Cost-Effectiveness of Scheduled Maintenance Treatment with Infliximab for Pediatric Crohn's Disease
ABSTRACT
Background: Infliximab recently became the only biologic approved for use in pediatric patients with severe active Crohn's disease (CD).
Objectives: To estimate the cost-effectiveness of scheduled maintenance treatment with infliximab compared with standard care in children suffering from severe active CD over 5 years from the UK National Health Service perspective.
Methods: A Markov model was constructed to simulate the progression of a hypothetical cohort of CD children through predefined health states on scheduled maintenance treatment with infliximab (5 mg/kg). The data to populate the model came from infliximab trials from Targan et al., ACCENT I, and REACH. The health states included in the model were remission, responding active disease, nonresponding active disease, surgery, postsurgery remission, postsurgery complications, and death. Standard care, comprising immunomodulators, and/or corticosteroids were used as a comparator. The primary outcome was quality-adjusted life-years (QALY) estimated using the EuroQol (EQ-5D) from a European CD population. To account for the weight-based dosing of infliximab, a baseline patient weight of 40 kg that increased by 5 kg/year up to 60 kg was used. The costs and outcomes were discounted at 3.5% over a period of 5 years. Probabilistic sensitivity analyses were performed by varying the infliximab efficacy estimates, costs, and utilities.
Results: The incremental cost-effectiveness ratio (ICER) for infliximab treatment was £14,607 compared with standard care. The sensitivity analyses revealed the treatment effect of infliximab to be the most influential parameter with ICERs ranging from £10,480 to £37,017. Assuming a willingness to pay of £30,000 per QALY, the probability of infliximab being cost-effective is 78.6%.
Conclusion: Scheduled maintenance treatment with infliximab (5 mg/kg) is likely to be a cost-effective treatment in children suffering from severe active CD under an 8-week maintenance program.
Introduction
Crohn's disease (CD) is a common chronic inflammatory bowel disorder of childhood and adolescence. Although the reported incidence of CD is lower in children than in adults at around 0.2 to 8.5 per 100,000, there are indications that the incidence is increasing [1–4]. CD significantly impacts children, often resulting in growth retardation and psychosocial issues, such as social isolation and behavioral problems. Children with CD also have a higher prevalence of psychiatric disorders, such as depression and anxiety, compared with healthy controls or children with other chronic conditions such as type-I diabetes [4].
The financial impact of adult CD has been reported in a study from the UK, which suggested that hospital admissions account for 75% of the total costs of CD, with mean costs over 6 months nearing £7000 for an adult hospitalized patient and £516 for an adult outpatient [5]. The findings of this study were consistent with research from Sweden and the United States reporting significant hospitalization costs [6,7].
Although, no other study has estimated this burden specifically in children, it is likely to be comparable to adults. Infliximab reduces this burden as observed in a retrospective study of 205 patients across seven centers in the UK. The study reported significant reductions in mean costs in the range of £138 per patient in 6 months post-infliximab compared with 6 months pre-infliximab [8]. Nevertheless, the treatment cost of infliximab remains high and there is a need to demonstrate its value in the CD treatment pathway.
The infliximab randomized controlled trial, REACH, demonstrated that 88% of moderate to severe CD children achieved clinical response after infliximab induction treatment (at weeks 0, 2, and 6) and more than half of these patients achieved and maintained a clinical remission on continued dosing regimen for 8 weeks up to 1 year [9]. Infliximab has also been recommended as an alternative treatment for pediatric patients with severe active CD (Harvey Bradshaw index [HBI] >8, CD activity index [CDAI] >300) who are refractory to or intolerant of steroids and immunosuppression, and ineligible for surgery by the European Crohn's and Colitis Organisation [10–12].
This economic analysis was performed to assess the cost-effectiveness of infliximab scheduled maintenance treatment, which is often labeled as expensive, compared with standard care in pediatric CD patients over 5 years from the UK National Health Service (NHS) perspective.
Methodology
Patient Population and Model Structure
The economic analysis was carried out in a hypothetical cohort of children aged 6 years to 17 years with moderate to severe active luminal CD, presenting with a pediatric CDAI (PCDAI) score of ≥30 at baseline [13]. As per the REACH trial population, the mean age assumed was 13 years (SD 2.5) and the mean patient weight was 43.8 kg (SD 14.6). On average, these children were suffering from CD for 2 years with a mean PCDAI score of 41.2 (SD 8.3). About one-third (34.9%) of the children were using corticosteroids at the time of starting infliximab treatment [9].
A Markov model was used to simulate the disease progression and track associated costs and outcomes over 5 years of treatment. The disease activity was characterized using CDAI instead of PCDAI to facilitate inclusion of adult CD estimates. An overview of the model is presented in Figure 1. The disease severity was characterized by two discrete “on-treatment” health states, namely, remission (CDAI ≤150) and responding active disease (CDAI >150). For children classified as nonresponders or discontinuers, the disease severity was characterized by the “off-treatment” health state of nonresponding active. The model also included a total of three additional health states—surgery, postsurgery remission, and postsurgery complications, as well as an absorbing health state of death.
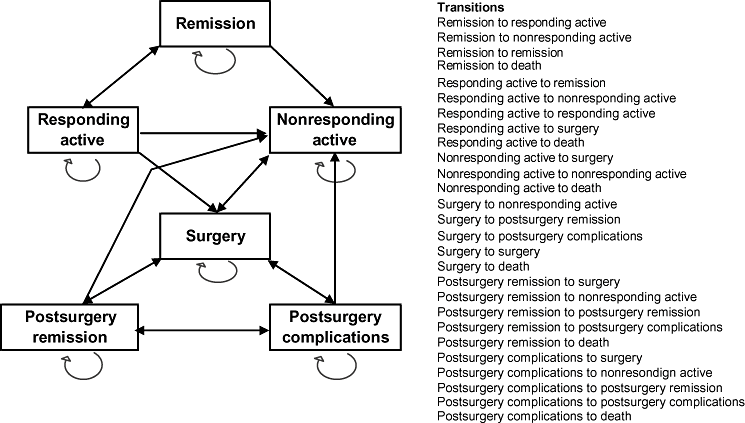
Markov model for moderate to severe pediatric Crohn's disease.
The cycle length used in the model was selected to facilitate transfer of efficacy estimates from clinical trial to infliximab dosing regimen. Therefore, the first cycle was 2 weeks (week 0–2), followed by an 8-week, and a 20-week cycle (weeks 2–10 and 10–30). The cycle length thereafter was 24 weeks.
All children started in the responding active health state. At the end of the first cycle of 2 weeks and each subsequent model cycle, children could either remain in the responding active health state or move to a different health state depending on the response. Responders achieving remission (CDAI ≤150) moved to remission, whereas those not achieving remission remained in the responding active health state. A response was defined as 70 or more point reduction in the CDAI score from baseline and at least a 25% reduction in the total score [14]. Nonresponders at week 2, responders losing response in subsequent cycles, or patients choosing to discontinue treatment moved to the nonresponding active state. Nonresponders were switched to standard care and could not return to infliximab treatment.
Children in the responding active and the nonresponding active health states could transition to surgery, which was an outcome of disease progression. The surgery state included surgical procedure and the postsurgical recovery included the associated hospitalization. In the subsequent model cycles, children could transition between surgery and the two postsurgery health states. These transitions are outlined in Figure 1. Because of unavailability of any evidence of infliximab re-treatment efficacy on infliximab failures, children with a recurrence of CD were not offered infliximab treatment. Patients in surgery and any of the postsurgery health states could not receive any biologic treatment for their CD but could receive treatment for their postsurgery complications.
Treatment Interventions and Transition Probabilities
The treatment interventions modeled and compared included an infusion at week 0 of either infliximab 5 mg/kg (infliximab scheduled maintenance) or placebo (standard care), followed by repeated infusions for responders at week 2, week 6, and every 8 weeks thereafter.
Table 1 displays the sources of transition probabilities. Because the REACH trial [9] did not include a placebo treatment arm, data from the two adult studies of infliximab Targan et al. [15] and ACCENT I [14] were used to estimate the transition probabilities. The transitions in the individual model cycles were estimated using patient-level data from the clinical trials mentioned above. The remission and response rates used to estimate these transition probabilities are displayed in Figure 2a.
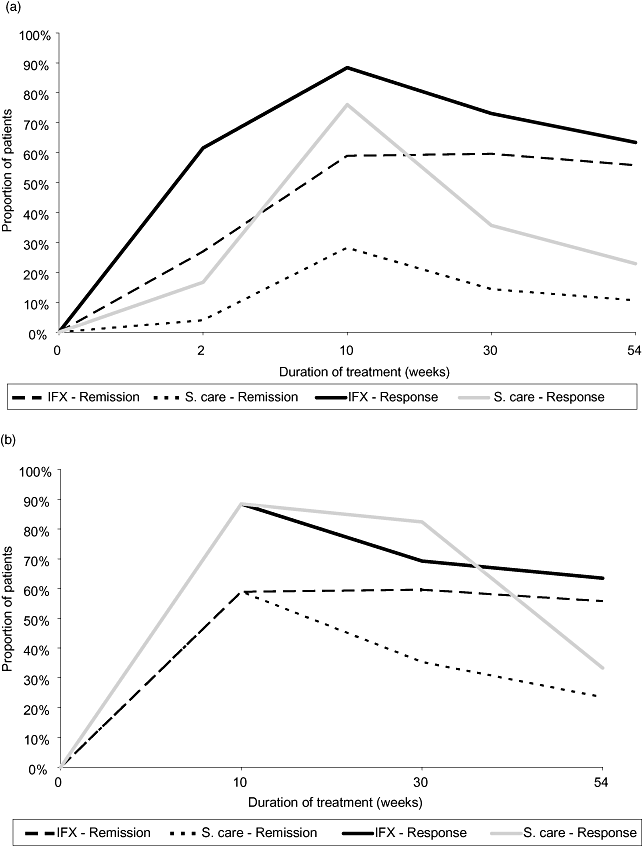
(a) Efficacy estimates in base-case. (b) Efficacy estimates in scenario B. IFX, infliximab; S. care, standard care.
The transition probabilities for the surgery and the postsurgical states were obtained from literature. The probability of surgery was based on an average surgery rate of 64% in 10 years obtained from a retrospective database study of 641 adult CD patients [16]. In absence of any details on varying risk over time, our analysis assumed a constant risk of surgery. The estimated probabilities of postsurgery complications were based on a cohort control study by Marchal and others, which compared postsurgery complications with and without infliximab [17]. The authors concluded that infliximab treatment did not have any significant impact on the frequency of postsurgery complications. Therefore, a complication rate of 20.5% estimated as a weighted average of complications observed in the comparative treatment arms was used [17]. Reports in the published literature were used to estimate the recurrence rates of 15.6% at 1 year in adult patients with severe active luminal CD and the repeat surgery rate of 16.7% at 1 year [18]. In absence of specific estimates in pediatric patients, the rates of surgery, postsurgery outcomes, and CD recurrence were assumed to be identical to the adult patients. It was assumed that the survival rates in pediatric CD were comparable to the survival in the general population [19] after adjustment on age and sex and, the standardized mortality ratio from all health states to death was 1.
Time Horizon
The efficacy observed in the 54-week trial period was extrapolated up to 5 years in the base-case analysis. These extrapolations were based on the transitions observed in the last treatment cycle (week 46–54) of the REACH trial. The last cycle transitions were selected for extrapolation to negate the additional beneficial effect of induction dose in the treatment regimen. A 5-year time horizon was assumed to be sufficient to capture all the relevant costs and effects of infliximab in pediatric CD. Other scenarios, including a 1-year and lifetime treatment, were explored in the sensitivity analyses.
Costs
The perspective adopted on the costs was that of the NHS in England and Wales. The reference year for the costs was 2006 to 2007.
The infliximab treatment cost was broken down into its acquisition costs and administration cost. The acquisition cost of infliximab was calculated using a baseline patient weight of 40 kg for an average 13-year-old patient at the beginning of the analysis and gradually increased it up to 60 kg (5 kg/year) during the follow-up period such that the average patient weight was 60 kg at adulthood. The drug administration cost of £96 per infusion was used, which incorporated all tests, assessments, and staffing costs associated with the infusion [20]. This resulted in a total cost per infusion of infliximab in the range of £935.24 (40 kg) to £1354.86 (60 kg).
The concomitant medication use was estimated using all patient baseline information in the REACH trial [9]. In our model, we assumed that throughout the analysis period, patients in presurgery health states maintained their baseline medication use except corticosteroids as observed in the REACH trial. A linear reduction in corticosteroid use, however, was assumed such that patients achieving and maintaining remission were assumed to be corticosteroid free by week 54. The proportion of children discontinuing active treatment due to serious adverse events was estimated using the REACH trial [9]. The model did not explicitly account for the costs of adverse events. Potential costs associated with infusion-related adverse events such as infusion reactions, headache, dizziness, nausea, injection site reaction, flushing chest pain, dyspnea, and pruritus were considered to be incorporated in the administration cost. Serious adverse events, related either to infliximab or other medications for CD, such as corticosteroids or immunomodulators, were assumed to be included as part of the hospitalization costs.
The estimated average cost per surgical intervention was £4190 based on the data published in the National Schedule of Reference Costs (NSRC) for 2006 to 2007 (Table 2) [21]. This was an average cost, taking into account elective and nonelective admissions, with or without complications. The treatment-related hospitalization and other assessments were estimated using the study by Jewell and others [8], whereas the individual health state costs were estimated using post hoc analysis of ACCENT I [22]. This study by Lichtenstein and others reported a 5.57-fold reduction in hospitalization for patients in remission and a threefold reduction in hospitalization for responders compared with nonresponders.
| Resource | Published estimate of resource use (n = 205)* | Cost per patient per cycle (24 weeks) | Unit cost (£) | Source | ||
|---|---|---|---|---|---|---|
| Pre-infliximab | Post-infliximab | Standard care (£) | Infliximab (£) | |||
| Diagnostic procedures | 162 | 63 | 356.04 | 138.45 | 488.11 | TDC [21] |
| Examination under anesthetic | 50 | 17 | 340.29 | 115.71 | 1511.52 | TEI, TNEI [21] |
| Inpatient days | 1435 | 342 | 1761.93 | 419.91 | 272.68 | TEI, TNEI [21] |
| Outpatient visits | 555 | 534 | 231.00 | 222.27 | 92.44 | TCLFUSNFF [21] |
| Total | 2689.29 | 896.34 | ||||
- * Resource use of 205 patients for 6 months [8].
- TDC, day cases Health Resource Group (HRG) data; TEI, elective inpatient HRG data; TNEI, nonelective inpatient HRG data; TCLFUSFF, consultant-led follow-up attendance outpatient face to face.
No published estimates were available for postsurgery health states. Therefore, the resource use such as diagnostic tests, surgical procedures, and hospitalizations associated with postsurgery remission and postsurgery complications was estimated by a panel of UK gastroenterologists using the Delphi method. Each panel member estimated the resource use independently, and values used in the economic model were averages of individual estimates. Individual health states were costed using NSRC for 2006 to 2007 [21].
Outcomes
The primary effectiveness measure used in these analyses was quality-adjusted life-years (QALYs). Because of lack of available data, the model did not account for any effect of infliximab on survival.
Once again, because of the lack of pediatric data, the health state preference values were adapted from a study in adult CD patients. A data set provided by Dr. Casellas, consisting of 201 adult Spanish moderate to severe CD patients from a published study [23], formed the basis for the estimates used in this analysis. The patients' EQ-5D responses were converted into the corresponding utilities using UK tariffs [24] and were attributed to individual health states using HBI [6]. No utility for the nonresponding active state was available from literature; therefore, a utility decrement of 0.1 was assigned to this state on consultation with a panel of UK gastroenterologists.
The preference values for the surgery and postsurgical health states were estimated from a secondary care database of 41 adult CD patients in the UK using EQ-5D [25]. The utility estimates obtained immediately after surgery (<2 months) were used for the health state of surgery and those obtained two or more months after surgery were used for postsurgery remission. There were no estimates available for patients in the postsurgery complications state; therefore, a utility value equivalent to the nonresponding active state was assigned to this state. This was based on the assumption that a postsurgery complication would lead to significant hospitalization along with symptoms of infection, resulting in an impaired quality of life (QoL). The utility estimates used for the corresponding health states in both treatment alternatives were identical. The treatment benefit achieved by infliximab was thus reflected only in the difference between the transition probabilities of treatment alternatives. Table 3 provides a summary of all utility estimates employed in the economic evaluation.
| Markov model health state | Corresponding state from the source | Source | Utility estimate |
|---|---|---|---|
| Remission | Remission (HBI < 3) | [23] | 0.83 |
| Active | Active (HBI > 3) | [23] | 0.55 |
| Nonresponding active | Not available; assigned | 0.55 | |
| Surgery | Postsurgery (<2 months) | [36] | 0.73 |
| Postsurgery remission | Postsurgery (>2 months) | [36] | 0.67 |
| Postsurgery complications | Not available; assigned | 0.55 | |
| Death | — | — | |
- HBI, Harvey Bradshaw index (14).
Cost-Effectiveness Analyses
The primary cost-effectiveness measure was the incremental cost per QALY gained. Costs and outcomes were discounted to present values at 3.5% per annum [25]. Half-cycle correction was applied to reflect the transitions occurring throughout each model cycle [26]. Multiple one-way sensitivity analyses were conducted by varying parameters such as patient age and weight, time horizon, discounting rate, and the administration cost of infliximab. The uncertainty surrounding other important variables such as transition probabilities, costs, and health state utilities was explored using probabilistic sensitivity analyses (PSA) with 10,000 simulations. The uncertainty surrounding the transition probabilities and the utility estimates was presented using the beta distributions. The distributions for the utility estimates were restricted to make sure that the resultant utilities represent health states that were clinically meaningful. For example, the resultant utility of a patient in active state had to be lower than that of a patient in remission in all the simulations. The resource use costs, because derived from NHS published cost estimates, were subjected to normal distributions. The means and standard deviations derived from data sources were used to estimate the distribution parameters.
Alternate Scenarios
The following scenario analyses were conducted to address the uncertainty around important parameters.
Scenario A: no treatment effect in the extrapolation phase. The base case assumed continued efficacy of infliximab beyond the trial period of 54 weeks. In scenario A, we explored no treatment effect beyond 54 weeks, thus resulting in subsequent transitions in the infliximab arm to be identical to those in the standard care treatment arm.
Scenario B: efficacy of standard care. The base case assumed the treatment effect of infliximab in children to be identical to that observed in adult patients. The uncertainty introduced by this assumption was explored in scenario B. The cost-effectiveness analysis in scenario B was entirely based on the REACH trial. During the first 10 weeks, the combined arm of the REACH trial was assumed to represent both the standard care and infliximab treatments in our analysis. Following the randomization in REACH at the 11th week, the 12-week and 8-week treatment arms of the trial represented the standard care and infliximab treatment arms, respectively, in our analysis. Figure 2b displays the efficacy thus derived from the REACH trial and used in scenario B.
All patients in the REACH trial received infliximab throughout 54 weeks. Therefore, the cost-effectiveness estimates in this scenario are highly conservative with a minimum incremental benefit attributed to infliximab.
Results
Cost-Effectiveness Analyses
In the base case, the scheduled maintenance therapy with infliximab derived a mean additional 0.55 QALYs at a mean additional cost of £8025 compared with standard care. The incremental cost per QALY gained for infliximab versus standard care was £14,607. The corresponding incremental cost-effectiveness ratios (ICERs) for infliximab under scenarios A and B were £18,768 and £37,017, respectively, as displayed in Table 4.
| Scenario | Standard care mean costs (£) | Infliximab mean costs (£) | Standard care mean QALYs | Infliximab mean QALYs | ICERs (£) |
|---|---|---|---|---|---|
| Base case | 25,987 | 34,012 | 2.675 | 3.224 | 14,607 |
| Scenario A | 25,987 | 31,010 | 2.675 | 2.943 | 18,768 |
| Scenario B | 23,186 | 37,181 | 3.090 | 3.468 | 37,017 |
- ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
Sensitivity Analyses
The results of the one-way sensitivity analyses demonstrated that the ICERs remained in the range of £10,480 to £37,017 at 5 years (Table 5). The treatment effect of infliximab (scenario B) had the highest impact on ICER (£37,017). The impact of change in the time horizon was less significant, with the ICERs for 1 year and lifetime horizons increasing to £18,624 and £21,223, respectively. The PSA showed that the results were robust with a very small proportion of simulations resulting in the southeast quadrant where infliximab is dominated by standard care. At a willingness to pay of £30,000 per QALY, the probability of infliximab scheduled maintenance treatment being cost-effective was 78.6%. The corresponding probabilities under scenarios A and B were 56.6% and 42.7%, respectively. The cost-effectiveness acceptability curves for the base case and alternative scenarios are shown in Figure 3.
| Parameter | Base-case estimate | Sensitivity estimate | Cost/QALY† (£) |
|---|---|---|---|
| Patient weight | 40 kg with progressive weight increase | 40 kg (no weight increase) | 10,480 |
| 50 kg (with vial sharing and no weight increase) | 17,036 | ||
| 60 kg (no weight increase) | 23,592 | ||
| Time horizon | 5 years | 1 year | 18,624 |
| Lifetime | 21,223 | ||
| Discount rate | Costs—3.5%QALYs—3.5% | Cost and QALYs—1.5% | 14,747 |
| Cost—1.5% and QALYs—6% | 12,935 | ||
| Cost—6% and QALYs—1.5% | 16,144 | ||
| Cost and QALYs—6% | 14,776 | ||
| Health state utilities | Increase by 10% | 13,279 | |
| Decrease by 10% | 16,230 | ||
| Infliximab administration cost | £96.00 | £124.00 | 15,482 |
- * All results except “time horizon” assumes the time horizon of 5 years used in the base-case.
- † Base-case: cost per QALY = £14,607.
- QALY, quality-adjusted life-year.
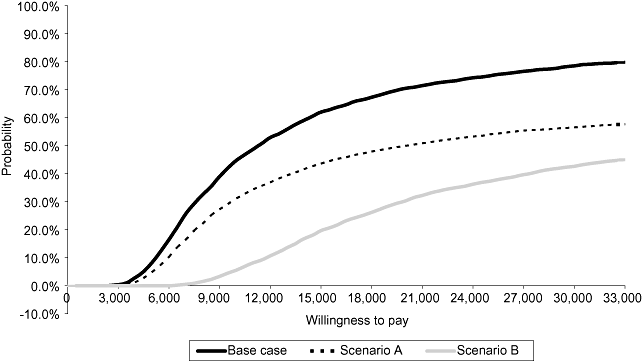
Cost-effectiveness acceptability curve in pediatric Crohn's disease.
Discussion
Scheduled maintenance treatment with infliximab is effective in achieving and maintaining remission in pediatric patients with moderate to severe active CD [9]. Infliximab trials in adults have also shown its benefit in reducing the surgeries and hospitalizations [14,27]. The purpose of this analysis was to assess the cost-effectiveness of scheduled maintenance treatment with infliximab in pediatric active CD patients over a 5-year time horizon.
Several published studies have estimated the cost-effectiveness of infliximab in adult CD patients [28–32]. To our knowledge, no study has estimated it in children. Therefore, a direct comparison of our results with the published estimates was not possible. Three of the adult studies used Silverstein patient cohort as a basis to estimate CD progression, whereas the other two used patient cohorts eligible to receive biologic to build their model frameworks. Because our study specifically focused on moderate to severe patients eligible to receive infliximab, we used the model structure and treatment pathway previously used by Lindsay and others [32]. This framework allowed inclusion of patient-level data from infliximab trials. The results of our base-case analysis suggested that infliximab is likely to be cost-effective using the National Institute of Health and Clinical Excellence (NICE) threshold of £20,000 to £30,000 per QALY [25]. The ICERs in our study are lower than most of the published estimates. This can be attributed to the observed higher efficacy in infliximab trials and lower infliximab costs in pediatric patients. In the REACH trial, 63.5% patients maintained their response and 55.8% patients maintained their remission by the end of week 54 [9]. These numbers are significantly higher compared with the efficacy observed in adult trials [14,27].
Although the base-case analysis resulted in an ICER within the acceptable limit of the NICE, the ICER for scenario B was above £30,000 per QALY. This scenario assumed minimum incremental benefit of infliximab by assigning the active treatment efficacy to standard care treatment. The efficacy thus obtained for standard care treatment is significantly higher than that observed in adult trials [14] and unlikely to be observed in clinical practice. We included this scenario in our analysis only to present the most conservative estimate of incremental treatment benefit of infliximab, which was the most important parameter affecting ICER. Therefore, this result warrants a careful interpretation of a worst-case scenario that is less likely to occur in a clinical setting.
The choice of time horizon is an important consideration. CD is a chronic condition, with more than half the patients undergoing a surgical intervention within 10 years of diagnosis [33]. This warranted analysis over a longer time horizon. Nevertheless, the efficacy estimates were derived from the trials with short treatment duration of 54 weeks. Therefore, in the base case, we conducted analysis over a period of 5 years. A comparison of a previous economic analysis with an observational study in adult CD patients indicated that infliximab was able to maintain its efficacy beyond the trial period, at least up to 4 years [32], and thus supports our assumption. Nevertheless, no such comparable data were available in children, and in the absence of any such comparison, we may have overestimated infliximab efficacy in the base case. This assumption was tested in scenario A. The worst-case scenario A assumed no treatment effect beyond 54 weeks. The resultant infliximab ICER of £18,768 further supported the efficacy extrapolation in the base case.
The other important parameter affecting infliximab efficacy was the choice of the data sources. We derived infliximab efficacy based on two adult and a single pediatric trial. In the absence of a placebo treatment arm in the REACH trial, we used placebo data from an adult trial to estimate the efficacy of the standard care treatment arm in our analysis [14]. Such comparisons have previously been drawn by regulatory authorities, including the Food and Drug Administration, while granting license to infliximab in the pediatric CD population [34]. We also explored other possible approaches such as meta-regression but decided to pursue a simplistic approach because of the heterogeneity in infliximab trials. This mix and match approach may have contributed to uncertainty around infliximab's absolute and/or relative treatment effect and we explored that through the scenario analyses and sensitivity analyses. In scenario B, we used efficacy estimates derived from the infliximab 12-week dosing arm of the REACH trial as proxy for standard care efficacy estimates. In the REACH trial, all patients received the full induction dose and the responders were randomized at week 10. This meant that in scenario B, the treatment effect of the induction dose was identical in the infliximab and standard care treatment arm. This may have significantly reduced the relative treatment effect of infliximab compared with standard care. In our sensitivity analysis, only in this very conservative scenario, infliximab ICER exceeded the acceptable limit of cost-effectiveness.
The other important parameters affecting ICERs were health state preferences and health state costs. Although the utility distributions were adjusted to avoid counterintuitive results, a small proportion of simulations (9.58%) in the PSA resulted in a negative incremental QALY gain. This is contrary to the trial evidence found in adult trials where infliximab has been shown to be effective in achieving and maintaining remission and associated QoL benefit. Again, this may be attributable to the multiple data sources used to derive efficacy estimates as well as health state preferences. The efficacy estimates for a true standard care treatment were not available in the REACH trial. Therefore, the efficacy estimates from the adult trials were used. The REACH trial also did not capture any QoL data. Health state preferences were available from other published studies that did not conform with the NICE's analysis framework within which this analysis was conducted [28,31,35]. Therefore, we used Casellas et al. patient cohort to derive presurgical utilities and the Health Outcomes Data Repository (HODaR) data set to derive postsurgery utilities [36]. This may have introduced uncertainty resulting in negative QALY gains for active treatment.
We estimated the health state resource use from the Jewell et al. study [8]. The study compared resource use among CD patients before and after introduction of infliximab in UK. Similar estimates were also available from two other studies [37,38]. We, however, preferred the Jewell et al. study as it represented UK clinical practice. The health states where resource use was unavailable were estimated by a panel of UK gastroenterologists. These experts represented major gastroenterology centers across UK and their estimates were subsequently pooled to derive mean values used in the analysis. This simplistic approach may have introduced uncertainty around the resource use estimates, which was further explored in PSA. The PSA showed that the predicted results of our model were comparable to other published estimates. The study by Saro and others found surgery rates of 9.8% and 4% 1 year prior and post-infliximab use. The corresponding rates in our model were 5.5% and 2.8% at the end of the first year [38]. In spite of this, the choice of resource use estimates and the elicitation method used to derive them where unavailable may have introduced uncertainty in the results and this remains a limitation of this analysis.
In conclusion, the economic analysis demonstrated that despite the uncertainty surrounding the results, infliximab scheduled maintenance treatment is likely to be a cost-effective treatment alternative for children suffering from moderate to severe CD.
Source of financial support: This study was funded in full by Schering-Plough, UK, and the writing of this article was funded in part by Schering-Plough, UK. Writing support was provided by Global Health Solutions and funded by Schering-Plough, UK. Yogesh Punekar is an employee of Schering-Plough, UK. Thomas Sunderland completed his summer internship from the University of Sheffield at Schering-Plough, UK. Neil Hawkins has served as a consultant and an advisory board member for Schering-Plough, UK. James Lindsay has served as a speaker, a consultant, and an advisory board member for Schering-Plough, Abbott Laboratories, and Shire UK, and has received research funding from Schering-Plough, Abbott Laboratories, and UCB Pharma.




