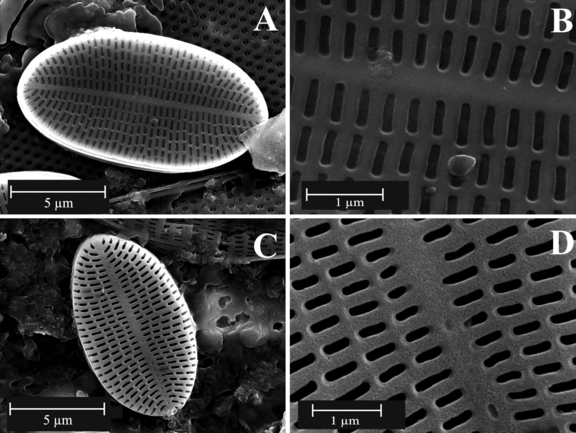
Field-emission scanning micrographs of examples of the frustule morphology of Cocconeis placentula from two sites (sites S3 and S4) in the Coorong wetlands. (A) and (B) correspond to 67 PSU, and (C) and (D) correspond to 100 PSU.
Then, the total number of pores per valve and the related percentage of open surface area were determined. This was to characterize the surface of exchange between the cell and the environment. Comparisons between the two sampling sites were conducted using the Wilcoxon–Mann–Whitney U-test (Zar 1999). In addition, the correlation between the size of the pores and the size of the diatoms was tested through the Spearman coefficient of rank correlation. Finally, the variability in the measurements of the length and width of the pores was assessed using the coefficient of variation (CV).
Results. The concentration of ammonium and phosphate, respectively, ranged between 20 and 103.33 μmol · L−1, and 2.11 and 6.32 μmol · L−1. The nutrients significantly increased between the two sites (Wilcoxon–Mann–Whitney, P < 0.01), with the highest concentrations consistently observed at 100 PSU. However, the concentration of nitrate was constant at both sites with 1.61 μmol · L−1, and nitrite concentrations ranged between 1.09 and 1.30 μmol · L−1. No differences were observed between the two sites for nitrate and nitrite (P > 0.05). The same observations were made for dissolved oxygen, which ranged between 3.86 and 4.29 mg · L−1.
The length (L) and width (W) of the pores of C. placentula were measured at L = 517 ± 8 nm (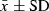 ) and W = 148 ± 2 nm for 100 PSU, and L = 490 ± 6 nm and W = 108 ± 2 nm for 67 PSU and showed significant differences between the two salinities at each site (Wilcoxon–Mann–Whitney, P < 0.01, Fig. 3). These findings indicate an increase in the size of the pores with salinity. Furthermore, the length/width pore ratio (RPore) at 100 PSU (RPore = 3.67 ± 0.08;
) and W = 148 ± 2 nm for 100 PSU, and L = 490 ± 6 nm and W = 108 ± 2 nm for 67 PSU and showed significant differences between the two salinities at each site (Wilcoxon–Mann–Whitney, P < 0.01, Fig. 3). These findings indicate an increase in the size of the pores with salinity. Furthermore, the length/width pore ratio (RPore) at 100 PSU (RPore = 3.67 ± 0.08; 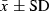 ) was significantly smaller than at 67 PSU (RPore = 4.78 ± 0.10, P < 0.01). Finally, the length/width valve ratio (RValve) did not vary between sites (P > 0.05). The variability (CV) in pore measurements was similar for both sites (CVlength = 17%–22% and CVwidth = 20%–21%).
) was significantly smaller than at 67 PSU (RPore = 4.78 ± 0.10, P < 0.01). Finally, the length/width valve ratio (RValve) did not vary between sites (P > 0.05). The variability (CV) in pore measurements was similar for both sites (CVlength = 17%–22% and CVwidth = 20%–21%).
Length and width of the pores located on the external side of Cocconeis placentula frustules for sites 3 (circles) and 4 (triangles), sampled along the Coorong wetlands. The length and width of individual pores have been normalized by the size (length and width) of the corresponding diatom frustule.
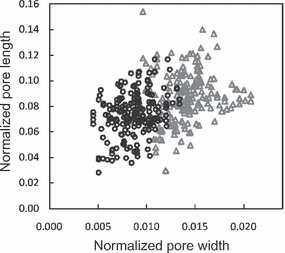
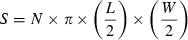 (1)
(1)
Surface of the pores located on the external side of Cocconeis placentula frustules for sites 3 (circles) and 4 (triangles), sampled along the Coorong wetlands. The x-axis represents the surface of the valve from which the pore measurements are taken.
The variation in pore size with salinity might first be related to the silicification process. In particular, an increase in silicification has been reported for various diatom species at low salinity (Paasche 1980, Tuchman et al. 1984, Romero 1994). Freshwater species then tend to have a higher silica content and biovolume compared to marine and brackish species (Conley et al. 1989). This difference could reflect higher silicon availability or transport coupling effects due to reduced salinity (Hildebrand 2008). In particular, because salinity can promote diatom growth (i.e., cell division rate; Guillard and Ryther 1962, Olsen and Paasche 1986), the difference in silica content between marine and freshwater diatoms might be a consequence of the hastened cell cycle in marine diatoms, which simply allows less time for silicon transport and deposition (Flynn and Martin-Jezequel 2000, Martin-Jezequel et al. 2000). In addition, an increase in silicification under fluctuating pH (Vrieling et al. 1999) and salinities (Gordon and Brodland 1990, Vrieling et al. 2007) could also be related to changes in the physicochemical condition of the silica deposition vesicle (SDV) where the diatom silica formation processes occur (Hildebrand 2008). These changes in silicification imply the formation of a thicker frustule at lower salinity, which may lead to the reduction in nanopore size. This trend is congruent with our results and with previous experiments, which showed that the pore size of cultured Navicula salinarum, Thalassiosira weissflogii, and Thalassiosira punctigera become smaller with decreasing salinity (Vrieling et al. 2000, 2007). However, salinity-induced osmotic stress leads to an increase in silicification in diatoms (Olsen and Paasche 1986, Romero 1994). This change is especially relevant at the extreme salinities encountered in our study, which trigger osmoregulation, and hence are likely to affect both growth and silicification processes (Vrieling et al. 2007) and thus the thickness and nanostructure of the diatom frustules.
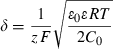 (2)
(2)This work suggests salinity as a main driver to the morphology of diatoms frustules, but other environmental factors, such as temperature, light, pH, nutrients, and metal ions, may also impact the physiological state of the cells and therefore regulate uptake and deposition rates of silica (Tuchman et al. 1984, Brzezinski 1985, Blank et al. 1986, Olsen and Paasche 1986, Romero 1994, Vrieling et al. 1999, 2000). Further work is nevertheless needed to generalize the present work and unambiguously identify the parameters and/or processes driving the morphological flexibility of diatoms. This is particularly relevant to provide further understanding of the quasi-ubiquitous ecological success of diatoms and their ecological competitiveness.
Acknowledgments
We thank the Department for Environment and Heritage for allowing us to undertake our research in the Coorong National Park. This project has been funded by the Australian Research Council and Flinders University. This research was supported under Australian Research Council’s Discovery Projects funding scheme (project numbers DP0664681 and DP0666420). Professor Seuront is the recipient of an Australian Professorial Fellowship (project number DP0988554).