Cost-Effectiveness of Screening and Optimal Management for Diabetes, Hypertension, and Chronic Kidney Disease: A Modeled Analysis
ABSTRACT
Objectives: Chronic kidney disease is, increasingly, both a contributor to premature deaths and a financial burden to the health system, and is estimated to affect between 10% and 15% of the adult population in Western countries. Hypertension and, in particular diabetes, are significant contributors to the global burden of chronic kidney disease. Although it might increase costs, screening for, and improved management of, persons at increased risk of progressive kidney disease could improve health outcomes. We therefore sought to estimate the costs and health outcomes of alternative strategies to prevent end-stage kidney disease, compared with usual care.
Methods: A Markov model comparing: 1) intensive management versus usual care for patients with suboptimally managed diabetes and hypertension; and 2) screening for and intensive treatment of diabetes, hypertension, and proteinuria versus usual care was developed. Intervention effectiveness was based on published meta-analyses and randomized controlled trial data; costs were measured from a central health-care funder perspective in 2008 Australian dollars ($A), and outcomes were reported in quality-adjusted life-years (QALYs).
Results: Intensive treatment of inadequately controlled diabetes was both less costly (an average lifetime saving of $A133) and more effective (with an additional 0.075 QALYs per patients) than conventional management. Intensive management of hypertension had an incremental cost-effectiveness ratio (ICER) $A2588 per QALY gained. Treating all known diabetics with angiotensin-converting enzyme (ACE) inhibitors was both less costly (an average lifetime saving of $A825 per patient) and more effective than current treatment (resulting in 0.124 additional QALYs per patient). Primary care screening for 50- to 69-year-olds plus intensive treatment of diabetes had an ICER of $A13,781 per QALY gained. Primary care screening for hypertension (between ages 50 and 69 years) plus intensive blood pressure management had an ICER of $A491 per QALY gained. Primary care screening for proteinuria (between ages 50 and 69 years) combined with prescription of an ACE inhibitor for all persons showing proteinuria and all known diabetics had an ICER of $A4793 per QALY gained.
Conclusions: Strategies combining primary care screening of 50- to 69-year-olds for proteinuria, diabetes, and hypertension followed by the routine use of ACE inhibitors, and optimal treatment of diabetes and hypertension, respectively, have the potential to reduce death and end-stage kidney disease and are likely to represent good value for money.
Introduction
Chronic kidney disease (CKD) is a significant global health concern [1] affecting approximately 10% to 15% of the adult populations of many Western countries such as Australia and the United States, and up to 20% of the adult Japanese population [2–4]. Health consequences are substantial, with sufferers at increased risk of cardiovascular disease and early death [5,6]. Some will progress to end-stage kidney disease (ESKD) requiring dialysis or transplantation [7]. The number of patients dependent on dialysis for survival exceeds 1.4 million globally and is increasing by 8% per annum [1]. The cost of dialysis in the United States alone is projected to exceed $US29 billion per annum by 2010 [5]. Given the relative proportions of CKD and ESKD, the cost of predialysis CKD is estimated to double that of dialysis [8].
Prevention, early detection, and intervention may prevent onset of CKD and reduce the likelihood of CKD progression. In most countries, diabetes is the primary cause of CKD and ESKD [9] with 20% to 40% of patients with type 1 or type 2 diabetes developing nephropathy [10]. Hypertension is strongly associated with CKD [11]. The onset and progression of CKD, as well as cardiovascular consequences might be reduced through intensive control of glycemia and hypertension [12–14].Where CKD occurs with proteinuria, angiotensin-converting enzyme (ACE) inhibitors or angiotensin II-receptor blockers (ARB) further reduce CKD progression, cardiovascular morbidity, and mortality [15–17].
International trial evidence suggests that early detection and management of CKD and risk factors such as diabetes, hypertension, and proteinuria may be cost-effective [18–24]. Despite this, substantial proportions of the Australian population have inadequately controlled diabetes and hypertension [25], with few Australian analyses that examine the cost-effectiveness of early detection and management of CKD risk factors [23].
The international nephrology community is increasingly advocating a comprehensive approach to the prevention of CKD, avoiding or delaying the onset of CKD in at-risk individuals, and intervening to prevent progression [1,26]. Nevertheless, this call to action lacks supporting cost-effectiveness data. Existing studies of isolated interventions have not considered the costs and health outcome implications of a comprehensive approach to prevention, early detection, and management, encompassing primary care screening coupled with improved pharmacotherapy. We assessed, from the perspective of a health-care funder, the health outcomes (measured in terms of quality-adjusted life-years [QALYs]) and incremental costs of intensive management of patients known to have diabetes and hypertension, with and without early detection of new patients at risk for CKD, compared with current practice.
Methods
We developed Markov models to simulate the annual progression of patients from the development of risk factors (specifically diabetes, hypertension, and proteinuria) through CKD, to ESKD and death (Fig. 1). We used these models to calculate the costs and health outcomes of CKD management strategies comprising of: (1) improved management of patients with diabetes and hypertension; and (2) primary care–based population screening of persons aged 25 or older for early detection of hypertension, diabetes (with or without microalbuminuria), and proteinuria (Table 1). Lacking evidence on which to base an estimate of the magnitude of the combined effectiveness of optimal control of diabetes, hypertension, and proteinuria, we modeled these interventions separately. Health outcomes were measured in terms of QALYs, and costs were measured in Australian dollars ($A) from a health funder perspective; all future costs and health outcomes were discounted at 5% per annum [27]. All rates were converted to annual probabilities. Additional details are available in reference [28] and as Supporting information at: http://www.ispor.org/Publications/value/ViHsupplementary/ViH13i2_Howard.asp
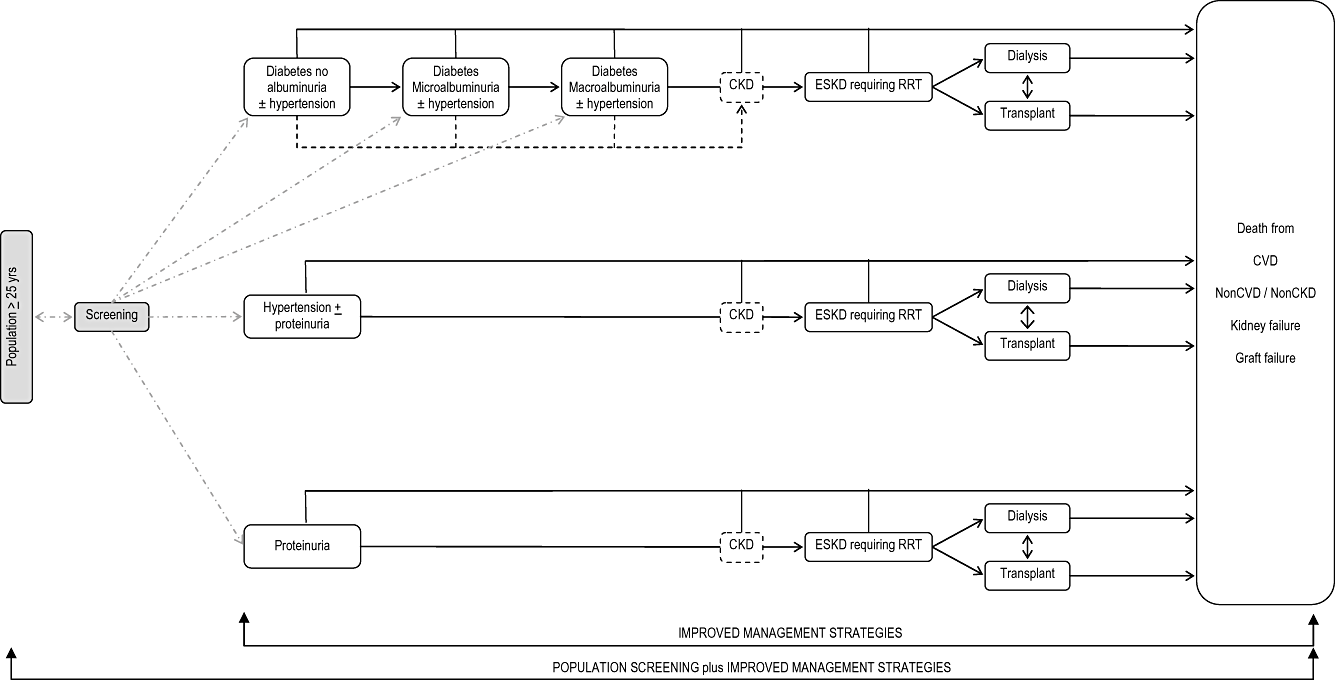
Model Schema: Screening for CKD risk factors (diabetes, hypertension, or proteinuria) PLUS better treatment of existing patients. CKD, chronic kidney disease; ESKD, end-stage kidney disease; RRT, renal replacement therapy.
| Patients | Intervention | Comparator |
|---|---|---|
| Improved management in known/existing patients | ||
| 1 Diabetics (±hypertension) | Intensive glucose control | Routine glucose control |
| 2 Diabetics (±hypertension) | Addition of ACEi | Current practice |
| 3 Hypertensive patients (±diabetes) | Intensive BP control | Routine BP control |
| Primary care–based screening for CKD risk factors | ||
| 4 Diabetes | Fasting BSL (with OGTT if screen-positive) + intensive glycemic control of new and known but uncontrolled patients | Current practice |
| 5 Hypertension | BP measurement in GP practice + intensive BP control of new and known but uncontrolled patients | Current practice |
| 6 Proteinuria | Protein detection with urine dipstick (with protein : creatinine ratio if screen-positive) + treatment with ACEi for new and known patients + ACEi for all diabetics | Current practice |
- ACEi, angiotensin-converting enzyme inhibitor; BP, blood pressure; BSL, blood sugar level; OGTT, oral glucose tolerance tests.
Model Structure and Assumptions
Structure. Markov models were constructed for each of the treatment and screening strategies in Table 1. Models incorporate uncertainty using Monte Carlo simulation and probabilistic sensitivity analysis, with a cycle length of 1 year; patients were followed over a lifetime (until death or age 95 years). Patients progress through a series of annual, age-specific transition probabilities, over their remaining lifetime, to determine whether they die, have a nonfatal cardiac event, stay in the current health state, or progress to ESKD. Patients with diabetes (with or without hypertension) progress through stages of diabetes with varying levels of nephropathy (diabetes with no albuminuria, to diabetes with microalbuminuria, to diabetes with macroalbuminuria), and eventually to ESKD requiring renal replacement therapy (RRT). In all diabetes health states, each year, patients have a chance of experiencing a nonfatal cardiac event, of dying from cardiovascular disease, dying from other causes, having progression of their nephropathy, or remaining in the same health state. For patients with diabetes and no albuminuria, progression of nephropathy meant the development of microalbuminuria; for patients with microalbuminuria, progression meant the development of macroalbuminuria, and for patients with existing macroalbuminuria, progression meant development of ESKD requiring RRT. For hypertension and proteinuria states, patients have an annual probability of experiencing a nonfatal cardiovascular event, of dying from cardiovascular disease, dying from noncardiovascular causes, remaining in the same health state, or progressing to ESKD requiring RRT. Figure 1 indicates CKD in a dotted box, indicating that it is not modeled explicitly as a health state in its own right, because it is generally asymptomatic, but rather to acknowledge that it is the clinical precursor to developing ESKD requiring RRT. Once patients reach ESKD requiring RRT, they receive dialysis or transplant. Patients on dialysis may receive a subsequent transplant, can experience a nonfatal cardiovascular event, die of cardiovascular or noncardiovascular causes, remain on dialysis, or can elect to discontinue active RRT, and instead receive conservative management until they die. Patients with a functioning transplant may return to dialysis after a graft failure, receive another transplant after graft failure, can experience a nonfatal cardiovascular event, die of cardiovascular or noncardiovascular causes, or remain alive with a functioning transplant.
For screening interventions, patients start in a “population” (undiagnosed) state from which they might be found to have the risk factor (by screening or clinical diagnosis); after diagnosis patients progress through the health states above. Patients can, in any cycle, experience a nonfatal cardiovascular event, or die of cardiovascular or noncardiovascular related causes.
Assumptions. For all interventions, the decision tree represented a choice between the intervention and current management. The AusDiab study was used to inform the modeled populations. AusDiab is an Australian population-representative cohort study that identifies, through oral glucose tolerance tests cases of diagnosed and undiagnosed type 2 diabetes, and elicits other risk factors for CKD [2,25,29,30]. The AusDiab study was also used to estimate: 1) the age-specific population prevalence of risk factors; 2) the prevalence of comorbidities (e.g., the number of people with diabetes and hypertension); 3) numbers of known (preexisting disease before AusDiab study entry) and new patients (disease diagnosed at AusDiab study entry); and 4) the proportion of patients with controlled and uncontrolled disease [25,30]. In AusDiab, disease control thresholds were defined as HbA1c of 7.0 or no for diabetes and systolic blood pressure of 140 mmHg or no, or diastolic pressure of 90 mmHg or no for hypertension. Patients with uncontrolled diabetes were at an increased risk of events, dependent on HbA1c levels [31,32]. Additional details on data sources are provided in the Supporting information and in reference [28].
Treatment. The age and risk factor profile of the modeled population for intensive treatment strategies was representative of the Australian treatment population aged 25 years or older, based on the AusDiab study. For treatment interventions, patients in the comparator arm were assumed to be managed according to current Australian practice. The proportion of patients who are “uncontrolled” on conventional management for each risk factor is based on AusDiab data. In the intervention arm, the benefits (in terms of reduction of events) are applied to only the patients who have uncontrolled risk factors. This assumes that those patients with controlled disease will gain no additional benefit from the intervention, in terms of avoidance of events. This assumption means that the estimation of benefit from more intensive intervention is likely to be a relatively conservative estimate of the population benefit.
Screening. For screening strategies, age and sex distributions of the entire Australian population aged 25 years or older formed the basis for the modeled population [33]. The proportion of the population with undiagnosed risk factors was based on AusDiab data (as above). For the screening interventions, it was assumed that a proportion of patients in the comparator arm would continue to receive clinical diagnoses; in the screening arm a patient could receive a diagnosis by screening, or by clinical diagnosis. As with treatment interventions, the benefits are applied to only the patients who have uncontrolled risk factors in the intervention arm. Discussion of the estimation of event rates in various patient populations is provided as Supporting information at: http://www.ispor.org/Publications/value/ViHsupplementary/ViH13i2_Howard.asp
All event rates, costs, and outcomes for patients requiring RRT are based on the actual treatment and outcome probabilities observed in the Australian national cohort of ESKD patients commencing treatment during 1996 to 2000 [11].
Strategies Modeled
Improved management of known patients with CKD risk factor (“treatment”). Three strategies for improving the management of these patients were modeled: 1) intensive glycemic control (compared with routine control); 2) the prescription of an ACE inhibitor for all diabetic patients (regardless of glycemic control or hypertension status); and 3) intensive blood pressure control (compared with routine blood pressure control). The effectiveness of interventions was based on data from meta-analyses or randomized controlled trials (Table 2) and was applied to the proportion of the population in the intervention arm whose disease remained uncontrolled with conventional management. Regimens are shown in Table 4.
| RR (95% CI) | Source data effectiveness estimate [supplementary data sources] | Source data underlying model of disease progression | |
|---|---|---|---|
| Diabetes | Diabetes | ||
| Intensive glycemic control | [31,55–61] | ||
| CVD death | 0.88 (0.74–1.04) | [14][[13,50]] | |
| CVD events | 0.98 (0.78–1.23) | [14][[13,50]] | |
| Progression from no albuminuria to microalbuminuria | 0.91 (0.85–0.98) | [14][[13,50]] | |
| Progression to microalbuminuria and to macroalbuminuria | 0.70 (0.57–0.85) | [14][[13,50]] | |
| Progression to macroalbuminuria and to ESKD | 0.64 (0.38–1.08) | [14][[13,50]] | |
| Intensive hypertension control | |||
| CVD death | 0.67 (0.40–1.12) | [62][[63,64]] | |
| CVD events | 0.75 (0.61–0.94) | [62][[63,64]] | |
| Progression from no albuminuria to microalbuminuria | 0.71 (0.51–0.99) | [12][[65]] | |
| Progression to microalbuminuria and to macroalbuminuria | 0.61 (0.31–1.21) | [12][[65]] | |
| Progression to macroalbuminuria and to ESKD | 0.61 (0.31–1.21) | [12] (assume as for micro to macro) [[65]] | |
| ACEi for all diabetics* | |||
| CVD death | 0.67 (0.40–1.12) | [62] (assume same as intensive vs. conventional) [[63,64]] | |
| CVD events | 0.59 (0.38–0.91) | [66] (assume same relative reduction for nonfatal events) [[63,64]] | |
| Progression from no albuminuria to microalbuminuria (no hypertension) | 0.72 (0.49–1.06) | [66][[65]] | |
| Progression from no albuminuria to microalbuminuria (with hypertension) | 0.52 (0.31–0.88) | [66][[65]] | |
| Progression from microalbuminuria to macroalbuminuria (all, regardless of hypertension) | 0.45 (0.28–0.71) | [16][[65]] | |
| Progression from macroalbuminuria to ESKD (all, regardless of hypertension) | 0.64 (0.40–1.03) | [16][[65]] | |
| Hypertension | Hypertension | ||
| Intensive hypertension control (no proteinuria) | [57,61,67–69] | ||
| CVD death | 0.93 (0.77–1.0) | [70][[71]] | |
| CVD events | 0.95 (0.76–0.95) | [70][[71]] | |
| Progression to ESKD | 0.69 (0.51–0.94) | [15] (assume same as ACEi vs. no ACEi in patients with proteinuria) | |
| ACEi vs. no ACEi (with or without protein) (used for intensive control in patients with proteinuria) | |||
| CVD death | 0.80 (0.71–0.80) | [70][[63,64]] | |
| CVD events | 0.80 (0.73–0.88) | [70][[63,64]] | |
| Progression to ESKD | 0.69 (0.51–0.94) | [15] | |
| Proteinuria, no diabetes, no hypertension | Proteinuria | ||
| ACEi vs. no ACEi | [58,67,68,72,73] | ||
| CVD death | 0.80 (0.71–0.80) | [62,70] assume RR is same as for hypertension no protein | |
| CVD events | 0.80 (0.73–0.88) | [62,70] (assume RR is same as for hypertension no protein) | |
| Progression to ESKD | 0.66 (0.51–0.85) | [15,23] | |
| Utilities (value for age 55) | Mean (SD) | Source | |
| Diabetes, no albuminuria | 0.754 (0.121) | [28] | |
| Diabetes, with microalbuminuria | 0.725 (0.125) | [28] | |
| Diabetes, with macroalbuminuria | 0.686 (0.159) | [28] | |
| Hypertension | 0.744 (0.122) | [28] | |
| Proteinuria | 0.711 (0.128) | [28] | |
| Hemodialyisis | 0.443 (0.317) | [44] | |
| Peritoneal dialysis | 0.569 (0.329) | [44] | |
| Transplant 0–12 months | 0.7325 (0.27) | [45] | |
| Transplant more than 12 months | 0.70 (0.27) | [45] | |
| CVD event (decrement in QOL for 3 months) | 0.33 (0.11) | [74] |
- * Figures for diabetics without albuminuria. All values are the same for diabetics with nephropathy, with the exception of relative risk of progression of kidney disease, as specified.
- ACEi, angiotensin-converting enzyme inhibitor; CI, confidence interval; CVD, cardiovascular disease; ESKD, end-stage kidney disease; QOL, quality of life; RR, relative risk.
| Proportion of patients | Average annual per patient cost for each class ($A 2008) | Total average annual treatment cost per patient ($A 2008) | Data source for utilization and unit costs | |
|---|---|---|---|---|
| Intensive glycemic control | ||||
| Drug costs | [14, (A. Patel and J. Chalmers. ADVANCE Study: management regimens in Australian and New Zealand patients. Personal Communication)][35] | |||
| Diet alone | 0.033 | $0.00 | $0.00 | |
| Sulfonylureas incl gliclazide | 0.9074 | $151.22 | $137.22 | |
| Metformin | 0.708 | $172.15 | $121.88 | |
| TZD | 0.179 | $1,089.91 | $195.09 | |
| Alpha-glucosidase inhibitors (acarbose) | 0.0644 | $874.41 | $56.31 | |
| Glinides | 0 | $0.00 | $0.00 | |
| Insulin | 0.3328 | $1,056.21 | $351.51 | |
| Total proportion-weighted cost per patient | $862 | |||
| Other outpatient health-care utilization | Use/patient/year | Unit cost | [36] | |
| Consultation visits | ||||
| Dietician | 1 | $57.55 | ||
| GP visits | 4—diet only | $33.55 | [18,36] | |
| 6—oral and/or insulin | ||||
| Endocrinologist or diabetes clinic | 1—diet only | $139.45 | ||
| 2—oral and/or insulin | ||||
| Podiatrist | 1 | $57.55 | ||
| Nephrologist consult | 0.1—diet only and oral | $139.45 | ||
| 0.3—insulin | ||||
| Ophthalmic consult | 1 | $139.45 | ||
| Diagnostic tests | ||||
| Home glucose test strips | 26—diet and oral (50% do 1/week) | $52.75 | ||
| 365—insulin (daily) | ||||
| HbA1c done quarterly | 4 | $16.90 | ||
| Other pathology (incl lipids, urinary albumin) | 1 | $15.75 | ||
| Total proportion-weighted cost per patient (3.3% diet, 63.4% oral, 33.3% insulin ± oral) | $910.20 | |||
| Conventional glycemic control | ||||
| Drug costs | [14, (A. Patel and J. Chalmers. ADVANCE Study: management regimens in Australian and New Zealand patients. Personal Communication)][35] | |||
| Diet alone | 0.106 | $0.00 | $0.00 | |
| Sulfonylureas incl gliclazide | 0.5674 | $151.22 | $85.80 | |
| Metformin | 0.7003 | $172.15 | $120.55 | |
| TZD | 0.1044 | $1,089.91 | $113.79 | |
| Alpha-glucosidase inhibitors (acarbose) | 0.0337 | $874.41 | $29.47 | |
| Glinides | 0 | $0.00 | $0.00 | |
| Insulin | 0.1835 | $1,056.21 | $193.81 | |
| Total proportion-weighted cost per patient | $543.43 | |||
| Other outpatient health-care utilization | ||||
| Regimens as per intensive for diet only, oral, and insulin | As above | As above | As above | [18,36] |
| Total proportion-weighted cost per patient (10.6% diet, 71% oral, 18.4% insulin ± oral) | $765.73 | |||
| Intensive hypertension control | ||||
| Drug costs | NEFRON [41]—proportions in patients with hypertension [35] | |||
| Thiazides or other diuretics | 0.36 | $151.43 | $53.86 | |
| Beta-blocker | 0.27 | $124.67 | $33.82 | |
| ACE inhibitor | 0.59 | $237.77 | $141.40 | |
| ARB | 0.37 | $350.47 | $130.41 | |
| Fixed dose low dose thiazide + ACE | 0.15 | $351.49 | $52.32 | |
| Fixed dose low dose thiazide + ARB | 0.29 | $393.23 | $117.06 | |
| CCB | 0.55 | $266.29 | $147.82 | |
| Alpha blocker | 0.04 | $264.51 | $10.14 | |
| Total proportion-weighted cost per patient | $686.82 | |||
| Other outpatient health-care utilization | Use/patient/year | Unit cost | [36] | |
| GP visits | 6 | $33.55 | $201.30 | |
| Other pathology (incl lipids, urinary albumin) | 1 | $15.75 | $15.75 | |
| Total annual cost per patient | $217.05 | |||
| Conventional hypertension control | ||||
| Drug costs | [40]; [35] | |||
| Thiazides or other diuretics | 0.14 | $151.43 | $21.20 | |
| Beta-blocker | 0.24 | $124.67 | $29.92 | |
| ACE inhibitor | 0.36 | $237.77 | $85.60 | |
| ARB | 0.22 | $350.47 | $77.10 | |
| Fixed dose low dose thiazide + ACE | 0.1 | $351.49 | $35.15 | |
| Fixed dose low dose thiazide + ARB | 0.14 | $393.23 | $55.05 | |
| CCB | 0.31 | $266.29 | $82.55 | |
| Alpha blocker | 0.03 | $264.51 | $7.94 | |
| Total proportion-weighted cost per patient | $394.51 | |||
| Other outpatient health-care utilization | Use/patient/year | Unit cost | [36] | |
| GP visits | 4 | $33.55 | $134.20 | |
| Other pathology (incl lipids, urinary albumin) | 1 | $15.75 | $15.75 | |
| Total annual cost per patient | $149.95 | |||
| Protein control (ACE inhibitor) | ||||
| Patients with proteinuria and hypertension—intensive management | ||||
| Drug costs (as for intensive hypertension management) | $686.82 | [35] | ||
| Other outpatient health-care utilization | $217.05 | [36] | ||
| Patients with proteinuria and hypertension—conventional management | ||||
| Drug costs (as for conventional hypertension management) | $394.51 | [35] | ||
| Other outpatient health-care utilization | $149.95 | [36] | ||
| Patients with proteinuria and no hypertension—intensive management | ||||
| Drug costs | $237.77 | [35] | ||
| ACE inhibitor only | ||||
| Other outpatient health-care utilization | $149.95 | [36] | ||
| Dialysis costs | ||||
| Hemodialysis | ||||
| Initial hemodialysis access | $15,490 | [34] | ||
| Hospital hemodialysis | $94,061 | [34] | ||
| Home hemodialysis | $51,782 | [77] | ||
| Satellite hemodialysis | $56,393 | [77] | ||
| Peritoneal dialysis | ||||
| Initial peritoneal dialysis access | $12,762 | [34] | ||
| Direct PD costs | $64,221 | [78] | ||
| Transplant costs | ||||
| Live donor transplant | ||||
| Surgery (recipient) | $35,962 | [34] | ||
| Surgery (donor) | $13,836 | [34] | ||
| Deceased donor transplant | ||||
| Surgery (recipient) | $35,962 | [34] | ||
| Surgery (donor) | $3,000 | Expert opinion | ||
| Other resources: all transplants | ||||
| Year of transplant | ||||
| Immunosuppressive drug costs | $19,038 | [35] | ||
| Other drug costs | $8,619 | [35] | ||
| Other health-care resource use | $6,428 | [36] | ||
| Subsequent years | ||||
| Immunosuppressive drug costs | $8,881 | [35] | ||
| Other drug costs | $724 | [35] | ||
| Other health-care resource use | $819 | [36] |
- ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker (also known as angiotensin II-receptor antagonists); CCB, calcium channel blocker; ESKD, end-stage kidney disease; GP, general practitioner; HbA1c, glycated hemoglobin; PD, peritoneal dialysis; TZD, thiazolidinediones.
Primary care–based screening strategies for CKD risk factors (“screening”). All screening strategies were based on annual primary care–based screening, offered to people aged between 50 and 69 years. In the base-case, it was assumed that 75% of people offered screening would participate [24]; the participation rate was varied in sensitivity analyses. Screening and diagnostic test characteristics are shown in Table 3. Because the AusDiab data formed the basis for the proportion of patients with undiagnosed disease, the thresholds for screen test positivity were also based on AusDiab [25,30].
| Value (%) | Data source | Total cost ($A 2008) | Unit cost source | |
|---|---|---|---|---|
| Population diabetes screening | ||||
| Screen test sensitivity (FBSL) | 86.7 | 89% [75]US 83% [76]Israel 95% [76] | $33.55 | MBS item 23 [36] |
| $11.75 | MBS item 66,503 [36] | |||
| Screen test specificity (FBSL) | 65.5 | 59% [75] | ||
| US 76% [76] | ||||
| Israel 47% [76] | ||||
| Diagnostic test for screen-positive (oral glucose tolerance test) | $33.55 | MBS item 23 [36] | ||
| $19.30 | MBS item 66,542 [36] | |||
| Population hypertension screening | ||||
| Screen test sensitivity (mean of 3 measurements) | 100 | (assumed) | $30.85 | MBS item 23 [36] |
| Screen test specificity (mean of 3 measurements) | 100 | (assumed) | ||
| Screening for proteinuria | ||||
| Screen test sensitivity (protein dipstick) | 89 | 90% [23] | $33.55 | MBS item 23 [36] |
| 90% (S. Chadban, personal communication, AusDiab) | $1 | [35] | ||
| Screen test specificity (protein dipstick) | 94 | 67% [23] | ||
| 94% (S. Chadban, personal communication, AusDiab) | ||||
| Diagnostic test for screen-positive (protein : creatinine ratio) | $33.55 | MBS item 23 [36] | ||
| $11.75 | MBS item 66,503 [36] |
- FBSL, fasting blood sugar level; MBS, Medical Benefits Schedule; US, United States.
The modeled screening test for diabetes was fasting blood sugar level. A level >5.5 mmol/L was classified as positive. After a positive screen, an oral glucose tolerance test was used as a diagnostic test. The screening test for hypertension was blood pressure measurement in a primary care setting (mean of three measurements), with a threshold of 140/90 mmHg, and was assumed to be 100% sensitive and specific (because this is what determines the provision of antihypertensives). Screening for proteinuria was conducted using a urine dipstick, followed by a confirmatory diagnostic test (spot urine protein : creatinine ratio >0.20 mg/mg) for a dipstick result of 1+ or greater.
It is assumed that all screen-positive patients undergo confirmatory diagnostic tests (diabetes and proteinuria). All screen-detected disease was treated intensively as described above.
Costs
Costs are reported in 2008 Australian dollars [34–38]. The analysis took a health-care funder perspective, because Australia has a centrally funded universal health-care system. Costs of screening strategies are in Table 3; costs of treatment interventions for risk factors and ESKD are summarized in Table 4.
Table 4 presents a proportion-weighted average annual treatment cost per patient. Nonpharmaceutical health-care resource utilization for diabetic patients is based on the care practices in the UKPDS study [18]; the proportion of patients on each class of hypoglycemic medication in the conventional and intensive management groups is based on the Australian and New Zealand ADVANCE study cohort at the end of follow-up (December 2007) [14, (A. Patel and J. Chalmers. ADVANCE Study: management regimens in Australian and New Zealand patients. Personal Communication)]. These data are broadly consistent with the NEFRON study [39]; and the average annual cost of each class of hypoglycemic used is based on 2008 Pharmaceutical Benefits Scheme (PBS) utilization and PBS unit cost data. Nonpharmaceutical health-care resource utilization for patients with hypertension is also based on UKPDS [22]; conventional hypertension management is based on a National Prescribing Service survey [40]. Intensive hypertension management (including patients with proteinuria) is based on data from the NEFRON study [41], which reported higher proportions of patients using all classes of antihypertensives compared with the National Prescribing Service data, and updated to reflect the current PBS use of fixed dose combinations of ACE/diuretic and ARB/diuretic. The average annual cost of each class of antihypertensive used is based on 2008 PBS utilization and PBS unit cost data. Patients with screen-detected proteinuria but no diabetes or hypertension were treated with an ACE inhibitor, the average annual cost of ACE inhibitors is based on 2008 PBS utilization and PBS unit cost data. In addition, costs of fatal and nonfatal cardiovascular events are based on data from an Australian population [42], inflated to 2008 values [38].
Health-Related Quality of Life (QOL)
Age and health state specific SF6D utility weights were calculated from individual patient SF-36 responses from the AusDiab study [43]; summary values of QOL for a 55-year-old are in Table 2 (additional detail available elsewhere [28]). Utility weights for dialysis and transplant health states were based on the published literature of pre- and post-transplant utility-based health-related QOL weights [44,45] (Table 2).
Sensitivity Analysis
Probabilistic modeling was conducted. The purpose of probabilistic sensitivity analysis is to reflect the uncertainty in all input parameters of a model simultaneously and to describe the implications of that uncertainty on costs, effects, and cost-effectiveness [46]. Model parameter distributions were applied as recommended: Gamma distributions were applied to costs, beta distributions were used for probabilities and utilities, and log-normal distributions were used for relative risks [46].
Results of probabilistic modeling are presented as cost-effectiveness acceptability curves that plot the likelihood that an intervention is cost-effective, over a range of decision-makers' willingness to pay thresholds for each additional health outcome (QALY) gained, and as scatter plots of the incremental costs and effects.
The effects of varying: 1) screening start age; and 2) screening participation on the cost-effectiveness of screening programs were also specifically assessed in deterministic one-way sensitivity analyses.
Results
Improved Management of Known Patients with Risk Factors for CKD (“Treatment”)
Over a patient's lifetime, intensive glycemic control of previously uncontrolled diabetic patients resulted in cost savings, on average, of $A133 compared with conventional management. It resulted in an additional benefit of 0.075 QALYs, meaning it was both less costly and more effective than conventional management. Cost savings were predominantly driven by the avoidance of costly health outcomes such as cardiovascular events, deaths, and the need for RRT. Over this time frame, for every 1000 patients managed intensively approximately six cardiovascular deaths were prevented, one noncardiovascular death was prevented, and there were approximately six fewer patients requiring RRT for ESKD.
Over a comparable time period, intensive control of previously inadequately controlled hypertension cost an extra $A352, and led to a gain of 0.136 QALYs, with an incremental cost-effectiveness ratio (ICER) of $A2588 per QALY gained. For every 1000 patients managed intensively over the duration of the model, 19 cardiovascular deaths were prevented, 5 noncardiovascular deaths were averted, and there were 10 fewer patients who required dialysis or transplant for ESKD.
The use of an ACE inhibitor by all diabetic patients led to average cost savings of $825 over a patient's lifetime, and a net health gain of 0.124 QALYs; it was both more effective and less expensive than current practice. For every 1000 diabetic patients who received an ACE inhibitor, there were 18 fewer cardiovascular deaths, 3 noncardiovascular deaths were prevented, and 9 fewer patients who developed ESKD and needed RRT. The additional small cost of an ACE inhibitor was more than offset by the reduction in expensive cardiovascular events and progressions to ESKD requiring RRT (Table 5).
| Intervention | Cost (intervention) ($A 2008) (95% CI) | Cost (comparator) ($A 2008) (95% CI) | Incremental cost ($A 2008) (95% CI) | QALYs (Intervention) (95% CI) | QALYs (comparator) (95% CI) | Incremental QALYs (95% CI) | ICER ($ per QALY) | Probability cost-saving | Probability cost-effective* | |
|---|---|---|---|---|---|---|---|---|---|---|
| Improved management in known/existing patients | ||||||||||
| 1 | Intensive glycemic control in known diabetic patients | $40,144 ($13,078–135,828) | $40,277 ($12,733–135,987) | −$133 (−$5,944–4,716) | 9.942 (5.122–15.037) | 9.867 (5.047–14.913) | 0.075 (–0.017–0.232) | Dominant† | 47% | 85% |
| 2 | Addition of an ACE inhibitor in known diabetics | $37,781 ($12,516–129,402) | $38,606 ($12,030–130,495) | −$825 (−$8,034–4,479) | 10.111 (5.275–15.192) | 9.987 (5.092–15.070) | 0.124 (–0.037–0.312) | Dominant† | 54% | 88% |
| 3 | Intensive blood pressure control in known hypertensive patients | $39,716 ($14,274–129,185) | $39,364 ($13,038–134,731) | $352 (−$9,668–9,718) | 10.070 (5.283–15.179) | 9.934 (5.095–15.057) | 0.136 (–0.053–0.350) | $2,588 | 44% | 82% |
| Primary care–based screening for CKD risk factors | ||||||||||
| 4 | Screening (50–69 years) for diabetes and intensive glycemic control in known and screen-detected diabetic patients | $17,832 ($3,027–70,025) | $16,487 ($1,875–68,202) | $1,345 (−$6,600–9,902) | 12.798 (4.321–17.720) | 12.701 (4.144–17.627) | 0.097 (–0.408–0.696) | $13,866 | 21% | 57% |
| 5 | Screening (50–69 years) for hypertension and intensive blood pressure control in known and screen-detected hypertensive patients | $14,061 ($1,178–61,009) | $14,004 ($1,402–63,661) | $57 (−$8,058–7,757) | 12.947 (4.768–18.037) | 12.831 (4.673–17.694) | 0.116 (–1.396–1.745) | $491 | 31% | 55% |
| 6 | Screening (50–69 years) for proteinuria and addition of an ACE inhibitor in all known diabetics and screen-detected patients with proteinuria | $16,974 ($1,867–65,239) | $16,821 ($1,641–64,826) | $153 (−$7,708–7,527) | 12.763 (4.871–17.806) | 12.731 (4.828–17.806) | 0.032 (–0.790–0.93) | $4,781 | 29% | 50% |
- * At willingness to pay threshold of $A50,000.
- † Dominant—intervention is less costly and more effective than comparator.
- 95% CI, 95% confidence interval; ACE, angiotensin-converting enzyme inhibitor; CKD, chronic kidney disease; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
Primary Care–Based Screening Strategies for CKD Risk Factors (“Screening”)
Primary care–based screening for risk factors and intensive management of new and already identified, but inadequately controlled, patients was also assessed.
Screening for diabetes between the ages of 50 and 69 years had an incremental lifetime cost of $A1345, compared with current practice, resulted in a gain of 0.098 QALYs, with an ICER of $A13,781 per QALY gained. For every 1000 patients screened for diabetes with detected cases managed with intensive glycemic control, there were approximately two fewer cardiovascular deaths, three fewer noncardiovascular deaths, and two fewer patients requiring RRT for ESKD.
Screening for hypertension plus intensive blood pressure management in new and already identified, but inadequately controlled patients, had an incremental cost of $A57, resulted in 0.116 incremental QALYs, giving an ICER of $A491 per QALY gained. For every 1000 patients screened for hypertension with detected cases managed with intensive blood pressure control, there were approximately nine fewer deaths from cardiovascular disease, four fewer noncardiovascular deaths, and approximately five fewer patients developing ESKD that needed RRT.
Screening for proteinuria plus the addition of an ACE inhibitor for all diabetics and for persons with screen-detected proteinuria had an ICER of $A4793 per QALY gained, compared with current practice. For every 1000 patients screened for proteinuria and treated with an ACE inhibitor, in combination with treating all diabetic patients with an ACE inhibitor, there were approximately three fewer deaths from cardiovascular disease, one fewer death from noncardiovascular causes, and three fewer patients that required RRT for ESKD (Table 5).
Sensitivity Analysis
Probabilistic sensitivity analysis—“treatment.” Cost-effectiveness acceptability curves (Fig. 2a) indicate the probability that an intervention is cost-effective, over a wide range of funders' willingness to pay for each additional health outcome. Table 5 indicates the probability that treatment of risk factors is cost-effective at a threshold of $A50,000 per QALY gained ranged from 84% to 88%. The probability that treatment interventions will save money ranged from 44% to 54% (Table 5 and Fig. 2a–d).
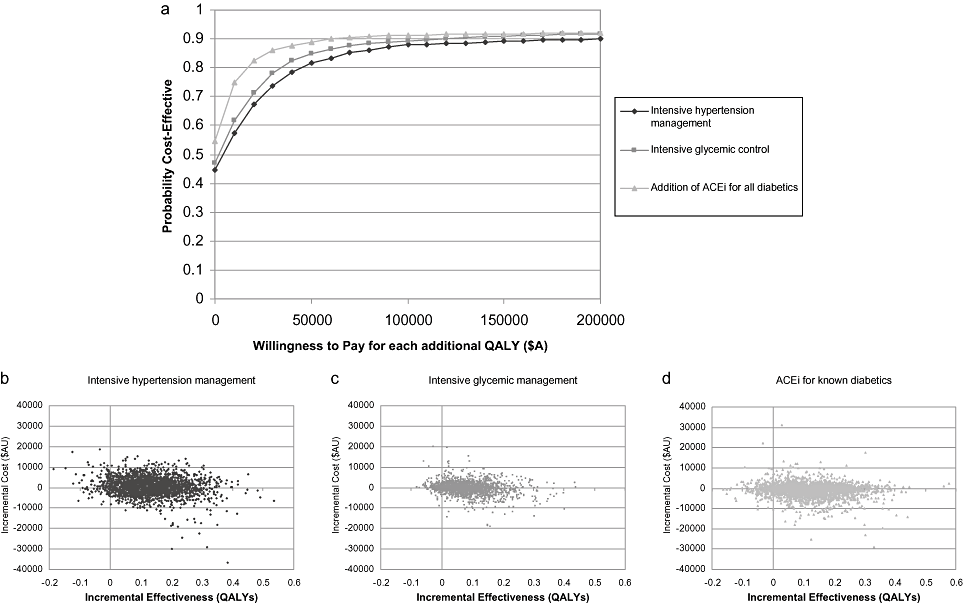
(a) Cost-effectiveness acceptability curves for improved management strategies for existing patients. (b–d) Scatter plots of incremental costs and incremental QALYs for improved management strategies for existing patients. ACEi, angiotensin-converting enzyme inhibitor; QALY, quality-adjusted life-year.
Probabilistic sensitivity analysis—“screening.” Figure 3a and Table 5 indicate that at a cost-effectiveness threshold of $A50,000 per QALY gained, there is between a 50% and 57% likelihood that screening and intensive management of risk factors will have an ICER of less than $A50,000 per QALY gained. The probability that screening interventions will save money ranged from 21% to 31% (Table 5). Despite this, as can be seen from Figure 3b–d, there is considerable uncertainty in the estimates of incremental costs and incremental QALYs. The uncertainty reflected here relates primarily to the uncertainty in the estimates of effectiveness of the interventions for screen-detected cases.
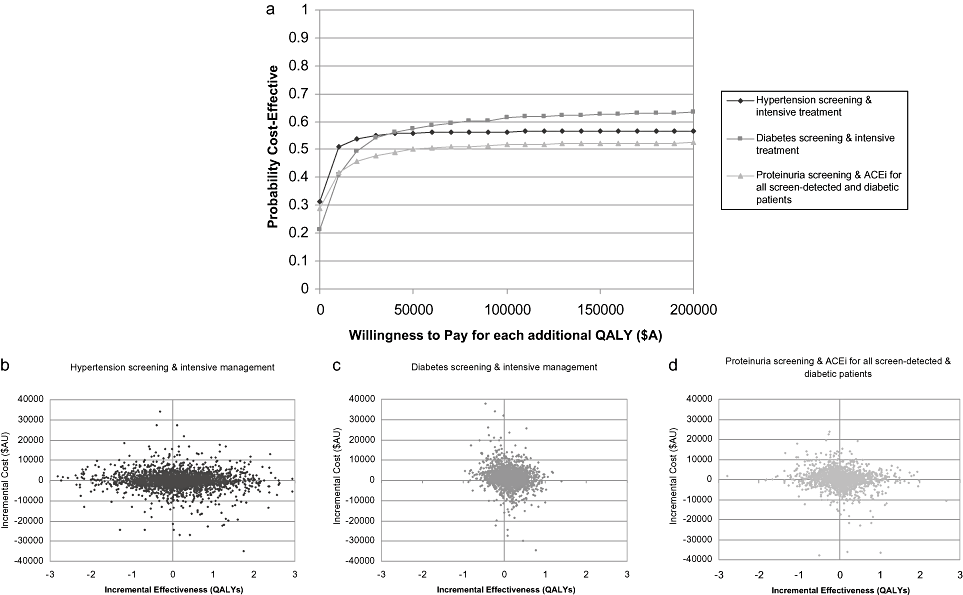
(a) Cost-effectiveness acceptability curves for screening and improved management for new and existing patients. (b–d) Scatter plots of incremental costs and incremental QALYs for screening and improved management for new and existing patients. ACEi, angiotensin-converting enzyme inhibitor; QALY, quality-adjusted life-year.
One-way sensitivity analyses. One-way sensitivity analyses indicated the cost-effectiveness of screening improved as the starting age increased, related primarily to increasing prevalence of hypertension, diabetes, and proteinuria with increasing age. The ICER increased slightly as participation increased, primarily driven by the higher costs associated with higher participation (Table 6).
| Screening intervention | Cost ($A 2008) (intervention) | Cost ($A 2008) (comparator) | Incremental cost ($A 2008) | QALYs (intervention) | QALYs (comparator) | Incremental QALYs | ICER ($ per QALY gained) |
|---|---|---|---|---|---|---|---|
| Diabetes screening | |||||||
| Starting age for screening | |||||||
| 30 | $18,231 | $16,487 | $1,744 | 12.808 | 12.701 | 0.107 | $16,299 |
| 40 | $18,097 | $16,487 | $1,610 | 12.805 | 12.701 | 0.104 | $15,481 |
| 50 (base-case) | $17,832 | $16,487 | $1,345 | 12.798 | 12.701 | 0.097 | $13,866 |
| 60 | $17,495 | $16,487 | $1,008 | 12.789 | 12.701 | 0.088 | $11,455 |
| Screening participation (%) | |||||||
| 25% | $17,419 | $16,487 | $932 | 12.794 | 12.701 | 0.093 | $10,022 |
| 50% | $17,671 | $16,487 | $1,184 | 12.797 | 12.701 | 0.096 | $12,333 |
| 75% (base-case) | $17,832 | $16,487 | $1,345 | 12.798 | 12.701 | 0.097 | $13,866 |
| 100% | $17,931 | $16,487 | $1,444 | 12.8 | 12.701 | 0.099 | $14,586 |
| Hypertension screening | |||||||
| Starting age for screening | |||||||
| 30 | $14,302 | $14,004 | $298 | 12.955 | 12.831 | 0.124 | $2,403 |
| 40 | $14,183 | $14,004 | $179 | 12.946 | 12.831 | 0.115 | $1,557 |
| 50 (base-case) | $14,061 | $14,004 | $57 | 12.947 | 12.831 | 0.116 | $491 |
| 60 | $13,677 | $14,004 | −$327 | 12.953 | 12.831 | 0.122 | Dominant |
| Screening participation (%) | |||||||
| 25% | $13,570 | $14,004 | −$434 | 12.953 | 12.831 | 0.122 | Dominant |
| 50% | $13,803 | $14,004 | −$201 | 12.951 | 12.831 | 0.12 | Dominant |
| 75% (base-case) | $14,061 | $14,004 | $57 | 12.947 | 12.831 | 0.116 | $491 |
| 100% | $14,194 | $14,004 | $190 | 12.953 | 12.831 | 0.122 | $1,557 |
| Proteinuria screening | |||||||
| Starting age for screening | |||||||
| 30 | $17,102 | $16,821 | $281 | 12.763 | 12.731 | 0.032 | $8,781 |
| 40 | $17,034 | $16,821 | $213 | 12.764 | 12.731 | 0.033 | $6,455 |
| 50 (base-case) | $16,974 | $16,821 | $153 | 12.763 | 12.731 | 0.032 | $4,781 |
| 60 | $16,897 | $16,821 | $76 | 12.764 | 12.731 | 0.033 | $2,303 |
| Screening participation (%) | |||||||
| 25% | $16,815 | $16,821 | −$6 | 12.764 | 12.731 | 0.033 | Dominant |
| 50% | $16,856 | $16,821 | $35 | 12.763 | 12.731 | 0.032 | $1,094 |
| 75% (base-case) | $16,974 | $16,821 | $153 | 12.763 | 12.731 | 0.032 | $4,781 |
| 100% | $17,065 | $16,821 | $244 | 12.764 | 12.731 | 0.033 | $7,394 |
- ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
Discussion
Our modeled analyses show that intensive treatment of patients with inadequately controlled hypertension and/or diabetes, and primary care–based screening for CKD and its major risk factors, followed by intensive treatment, can lead to improved health outcomes and are likely to represent good value for money. In patients with existing diabetes, intensive management of patients with uncontrolled blood glucose and the addition of an ACE inhibitor in all patients appear to offer improved health outcomes and lower long-term costs. Intensive hypertension management also offers good value for money. In addition, primary care–based screening and treatment for diabetes and for hypertension and proteinuria also seem to offer better value for money compared with many population screening programs already funded in much of the developed world [47,48].
In adopting a risk factor-based approach, our analyses of primary care–based interventions for CKD considered the combined effects of screening and improving the current suboptimal management of people with two chronic diseases. We modeled a true population perspective by explicitly considering the age and sex distribution of the population, the proportion of patients with diagnosed and undiagnosed risk factors for CKD, and the proportion of suboptimally controlled patients. We have applied best-practice economic modeling techniques [49], utilizing a probabilistic approach to characterize the joint parameter uncertainty by incorporating distributions of both probabilities of events, and of treatment effectiveness. Given the recent controversy surrounding the benefits of intensive glycemic control [14,50], this probabilistic approach has explicitly modeled the possibility of no treatment benefit. As such, our analysis more accurately estimates the costs and health outcomes at a whole of population level.
There are few other cost-effectiveness estimates of these interventions in an Australian context. Our results are broadly consistent with previous cost-effectiveness analyses of treatment interventions for individual risk factors such as diabetes, hypertension, and proteinuria for CKD [18–20,22,51], which have reported ICERs ranging from interventions being both less costly and more effective to ICERs greater than $US40,000 per QALY gained for intensive glycemic control [19].
The results of our models also suggest that primary care screening of 50- to 69-year-olds for diabetes, hypertension, and proteinuria, with subsequent intensive management for all screen-detected and suboptimally controlled patients, is likely to offer good value for money in the Australian context, albeit subject to some underlying uncertainty. Differences—in the underlying modeled populations, in health systems, in delivery of primary care, in the cost of care, and in the health outcomes considered—all contribute to the variability in published ICERs. Despite these differences, previous analyses also suggest that screening for single risk factors may offer good value for money, particularly for specific population subgroups. For example, a trial-based analysis of screening for albuminuria with subsequent fosinopril (PREVEND) reported an ICER of €16,700 per life-year gained [52], although proteinuria screening for patients with diabetes and comorbid hypertension had an ICER of $US20,011 per QALY gained [53]. ICERs for proteinuria screening, with subsequent ACE inhibitor or ARB treatment ranged from $US18,621 per QALY gained for patients with hypertension to $US282,818 per QALY gained for people aged 50 years without hypertension or diabetes [24].
Why is our estimate of the cost-effectiveness of screening for proteinuria, then providing ACE inhibitor treatment for screen-detected patients with proteinuria and known diabetics, more favorable? First, the actual interventions and patient populations modeled are different. Our intervention modeled ACE inhibitor treatment for all screen-detected patients with proteinuria plus all diabetic patients. We also report that ACE inhibitor treatment for all patients with diabetes resulted in both a cost-saving and additional QALYs. Second, we modeled general practice screening and management, meaning the total cost of diagnostic assessment for all screen-positive patients was $A45.30 (Table 3), compared with higher estimates of specialist-based diagnostic assessment $US2372 to $US2742 [24]. Third, the costs of pharmaceutical management in our model were substantially lower (cost of ACE inhibitors $A237 compared with $US390 to $US511 [24]).
Limited evidence regarding complex interplay between diabetes, hypertension, and proteinuria in CKD prevalence, risk of progression, and effectiveness of screening and better management, meant that our study was restricted to modeling costs and effects of single risk factor-based interventions. No data are available on the combined effectiveness of multiple concurrent strategies of risk factor management. Cost-effectiveness analyses would ideally be based on randomized trial evidence of the effectiveness of a “package” of interventions (screening and better management) compared with routine management. Such a trial has, however, has not been performed. Instead, we found it necessary to synthesize the results of several independent trials and meta-analyses. Robust data on the natural progression of CKD from large-scale, population-based studies are only beginning to become available. They will be crucial to the development of screening and intervention strategies such as those modeled here [54].
Using a health-care funder perspective meant that costs related to productivity changes, and out of pocket costs to patients and families, have not been included. It is possible that cost-offsets from improved productivity as a result of avoiding, or more actively managing, chronic disease may have been underestimated; however, these cost-offsets need to be balanced against the additional costs to patients and families also not captured by a health-care funder perspective. Our analyses specifically consider the prevalence of risk factors in a representative Australian population; in populations with a higher prevalence of CKD risk factors, screening may well be more effective and cost-effective. Similarly, our analysis is based on disease thresholds that may be higher than would be used in current clinical practice. Lowering the thresholds for risk factors would effectively increase the size of the population considered to have the risk factor (in the context of screening) or be “uncontrolled,” and therefore able to benefit from intensive intervention. The effect on the ICER of lowering disease thresholds would depend upon both the relative effectiveness of the interventions and the absolute risk of events in this expanded population.
Conclusions
The rising prevalence of CKD and the ever-increasing demand for high-cost dialysis and kidney transplant therapy require a full exploration of a population-based approach to screening for, and intensive treatment of, its risk factors. If a funder is willing to spend up to $A50,000 for each additional QALY gained, a range of strategies addressing intensive blood glucose and blood pressure control among already identified patients, combined with primary care screening of asymptomatic 50- to 69-year-olds for diabetes, hypertension, and proteinuria, and subsequent optimal care, should be strongly considered.
Acknowledgments
Special thanks to the ANZDATA Registry staff, in particular to Victoria Shtangey for her prompt and generous responses to data requests. KH had full access to all of the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis. AC receives salary support from a NHMRC Senior Research Fellowship.
Source of financial support: Financial support for this study was provided in part by a grant from Kidney Health Australia, a not-for-profit organization providing patient education and support; Dr Howard and Professor Salkeld received a personal payment, and Dr White received educational support for their involvement in the project. The funding agreement ensured the authors' independence in designing the study, interpreting the data, writing and publishing the report, and publication was not contingent on sponsor approval.




