DNA BARCODING AND GENETIC DIVERGENCE IN THE GIANT KELP MACROCYSTIS (LAMINARIALES)1
Received 29 September 2009. Accepted 28 January 2010.
Abstract
The brown alga Macrocystis C. Agardh is widely distributed throughout the cold temperate waters of the Northern and Southern hemispheres, forming ecologically diverse and productive kelp forests. The taxonomy of this alga has been under constant discussion. Since the first description, species have been mostly described by holdfast and blade morphology; however, the importance of these taxonomic characters has been questioned. Based on a morphological study, the genus has recently been synonymized into a single species, M. pyrifera (L.) C. Agardh, but additional genetic evidence is still lacking. Using the “DNA-barcoding” gene (COI), we examined the taxonomy of Macrocystis collected from 19 sites worldwide, covering the distribution of the four ecomorphs (M. “pyrifera,” M. “angustifolia,” M “integrifolia,” and M. “laevis”). Our molecular data strongly support the recognition of a single species; therefore, the genus should contain only one species, M. pyrifera, the oldest name. Results also reveal shared haplotypes in several distant sites around the Southern Hemisphere and very low variability among samples. Additionally, samples of the ecomorphs M. “integrifolia” and M. “pyrifera” from a sympatric population in California had the same haplotype. The revised taxonomy changes questions of Macrocystis distribution from interspecific dispersal and evolutionary questions to intraspecific ecological questions on the maintenance of Macrocystis in certain environments that produce particular morphologies.
Abbreviations:
-
- bp
-
- base pair
-
- COI
-
- cytochrome c oxidase subunit I
-
- CTAB
-
- cetyl trimethylammonium bromide
The genus Macrocystis was created by Agardh in 1820, although it was known long before as Bulbus marinus crinitus (Bahuine 1651) and described by Linnaeus (1771) as Fucus pyriferus from specimens collected by Koenig in “Oceano Aethiopico” during a voyage between Europe and India (Womersley 1954). The exact locality of these specimens is not clear, and different localities have been mentioned by different authors: Cape of Good Hope by Hooker (1847); Kerguelen or Crozet Islands by Setchell (1932); Tristan da Cunha, Gough Island, or Falkland Islands by Womersley (1954). As the locality cannot be designated, a neotype has been selected from the western Falkland Islands, King George’s Sound (Spencer et al. 2009).
Since the first description, several species of Macrocystis have been described (Table S1 in the supplementary material). At first, most of these descriptions were based on drift specimens, and pneumatocyst and blade morphology were considered the most important taxonomic features. Hooker (1847) combined all the species reported by the mid-19th century into a single taxon, M. pyrifera, believing that variations among species were environmentally induced. This unification of Macrocystis species found general agreement (Harvey 1862, Skottsberg 1907). However, Areschoug (1883) and De Toni (1895) recognized the importance of the holdfast as a taxonomic character (Fig. 1). Howe (1914) reported M. integrifolia Bory from Peru on the basis of holdfast morphology, while Setchell (1932) concluded that this species also occurred in the Northern Hemisphere (NE Pacific). Womersley (1954) continued reliance on holdfast features, reporting M. angustifolia Bory in southern Australia, northern Tasmania, and South Africa. The general agreement on the importance of the holdfast as a taxonomic feature was questioned when Hay (1986) described a fourth species, M. laevis C. H. Hay, present at Marion Island (SW Indian Ocean), primarily based on the morphology of its smooth blades and vesiculate sporophylls; however, it possessed a conical holdfast similar to M. pyrifera. Aguilar-Rosas et al. (2003) also collected M. laevis in southern Chile, but this record has been questioned (Gutierrez et al. 2006). North (1994) observed that if using blade features finds general acceptance by algal taxonomists, possibilities exist for the creation of additional species within the genus due to the high plasticity in blade and holdfast morphologies. Thus, four species remained for more than 20 years, but recently, Demes et al. (2009) proposed the conspecificity of M. pyrifera, M. integrifolia, M. laevis, and M. angustifolia, with M. pyrifera as the only species. They demonstrated that environmental conditions influenced holdfast morphology of M. pyrifera and M. integrifolia in California and that this character could therefore not be used to separate the species (Demes et al. 2009). For the purpose of this article and following Demes et al. (2009), we will call the former four species “ecomorphs” (namely: M. “integrifolia,” M. “laevis,” M. “angustifolia,” and M. “pyrifera”) and use M. pyrifera to refer to the taxonomic species.
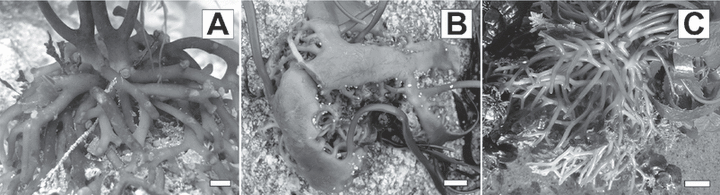
Macrocystis holdfast, the main morphological feature used to distinguish the former four species. (A) Macrocystis “pyrifera” with a conical holdfast (individual from North Island, New Zealand), (B) M. “integrifolia” with a rhizomatous holdfast (individual from northern Chile), and (C) M. “angustifolia” with a mounding rhizomatous holdfast (individual from southern Australia). The holdfast of M. “laevis” is the same as for M. “pyrifera.” Scale bar = 2.5 cm.
Previous studies have shown interfertility among the three most widely distributed ecomorphs (M. “pyrifera,” M. “angustifolia,” and M. “integrifolia”) (Lewis et al. 1986, Lewis and Neushul 1994, 1995, Druehl et al. 2005, Westermeier et al. 2007). In an early study, Coyer et al. (2001) addressed the taxonomy of Macrocystis using molecular methods. Using the internal transcribed spacer (ITS1 and ITS2), they suggested that the genus is monospecific as their data showed that the four species were not resolved in their phylogenetic analyses, but most of the 24 individuals analyzed corresponded to M. “pyrifera” (70% of the samples) and their data did not include M. “integrifolia” from the Southern Hemisphere.
Recently, the use of a mitochondrial gene, cytochrome c oxidase subunit I (COI) as a standardized marker has been suggested as useful for species identification in macroalgae (Saunders 2005, Robba et al. 2006, Lane et al. 2007, Kucera and Saunders 2008, McDevit and Saunders 2009). The COI gene is a relatively short piece of DNA that can be readily amplified and sequenced with one set of primers (Robba et al. 2006) and has the advantage of being an objective species identification tool in cases where identification is ambiguous (Kucera and Saunders 2008). According to McDevit and Saunders (2009), the ITS region is difficult to align above the genus level due to the large number of insertions and deletions, a problem not encountered in COI.
The origin and presence of M. “integrifolia” in both hemispheres remain unresolved; on the basis of chloroplast DNA, Druehl and Saunders (1992) suggested that M. “pyrifera” and M.“integrifolia” were either separated prior to trans-hemispheric dispersal or diverged subsequent to dispersal through the equator during the Pleistocene glaciations. Druehl and Saunders (1992) revealed low sequence divergence between M. “pyrifera” and M.“integrifolia” from the Northern Hemisphere (0.08%), but samples of M.“integrifolia” between hemispheres had higher divergence (0.3%).
Given the problematic morphology-based taxonomy, we investigated the species status among multiple Macrocystis samples collected worldwide using the DNA-barcoding COI sequence. We analyzed sequences from 118 samples of the four Macrocystis ecomorphs enhancing sample size and geographic coverage from the previously published analysis (Coyer et al. 2001).
Materials and methods
Sampling sites and collection. One hundred and eighteen samples of Macrocystis were collected from 19 sites around the world, representing the distributional range of the four former described species (Fig. 2 and Table S2 in the supplementary material). At these sites, multiple individuals (5–7) were collected haphazardly in an area of ≥200 m2. Healthy apical tips (2–3 cm2) without epiphytes or epibionts were excised and preserved in sealed bags with silica gel until DNA extraction.
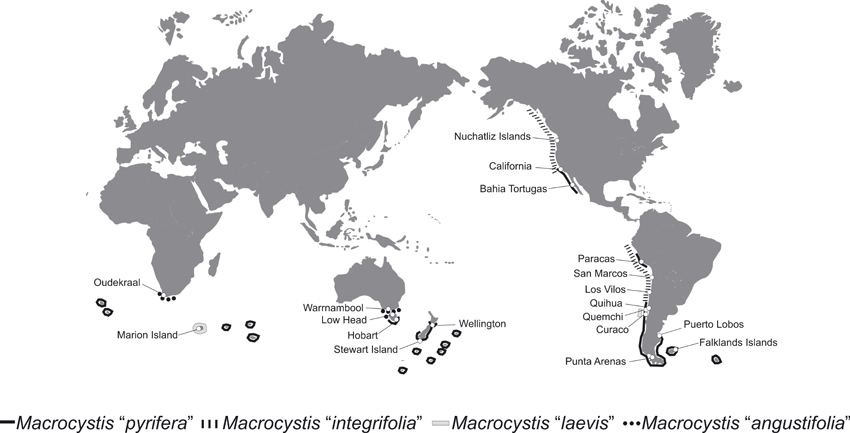
Map showing the worldwide distribution of Macrocystis ecomorphs and sites where the individuals were collected (also Table S2, see supplementary material).
Samples were assigned to each ecomorph according to morphological features, predominantly based on holdfast morphology (Fig. 1). In most of the collecting sites, one ecomorph was present, and no ambiguous morphologies were found. Samples with noncorrugated blades, conical holdfast, and sporophylls possessing pneumatocysts were assigned to M. “laevis” (Marion Island, type locality; Quihua and Curaco, southern Chile localities described by Aguilar-Rosas et al. 2003). Samples with a rhizomatous holdfast were assigned to M. “integrifolia,” while samples with mounding rhizomatous holdfast were assigned to M. “angustifolia.” Samples with a conical holdfast and corrugated blades were assigned to M. “pyrifera.” Sympatric samples of M. “pyrifera” and M. “integrifolia” were collected at Stillwater Cove, Carmel Bay, California. Individuals of M. “integrifolia” were collected intertidally, whereas individuals of M. “pyrifera” were collected subtidally, and the two populations were separated by 100 m.
DNA extraction and COI amplification. DNA was extracted following a modified cetyl trimethylammonium bromide (CTAB) method (Zuccarello and Lokhorst 2005). The COI region was amplified using the primers GAZF2 and GAZR2 (Lane et al. 2007), which amplifies an ∼610 bp fragment of the 5′-end of the COI gene.
The PCR amplifications were performed in a 30 μL reaction volume consisting of 1X buffer (New England Biolabs, Beverly, MA, USA), 2.5 mM dNTPs, 2.5 mM MgCl2, 0.025% BSA, 10 nM of each primer and 1 U Taq polymerase (New England Biolabs), plus 1.5 μL of template DNA. The PCR cycle had an initial denaturation step at 95°C for 5 min, followed by 5 cycles of 30 s at 95°C, 30 s at 60°C reduced by 1°C each cycle, and 45 s at 72°C, followed by 30 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 45 s with a final extension period of 10 min at 72°C. PCR products were cleaned using ExoSAP-IT (USB, Cleveland, OH, USA) and commercially sequenced (Macrogen Inc., Seoul, South Korea).
Data analysis. COI sequences were aligned using ClustalW in the BIOEDIT program (Hall 1999). Haplotype frequencies were calculated using the software DnaSP Version 4.0 (Rozas and Rozas 1995). Estimates of haplotypic (He) and nucleotide (π) diversity were calculated for each species group and for the entire data set using ARLEQUIN version 3.1 (Excoffier et al. 2005). Haplotype genealogies were reconstructed with a median-joining network by using NETWORK v 4.5 (Bandelt et al. 1999). Sequence divergences among species were calculated using MEGA 4.0 (Tamura et al. 2007).
Results
The COI sequences of the 118 analyzed individuals were as follows: 56 of M. “pyrifera,” 26 of M. “integrifolia,” 16 of M. “angustifolia,” and 20 of M. “laevis” (ecomorphs assigned according to morphological characteristics, see Materials and Methods section for more details). The 613 aligned bp showed 11 variable sites that yielded nine unique haplotypes (GenBank accession nos. HM153257 to HM153265) (Fig. 3). The ecomorph with the most haplotypes was M. “pyrifera” with seven; this ecomorph also displayed the highest haplotype diversity, He = 0.71558 (Table 1). All samples of M. “laevis” had the same haplotype (H1), despite the wide geographic range of collection for this ecomorph (southern Chile and Marion Island separated by ∼8,000 km). M. “integrifolia” displayed the highest nucleotide diversity value, π = 0.00320 (Table 1).
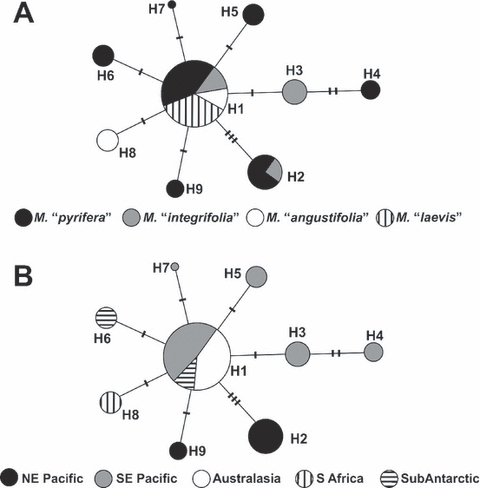
Haplotype network for cytochrome c oxidase subunit I (COI) of Macrocystis individuals, with circle size proportional to haplotype frequency. Lines connecting the haplotypes represent single bp mutations. Short cross lines represent undetected/hypothetical haplotypes. (A) Haplotypes are shaded according to the respective four ecomorphs. (B) Haplotypes are shaded according to the geographic origin (NE Pacific = Nuchatliz Islands, California, and Bahia Tortugas; SE Pacific = Paracas, San Marcos, Los Vilos, Quihua, Quemchi, Curaco, and Puerto Lobos; Australasia = Warnnambool, Low Head, Hobart, Wellington, and Stewart Island; S Africa = Oudekraal; SubAntarctic = Marion Island and Falkland Islands).
| N | H | He | π | |
|---|---|---|---|---|
| Macrocystis “integrifolia” | 26 | 3 | 0.66769 | 0.00320 |
| M. “pyrifera” | 56 | 7 | 0.71558 | 0.00232 |
| M. “laevis” | 16 | 1 | 0.00000 | 0.00000 |
| M. “angustifolia” | 20 | 2 | 0.50000 | 0.00082 |
- N, sample size; H, numbers of haplotypes; He, haplotype diversity; π, nucleotide diversity.
The hypothesized ancestral haplotype (H1) was found in all ecomorphs (Fig. 3A) and had a wide distribution, including samples from southern Australia; northern, central, and southern Chile; Marion Island; and Argentina (Fig. 3B). Some haplotypes were unique to a particular population (H4 = Paracas, Peru; H3 = San Marcos, northern Chile; H5 = Punta Arenas, southern Chile; H6 = Falklands Is.; H7 = Puerto Lobos, Argentina; H8 = Oudekraal, South Africa; H9 = Bahia Tortugas, Mexico) (Fig. 3B). Sequence divergence between haplotypes is low (Table 2), ranging from 0.00126 to 0.00348 as expected, since six of the eight haplotypes vary with respect to H1 by only one substitution. Samples of M. “pyrifera” and M. “integrifolia” collected in California had the same haplotype (H2), which was also shared with samples of M. “integrifolia” from Canada. Samples of M. “integrifolia” from the Northern Hemisphere (H2) and Southern Hemisphere (H1) differ by five substitutions (0.82% divergence), and interestingly, the divergence between M. “integrifolia” and M. “pyrifera” in both hemispheres was similar or lower.
| M. “integrifolia” | M. “pyrifera” | M. “laevis” | M. “angustifolia” | |
|---|---|---|---|---|
| M. “integrifolia” | – | |||
| M. “pyrifera” | 0.00348 | – | ||
| M. “laevis” | 0.00270 | 0.00126 | – | |
| M. “angustifolia” | 0.00332 | 0.00187 | 0.00187 | – |
Discussion
Our molecular data confirm previous suggestions (Hooker 1847, Skottsberg 1907, Graham et al. 2007, Demes et al. 2009) that only one species of Macrocystis, M. pyrifera, should be recognized. The low genetic variation detected in our worldwide collections and shared haplotypes between all ecomorphs suggest that all these ecomorphs share a very recent common ancestor. We also observed no genetic distinction between two sympatric populations of M. “pyrifera” and M. “integrifolia” from California.
Previous taxonomy is not concordant with molecular evidence. The main morphological features applied to discriminate species, holdfast and blade morphology, have been reported as phenotypically plastic under different environmental conditions, such as temperature (North 1971), wave-exposure (Brandt 1923, Druehl 1978), currents (Wheeler 1980, Kain 1982, Hurd et al. 1996), and depth (Clendenning 1964, van Tussenbroeck 1989a). Demes et al. (2009), based on observations in Chile and California, suggest that polymorphism in Macrocystis holdfast morphology is determined by the depth at which the sporophytes grow (fig. 1 in Demes et al. 2009). They also observed that the height of the basal stipe increases with depth. Furthermore, they carried out transplants of intertidal M. “integrifolia” to the subtidal (2.5 m), which resulted in a switch in holdfast morphology to that of M. “pyrifera.”
One of the main morphological features used to describe M. “laevis” is its noncorrugated blades. Smooth blades have also been described from the Falklands Islands (Skottsberg 1921, van Tussenbroeck 1989b) and from French Farm, South Island, New Zealand (Fig. 4). Our results show no genetic difference among samples of M. “laevis” collected from southern Chile and Marion Island and M. “pyrifera” from different sites in the Southern Hemisphere.

Macrocystis pyrifera samples collected at French Farm, South Island, New Zealand. (A) Smooth and corrugated blades, and (B) apical section of a smooth M. pyrifera individual. Scale bar = 2 cm.
Additional evidence to consider Macrocystis as monospecific comes from interfertility studies (Lewis et al. 1986, Lewis and Neushul 1994, 1995, Druehl et al. 2005, Westermeier et al. 2007). Laboratory crosses between ecomorphs have been reported in the Northern Hemisphere (Lewis et al. 1986) and Southern Hemisphere (Westermeier et al. 2007), although many of these studies did not check for hybrid viability in the field or over multiple generations. These data would strongly suggest that reproductive isolation and the biological species criterion do not apply to the previously named species of Macrocystis.
The divergence values in COI among species determined in this study (0.00–1.80%) are under the threshold generally used for the characterization of species in barcoding studies of macroalgae (Lane et al. 2007, Kucera and Saunders 2008, McDevit and Saunders 2009, Saunders 2009). Using COI, Fraser et al. (2009a) found divergence values between 3.0% and 3.8% in two forms of Durvillaea antarctica in New Zealand. Divergence values among Macrocystis ecomorphs are much lower than interspecific COI variation (2.2%–4.7%) detected within the brown algal genus Alaria (Lane et al. 2007). Previous molecular studies carried out in Macrocystis have also shown low genetic diversity but usually using restricted sampling (few samples and collection sites). Using chloroplast DNA, Yoon et al. (2001) reported low genetic divergence among three Macrocystis ecomorphs collected in California and Argentina, but they included few samples. Using the ITS1 and ITS2 regions, Coyer et al. (2001) were unable to differentiate M. pyrifera and M. integrifolia from the Northern Hemisphere, and samples from the Southern Hemisphere displayed very low sequence divergence. Our results, obtained from a wider sampling area and more samples, are consistent with these studies; these data also indicate more COI haplotypes in the Southern Hemisphere than in the Northern Hemisphere.
Possible explanations for the observed low genetic divergence are either a recent common ancestor and rapid dispersal and/or high levels of gene flow between populations. The Laminariales are thought to have arisen in the Northern Hemisphere (Estes and Steinberg 1988, Lüning and tom Dieck 1990, Vermeij 1992, 2001, Coyer et al. 2001), and specifically, Macrocystis is thought to have spread southward along the west Pacific coast (see Coyer et al. 2001 and references therein). Transtropical dispersal probably occurred during the cooler Pleistocene (∼20,000 years ago) when the tropics were compressed (Coyer et al. 2001), suggesting that this event was recent, although our data were not able to locate the putative ancestral haplotype in Northern Hemisphere populations.
High levels of gene flow are related to the high dispersal potential in some brown algae. Fraser et al. (2009b) revealed colonization of a single haplotype of the floating kelp D. antarctica into recently deglaciated areas around the Southern Ocean. Other studies have also produced evidence of reproductive viability of detached Macrocystis (Macaya et al. 2005, Hernández-Carmona et al. 2006). Muhlin et al. (2008) demonstrated the importance of detached thalli on genetic differentiation in Fucus vesiculosus. Connectivity through floating kelp along the Antarctic Circumpolar Current might explain the low divergence among samples from Chile, South Africa, Australia, Tasmania, and New Zealand in Macrocystis. Further analysis including an intensive sampling along the Southern Ocean and more variable markers might corroborate this hypothesis.
The revised taxonomy changes questions of Macrocystis distribution from interspecific dispersal and speciation to intraspecific ecological questions about the causes that trigger variation in Macrocystis morphologies. In addition, management and fisheries statistics as well as governmental policies have to be changed to M. pyrifera in countries were two or more ecomorphs are present (e.g., Chile, Australia, Peru).
DNA barcoding has been used to resolve taxonomic ambiguity in a variety of different organisms but has also stimulated intense debate about its reliability at the species level (Plaisance et al. 2009). It should not be used as the only taxonomic tool; more traditional approaches are also necessary. To date, the International Code of Botanical Nomenclature (ICBN) does not include information about the use of DNA barcoding; however, we believe that this study together with previous evidence supports (e.g., Demes et al. 2009) the recognition of a single Macrocystis species.
In conclusion, shared haplotypes between several distinct ecomorphs of Macrocystis (indicating a recent ancestor or high gene flow between species), low genetic variation among samples collected worldwide, the known plasticity of holdfast morphology (a main criterion for former species designation), and reproductive compatibility between all ecomorphs corroborate previous suggestions that the four ecomorphs must be considered as a single species. Henceforth, the only valid name should be M. pyrifera.
Acknowledgments
We are grateful to R. Anderson, O. Cerda, K. Demes, C. Fraser, M. Graham, I. Hinojosa, A. Mansilla, J. Mee, J. S. Moore, L. Orensanz, A. Perez-Matus, J. Pompert, R. Riosmena-Rodríguez, and M. Thiel for kindly providing algal material. We thank Martin Thiel and one anonymous reviewer for their comments on earlier versions of this manuscript. This research was partially funded by the Department of Conservation New Zealand and a New Zealand Study Abroad Scholarship and CONICYT-VUW PhD scholarship to E. C. M.