Rapid shape divergences between natural and introduced populations of a horned beetle partly mirror divergences between species
Abstract
SUMMARY Onthophagus taurus is a polyphenic beetle in which males express alternative major (horned) and minor (hornless) morphologies largely dependent on larval nutrition. O. taurus was originally limited to a Turanic–European–Mediterranean distribution, but became introduced to several exotic regions in the late 1960s. Using geometric morphometrics, we investigate the present-day morphological shape differentiation patterns among native (Italian) and introduced (Western Australian and Eastern US) populations. We then contrast these divergences to those observed between native O. taurus and its sympatric sister species O. illyricus. Our analysis failed to find significant divergences between O. taurus populations in external morphological traits (head, pronotum) when analyses were conducted separately for each sex. However, when sexes and male morphs were analyzed together, three important differences among populations emerged. First, relative warp analyses showed that native and introduced populations diverged in certain shape components that normally distinguish major and minor male morphs. Second, comparison of covariation of body regions (head vs. pronotum) in the three populations showed that populations diverged in the nature of this covariation, suggesting that different body regions are not totally constrained to evolve in concert. Lastly, and most importantly, the analysis of genitalic shape revealed little to no divergence of female genitalia, but unexpected substantial differentiation of male genitalia among the three O. taurus populations. This suggests that genitalic shape divergence can occur extremely rapidly even in the absence of sympatry and possible reinforcement, and that the genitalia of males and females may diverge independent of one another, at least during the early stage of interpopulational divergence. Interpopulation divergences in O. taurus mirrored aspects of interspecific divergences between O. taurus and O. illyricus in some cases but not others.
INTRODUCTION
Polyphenic development allows for the production of discrete morphological variants within populations in response to changes in environmental conditions, and represents an extreme yet common case of adaptive phenotypic plasticity (Nijhout 1999; Pigliucci 2001). Polyphenic development is thought to play an important role in speciation and in the evolution of morphological and behavioural novelties (West-Eberhard 2003). Specifically, polyphenic organisms provide opportunities for diversification unavailable in monomorphic species, such as evolutionary changes in developmental thresholds that separate alternative morphs, or the differential gain, loss, divergence, and recurrence of alternative phenotypes (West-Eberhard 2003; Moczek 2007). Here, we investigate morphological diversification in two polyphenic species of the beetle genus Onthophagus, a taxon that has recently emerged as a successful model system for integrating genetic, developmental, and ecological components of phenotypic diversification (reviewed in Emlen 2000; Moczek 2006a, b).
Male Onthophagus are commonly dimorphic with respect to horn expression, with large “major” males expressing disproportionately long horns whereas smaller, minor males remain hornless or express greatly reduced horns (Paulian 1935; Balthasar 1963; Simmons et al. 1999). In many species the scaling relationship between body size and male horn length is markedly sigmoidal, that is, male morphs are separated by a sharp body size threshold marked by dramatic changes in horn length occurring over a narrow body size range. Body size and horn length are determined largely by larval feeding conditions (quantity and quality of dung) and independent of paternal genotype, although males may differ heritably in other aspects of horn expression such as the threshold size separating major and minor morphs (Emlen 1994; Hunt and Simmons 1998; Moczek 1998; Emlen and Nijhout 1999, 2001; Moczek and Emlen 1999; Moczek et al. 2002). Alternative male morphs use their alternative morphologies in the context of alternative reproductive tactics. Major males use horns as weapons in male–male combat whereas minor males rely on nonaggressive sneaking tactics to secure mating opportunities (Cook 1987; Siva-Jothy 1987; Emlen 1997; Moczek and Emlen 2000). Recent studies have shown that populations that differ in degree and nature of competition for mating opportunities often also diverge heritably in the critical threshold body size that separates alternative male morphologies (Moczek and Nijhout 2003). Here, we quantify morphological divergences between native and recently established exotic populations of the polyphenic beetle Onthophagus taurus that are experiencing markedly different ecological conditions (Moczek 2003). We then contrast these interpopulational differences to those observed between native O. taurus and its sympatric sister species, O. illyricus.
O. taurus originally exhibited a typical Turanic–European–Mediterranean distribution (Balthasar 1963). However, in the late 1960s, O. taurus was introduced into Western Australia as part of a biocontrol program as well as into the Eastern United States due to an accidental release. Origin and dynamic of the Western Australian releases are well documented, but the exact origin of the Eastern United States release is unknown. Comparisons of extant exotic and native O. taurus populations to archival museum collections suggest that Eastern US and Western Australian populations have diverged dramatically in the critical size threshold that separates alternative male morphs in <50 years since introduction to a novel habitat (Moczek and Nijhout 2003). This divergence occurred in opposite directions relative to the ancestral, Mediterranean body size threshold, which despite the wide native distribution of this species turned out to be remarkably invariable. Threshold divergences are maintained across generations in common garden rearing and reflect evolved differences in the developmental machinery that governs male morph expression (Moczek et al. 2002). As a consequence, present-day US populations develop horns at much smaller body sizes compared with their European ancestors, whereas Western Australia populations switch to the horned morphs only at much larger body sizes. Moreover, kind and magnitude of these differences mirrored divergences between O. taurus and its sister species O. illyricus, suggesting that in a remarkably short period exotic O. taurus populations have been undergoing evolutionary modifications at least in part similar to those that characterize patterns of allometric differentiation between species. Here, we investigate the degree to which threshold sizes diverged in isolation from, or in concert with, other morphological traits, using geometric morphometrics, an analytical technique capable of detecting even very subtle divergences in shape. Specifically, we examined traits and body regions in immediate or close vicinity to horn expression (head, pronotum), which may be likely to be at least in part functionally integrated, as well as distant body regions that should be, at least at first sight, functionally decoupled from horns (genitalia) (Nijhout & Emlen, 1998). Secondly, we quantified morphological divergence in both sexes to determine the degree to which sexes and their parts can evolve independent of one another. Lastly, we contrast divergences between native and exotic O. taurus populations to divergences observed among sympatric populations of native O. taurus and O. illyricus. We use our results to explore the importance of developmental and ecological mechanisms in driving, as well as biasing, phenotypic diversification in natural populations.
METHODS
Study species
Geometric morphometric analysis was based on head, prothorax, right elytron, and genitalia of female and male O. taurus collected in La Mandria Natural Park, North-Western Italy (LM), near Narrikup, Western Australia (WA), and in Durham and Orange Counties, North Carolina, USA (NC), as well as O. illyricus collected in North-Western Italy (Table 1). Images of each anatomical structure were captured using a Leica DFC320 digital camera connected to a stereoscopic dissecting scope (Leica Z16Apo, Leica Microsystems AG, Wetzlar, Germany). In landmark-based morphometric analyses, the morphology of an object is represented by coordinates of sets of landmarks points (Bookstein 1991). Landmarks were chosen for their ease of identification, their homology in the two species, and for the ability to capture the general shape of each morphological structure. Landmarks were digitized using TpsDig 1.40 (Rohlf 2006a). To evaluate the confidence of the landmark configuration, a repeatability test was conducted by digitizing the landmarks 10 times on the same specimen at 10 different moments and then computing the ratio between the variance on the same specimen and the variance of the total sample (Variance=(Procrustes distances)2/N−1, where N is the number of objects considered in each set of measures). We accepted the landmark configuration only if the ratio was ≤0.05. Landmarks were positioned on the external anatomical structure as shown in Fig. 1. The landmarks for head (N=5), prothorax (N=4), and elytra (N=7) were digitized on half of each structure to remove the variability introduced by an eventual asymmetry. Genitalia were photographed on a thin film of glycerol to avoid deformation of parts due to compression under cover slides and landmarks were digitized as shown in 2, 3.
| Analysis | Trait | Onthophagus taurus | O. illyricus | TOT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Italy | North Carolina | Western Australia | Italy | |||||||||||
| F | M | m | F | M | m | F | M | m | F | M | m | |||
| RW analysis | Head | 40 | 15 | 15 | 20 | 21 | 9 | 16 | 15 | 15 | 20 | 15 | 15 | 120 |
| Prothorax | 40 | 15 | 15 | 20 | 21 | 9 | 16 | 15 | 15 | 20 | 15 | 15 | 120 | |
| Right elytron | 40 | 15 | 15 | 20 | 21 | 9 | 16 | 15 | 15 | 20 | 15 | 15 | 120 | |
| Aedeagus | — | 69 | — | 33 | — | 22 | — | 93 | 217 | |||||
| Vagina | 21 | — | — | 18 | — | — | 16 | — | — | 62 | — | — | 117 | |
| PLS analysis | Head versus Prothorax | 10 | 15 | 15 | 15 | 21 | 9 | 15 | 15 | 15 | — | — | — | 130 |
- F, females; M, major males; m, minor males.
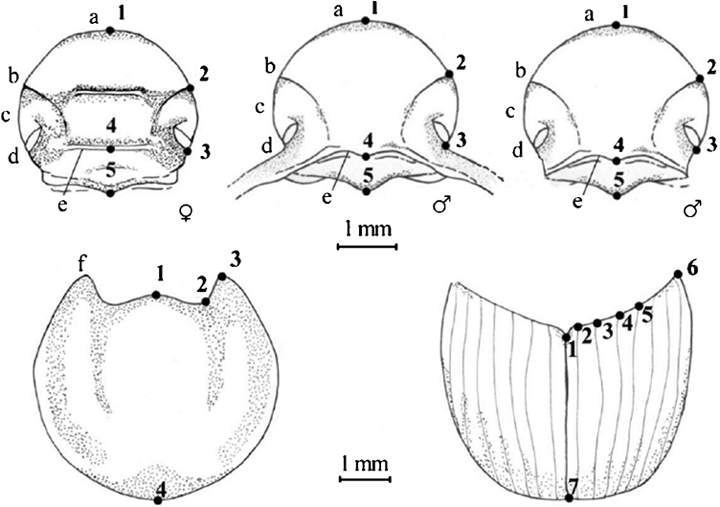
Drawings of head of both sexes, prothorax, and elytra of Onthophagus taurus. Photographs of pronota and right elytra were taken at × 12.5 magnification, and heads at × 32 magnification. The landmarks for head (N=5), prothorax (N=4), and right elytron (N=7) were digitized on half of each structure to remove the variability introduced by an eventual asymmetry. a, anterior margin of the clypeus; b, genal suture; c, gena; d, eye; e, vertex carina; and f, anterior angle of prothorax.
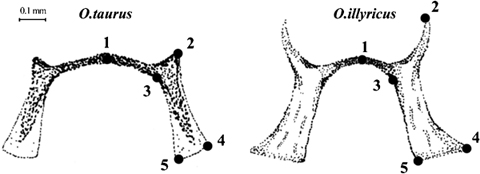
Female genitalia of Onthophagus taurus (left) and O. illyricus (right). Drawings show the sclerotized area of the vagina and the landmarks (N=5) used in geometric morphometric analysis.
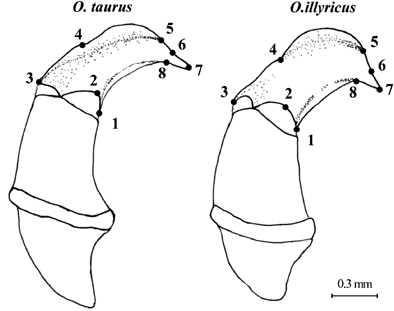
Male genitalia of Onthophagus taurus (left) and O. illyricus (right). Drawings illustrate the aedeagus and the position of the landmarks (N=8) on the left paramer used in geometric morphometric analysis.
Generalized procrustes analysis (GPA) and relative warp analysis
The landmarks of each specimen were optimally aligned using a GPA to remove the nonshape effects of translation, rotation, and scale (Rohlf 1990, 1999; Rohlf and Slice 1990). As long as variation in shape space is small, the data in tangent space are an almost perfect approximation of the data in shape space, and we tested this approximation with the program tpsSmall 1.20 (Rohlf 2003). We then used a Thin-Plate Spline (TPS) approach (Bookstein 1989, 1991), which translates in a mathematically rigorous way Thompson's (1917) idea of transformation grids (where one object is “warped” into another), and which generates shape variables that allow to map deformation in shape of an object onto another. Lastly, we described shape variations using GPA, multivariate descriptions of shape variables, Relative Warp Analysis (the principal component analysis of the partial warp scores generated by the TPS analysis to identify the major axis of shape variation) and visualization of transformation grids. All analyses were performed using TpsRelw 1.39 (Rohlf 2006b). Each type of analysis, and for each structure under consideration, was initially conducted separately for females, and minor and major male morphs of the three populations. This was followed by a second round of analyses that combined both male morphs within populations, and finally a third round which combined morphs and sexes within populations. Morphospatial representation of classes (populations, species, sexes, and morphs) on an axis system was conducted using NTSYSpc 2.20 (Rohlf 2005b) and SPSS 14.0 software (SPSS Inc. 2005). Here, we used Discriminant Analysis on relative warp scores (in keeping with Fulford and Rutherford 2000; Janz̆ekovic and Kryštufek 2004; Kryštufek and Janz̆ekovic 2005; Pizzo et al. 2006a) to obtain a classification matrix based on shape variation. The percentages of correct classifications were used to evaluate the inter- and intraspecific (among populations) discriminatory power of each anatomical structure.
Partial least-square (PLS) analysis
PLS methods are extensively used in the social sciences, ecology, and clinical studies, but are rarely used in biological research despite their potentially wide range of applications (Streissguth et al. 1993; McIntosh et al. 1996; Rohlf and Corti 2000; Klingenberg et al. 2001; Abdi 2003; Roggero 2004; Zelditch et al. 2004). Their use differs from regression in that variables are treated equally: the PLS approach does not assume that one is the cause of the variation in the other. In Geometric Morphometrics, PLS Analysis is used to describe covariation between two shapes, or between a set of variables and shapes, recorded for the same individuals (Rohlf 2005a). Based on two different sets of shape variables, this method permits the construction of pairs of shape vectors that can capture all possible covariances, and allows identification of whatever pattern of covariation may exist between different body parts. Scatterplots can visualize the correlation between the two sets of shape vectors, with each point corresponding to an individual. When most of the covariation appears to be concentrated into just a few dimensions, permutation tests can be used to determine whether observed correlations between any pairs of dimensions are larger than what can be expected by chance. As pointed out by Rohlf (2005a), only if the paired vectors are highly correlated and account for an appreciable proportion of the covariation, a biological meaning may be assumed. Here, we used tpsPLS 1.17 (Rohlf 2005a) to test for covariation between head and prothorax.
RESULTS
Values of the two first relative warp scores obtained from Relative Warp Analysis of each anatomical structure were plotted on an axis system to visualize the position of each individual in morphospace, and to assess eventual differences among populations, sexes, and morpho-types. As a general rule, within each sex (with no distinction between morphs), the simple Relative Warp Analysis of all external morphological structures considered did not reveal any marked phenotypic differentiation, both between native and introduced O. taurus populations or between O. taurus and O. illyricus. As an example, the relative warp plot resulting from the Relative Warp Analysis of male head is shown in Fig. 4A. Discriminant Analysis performed on the relative warp scores produced consistently low percentages of correct classifications (Table 2).
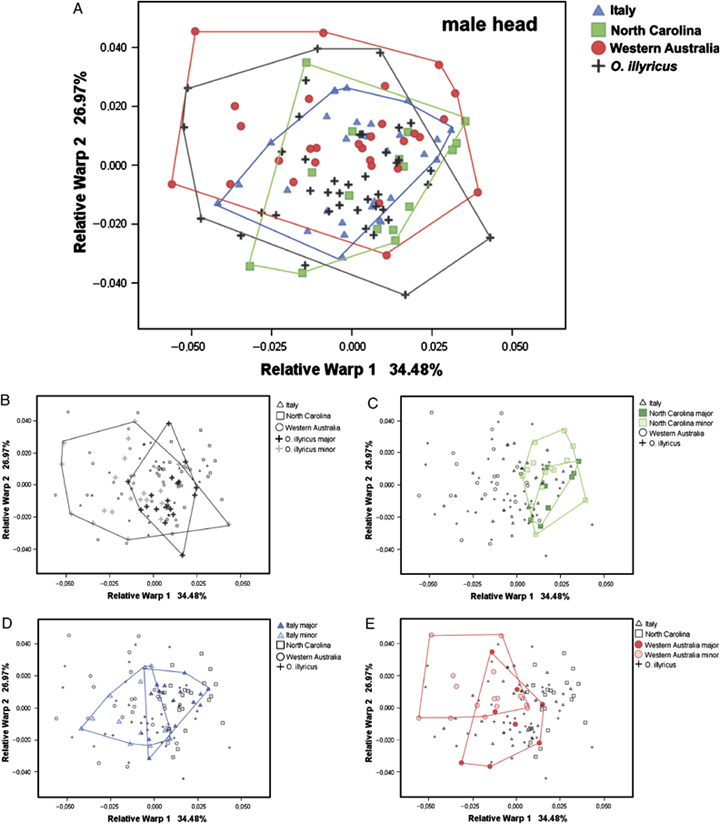
Scatterplot of the two first relative warp scores obtained from the Relative Warp Analysis of male head shape of male Onthophagus illyricus and three populations of male O. taurus. Plot (A) is presented as an example of the relative warp plots resulting from the shape analysis of external morphological structures when morphs are not considered separately, which does not permit any interpopulational discrimination. Plots (B–E) show the same relationships; however, this time male morphs are considered separately (B: O. illyricus; C: O. taurus (Italy); D: O. taurus (North Carolina); E: O. taurus (Western Australia).
| Trait | Species/populations | Onthophagus illyricus (Italy) | O. taurus (Italy) | O. taurus (North Carolina) | O. taurus (Western Australia) |
|---|---|---|---|---|---|
| Male head | O. illyricus (Italy) | 25 | 17.5 | 37.5 | 20 |
| O. taurus (Italy) | 12.9 | 67.7 | 12.9 | 6.5 | |
| O. taurus (North Carolina) | 25 | 25 | 25 | 25 | |
| O. taurus (Western Australia) | 6.9 | 10.3 | 17.2 | 65.5 |
- Percentages of correct classifications are highlighted in bold.
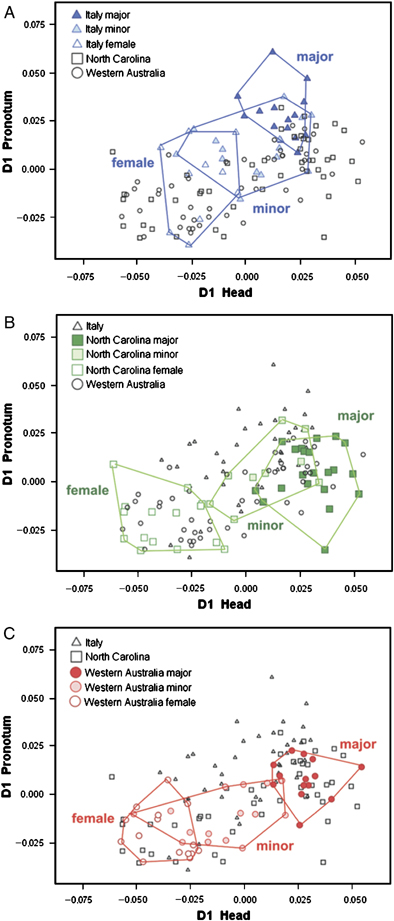
Male morph-specific differences diverge across populations
Even though sexes could not be distinguished across populations or species, several interesting differences among populations emerged once the relative positions of minor and major males were considered (Fig. 4, B–E). The first relative warp (which allowed a partial discrimination between major and minor morpho-types) explained 34.48% of the total amount of the sample variance, whereas the second relative warp explained 26.97%. Plots showed that the morpho-spatial relationships between the two morphs were different in the three populations. The two areas occupied by North Carolina minor and major males greatly overlapped, whereas minor and major males from Western Australia were more differentiated. The relative minor–major position pattern of O. illyricus (Fig. 4B) (majors mostly clustered within minors) resembled the pattern observed in the North Carolinian sample (Fig. 4D), whereas the relative minor–major position pattern of Italian O. taurus (Fig. 4C) (minors and majors rather differentiated) resembled the pattern observed in the Western Australian sample (Fig. 4E).
Nature and degree of covariation between body regions can diverge across populations
PLS Analyses require that samples are homogeneous and, therefore, they were conducted on individuals of O. taurus only. Covariation between O. taurus head shape and prothorax shape revealed that shape changes in the head were related to shape change in the prothorax (and vice-versa). The percent covariation value for the first dimension was very high (93.4%) and permutation tests (0.1% was the percent of correlation observed) confirmed that the covariation concentrated in the first dimension was significant and not produced by chance.
In the scatterplot of the first pair of shape vectors we distinguished females, minor, and major males of each population, and our results suggest that these three phenotype classes can be separated according to a particular head-prothorax shape covariation (Fig. 5). In each plot, minor males were always placed between females and major males, suggesting a gradual shape variation from females to majors, passing from minors with feminine facies (without horns) to minors that largely resembled majors (with reduced horns). However, our data also suggest that the three populations have diverged in the relative morphospatial positions of females, minor and major males. In the “ancestral” European population, minor males were situated exactly in the middle between females and majors (Fig. 5A); minor and major males from North Carolina appeared poorly differentiated, but well separated from females (Fig. 5B), whereas a marked overlap between females and minors, and a fable superimposition with major males, was apparent for the Western Australian population (Fig. 5C). Combined, these results suggest that degree and nature of covariation between body regions can diverge in a relatively short amount of time between natural, geographically isolated populations, and by inference, that different body regions are not totally constrained to always evolve in concert.
Relative position of female, minor males and major males in the scatterplot of the first dimension of the head and the prothorax obtained from the shape (head) versus shape (prothorax) Partial Least Square (PLS) Analysis. The same plot is shown three times to describe the relative morphospatial positions of Onthophagus taurus from (A) Italy, (B) North Carolina, and (C) Western Australia.
Genitalia diverge rapidly in males but not females across populations
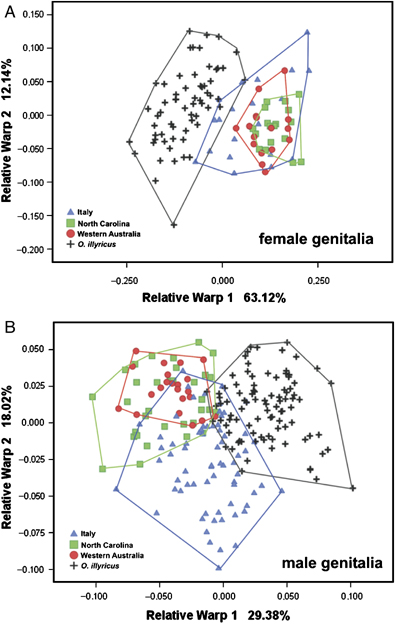
Relative Warp Analysis of vagina shape (Fig. 6A) showed a marked differentiation between Italian O. taurus and O. illyricus along the first relative warp axis (63.1% of variance explained). In contrast, vagina shape of O. taurus populations from North Carolina and Western Australia could not be distinguished from that of their Mediterranean counterparts and instead was less variable and much more concentrated in morpho-space. This was further supported by discriminant analysis conducted on the relative warp scores of vagina shape. Substantial divergences between species allowed for correct classification of 98.4% of O. illyricus individuals, whereas much poorer divergences among O. taurus populations permitted only a relatively moderate proportion of correct classifications (percentages of correct classification were 71.4, 72.2, and 43.8 for Italy, North Carolina, and Western Australia, respectively; Table 3).
Scatterplot of the two first relative warps obtained from the Relative Warp Analysis of female (A) and male (B) genitalia shape.
| Trait | Species/populations | Onthophagus illyricus (Italy) | O. taurus (Italy) | O. taurus (North Carolina) | O. taurus (Western Australia) |
|---|---|---|---|---|---|
| Vagina | O. illyricus (Italy) | 98.4 | 1.6 | 0 | 0 |
| O. taurus (Italy) | 0 | 71.4 | 9.5 | 19 | |
| O. taurus (North Carolina) | 0 | 0 | 72.2 | 27.8 | |
| O. taurus (Western Australia) | 0 | 25 | 31.3 | 43.8 | |
| Aedeagus | O. illyricus (Italy) | 97.8 | 0 | 1.1 | 1.1 |
| O. taurus (Italy) | 3 | 72.7 | 21.2 | 3 | |
| O. taurus (North Carolina) | 0 | 18.2 | 81.8 | 0 | |
| O. taurus (Western Australia) | 4.3 | 7.2 | 4.3 | 84.1 |
- Percentages of correct classifications highlighted in bold.
However, these results were in marked contrast to those obtained from male genitalia. Relative warp scores of the aedeagus not only clearly distinguished O. illyricus from O. taurus from any of the three populations but also separated native from both exotic O. taurus populations (Fig. 6B). Interestingly, Relative Warp Analysis separated aedeagus shape only along the second (18%) but not the first (29.4%) warp axis, whereas interspecific differences were manifest along both. Again, discriminant analysis conducted on relative warp scores of the aedeagus substantiated this picture of divergence, and permitted high percentages of correct classifications both between species and between O. taurus populations (O. illyricus: 97.8%; O. taurus: Italy 72.7%, North Carolina 81.8%, and Western Australia 84.1%, Table 3). These results suggest that male genitalic shape can diverge rapidly between allopatric populations in a manner similar to divergences observed between species but without requiring sympatry, reinforcement, and obvious parallel changes in female genitalic shape.
DISCUSSION
The diversification of morphological shape is a classic topic in evolutionary biology, and a diverse literature explores the relative contributions of genetic architecture, developmental processes, and behavioral and ecological mechanisms in the genesis of morphological diversity (e.g., Thompson 1942; Meinhardt 1995; Raff 1996). Recent work specifically emphasizes polyphenic organisms, that is, organisms capable of expressing two or more discretely different phenotypes in response to change in environmental conditions, as especially powerful agents of morphological diversification. By virtue of their developmental plasticity, polyphenic organisms more than others may be prepositioned to undergo rapid diversification both within and between species, with the potential to mediate the origin of major novel traits (Schlichting and Pigliucci 1998; West-Eberhard 2003). Here, we investigate the micro- and early macroevolution of shape in two polyphenic beetle species. Specifically, we investigate shape evolution between native and two very recently founded exotic populations of O. taurus on one side, and O. taurus and its sympatric sister species O. illyricus on the other. Using a geometric morphometric approach to study head, prothorax, and genital shape of females and male morphs our analyses reveal a complex, and partly parallel pattern of shape differentiation within and between species, and we discuss our most significant findings below.
When male morphs were not considered separately our analyses failed to detect significant intra- and interspecific differences, the latter of which was consistent with the results of earlier studies (Pizzo et al. 2006a, b). However, once the morpho-spatial position of minor and major male morphs were examined separately, three important intraspecific differences emerged. First, the morpho-spatial relationships between both male morphs were different in all three O. taurus populations, with the differentiation pattern of one of the exotic populations (Western Australia) resembling the pattern found among native O. taurus, whereas the differentiation pattern of the other exotic population (North Carolina) resembled the pattern found among native O. illyricus. These findings suggest that the shape differences that distinguish morphs within populations can change across populations, including changes that resemble those normally found among species.
Second, a partly similar result was obtained with respect to the covariation between O. taurus head shape and prothorax shape. This analysis showed that shape changes in the head were correlated with shape change in the prothorax, which was not surprising and in fact matches the results of an earlier study (Pizzo et al. 2006a). What was surprising, however, was that in each population, females, minor and major phenotypes could be characterized and classified based on a unique covariation between head- and prothorax shape. Combined, these results suggest that degree and nature of covariation between head and prothorax are surprisingly labile and can diverge in a relatively short amount of time between natural, geographically isolated populations. By inference, these results also underscore the modular character of different insect body regions, permitting them to diverge, at least partly, independent of one another.
Lastly, our study revealed a substantial, and completely unexpected, divergence in the shape of male genitalia across the three O. taurus populations. Importantly, this divergence only occurred along one of the two major warp axes that separate male genitalic shapes between species (Fig. 6B), and it was not paralleled by obvious corresponding divergences in female genitalic shape across populations even though female genitalic shape clearly distinguished O. taurus from its sister species O. illyricus (Fig. 6A). Averaged over all three populations, the population of origin of over 75% of all males could be determined based solely on genitalic shape. This result was entirely unexpected because O. taurus and O. illyricus still exhibit partial overlap in the morpho-spatial position of male genitalia, despite having become reproductively isolated approximately 3–4 MYA (Pizzo et al. 2006a), whereas exotic O. taurus populations were founded <50 years ago.
Our results suggest that at least some aspects of male genitalic shape can diverge extraordinarily rapidly between allopatric populations in a manner similar to at least some of the divergences observed between species. Moreover, our results suggest that rapid genitalic divergence can evolve in the absence of sympatry, reinforcement, and selection for assortative mating, which combined have generally been assumed to drive rapid genitalic divergence in nature (Kawano 2002, 2003, 2004; Eberhard 2004a, b; Eberhard and Ramirez 2004). The latter observation therefore excludes the most common traditional explanation for early genitalic divergence, and begs the question what forces could have driven the unusual degree of genital divergence observed between allopatric O. taurus populations.
One possible alternative explanation could be sexual selection acting directly on male genitalia and selecting for different genitalic shapes in different populations due to differences in female preferences. However, the function of male and female genitalia is widely believed to be that of lock and key, and evolutionary changes in females preferences for male genital shape should be reflected at least in part in corresponding changes in female genital shape, which our analysis failed to detect. Alternatively, male genitalia could have diverged as a by-product of natural or sexual selection operating on a different, but developmentally correlated, trait. Interestingly, earlier studies have shown that the O. taurus populations studied here have diverged substantially, and heritably, in the critical body size above which males express the horned, fighter morph (Moczek and Nijhout 2003), and that between-population divergences in horn expression appear to have been driven by population-wide differences in the intensity of competition for mating opportunities (Moczek 2003). At the same time, a growing series of developmental studies suggest that evolutionary changes in horn expression have the capacity to bring about correlated changes in other traits (wings, antennae, mouthparts: Emlen 2001; copulatory organs: Moczek and Nijhout 2004; testes: Simmons and Emlen, 2006; flight muscles: A. Gibbs and A. Moczek, personal observation). Such correlated changes may be caused by resource allocation trade-offs during development, which enforce compensatory changes in trait expression among traits that share the same and limited pool of resources to support their growth. This raises the intriguing possibility that genitalic shape divergence between allopatric O. taurus populations documented here may be the by-product of selection operating on secondary sexual traits. Studies are now under way to explore if and how the development of secondary and primary sexual traits may be linked, if this linkage has the capacity to bias patterns of genitalic shape divergence across taxa, as well as the functional consequences of the kind of genitalic divergence documented here.
Acknowledgments
We wish to thank F. J. Rohlf and A. Cardini for their useful suggestion on application of PLS Analysis. The research was supported by funds from Turin University. A. P. was funded through a grant from the Dipartimento di Biologia Animale e dell'Uomo of Turin, and APM through National Science Foundation grants IOS 0445661 and IOS 0718522.




