Cost-Effectiveness of Using Recombinant Human Thyroid-Stimulating Hormone before Radioiodine Ablation for Thyroid Cancer: The Canadian Perspective
ABSTRACT
Objectives: Radioiodine ablation for the treatment of thyroid cancer is traditionally performed after preparing patients by inducing hypothyroidism. Exogenous stimulation of thyroid-stimulating hormone (TSH) using recombinant human TSH (rhTSH) avoids hypothyroidism and hastens the clearance of radioiodine from the patient. These advantages are achieved without jeopardizing the success rate of remnant ablation. An economic analysis was performed to place the increased acquisition cost of rhTSH in the context of the health benefits achieved and the earlier discharge from radioprotection.
Methods: Markov modeling, using 17 individual weekly cycles, was used to assess the incremental cost per quality-adjusted life-year (QALY) associated with exogenous stimulation. Clinical inputs were largely sourced from a multicenter, randomized, controlled trial comparing remnant ablation success after either rhTSH or hypothyroid preparation. The model applied Canadian unit costs, taking a societal perspective. Additional costs associated with rhTSH were considered in the context of the clinical benefits and cost offsets. These included avoidance of hypothyroidism, increased work productivity, earlier administration of ablation after surgery, and earlier discharge from the radio-protective ward because of faster radioiodine clearance following rhTSH preparation. The model duration avoided the need for discounting.
Results: The additional benefits of rhTSH (0.0576 QALY) are obtained with an incremental cost of CDN$87, generating an incremental cost per QALY of CDN$1520. Deterministic one-way and two-way sensitivity analyses demonstrated the result to be robust.
Conclusions: The use of rhTSH before radioiodine ablation represents a reasonable allocation of costs, with the benefits to patients, hospitals, and society as a whole, obtained at modest cost.
Introduction
Surgical intervention using thyroidectomy followed by 131I radioiodine ablation remains the standard treatment for stages 3 and 4, as well as many patients with stages 1 and 2 differentiated thyroid cancer [1]. The aim of the radioiodine ablation is to destroy thyroid remnants through selective uptake of iodine into the thyroid tissue. This is traditionally achieved by rendering patients hypothyroid by denying thyroid hormone replacement therapy with thyroxine (T4) from thyroidectomized patients for several weeks before ablation. Although inducement of hypothyroidism elevates serum thyroid-stimulating hormone (TSH) such that radioiodine uptake by remnants is optimal [2], it is associated with significant morbidity. Commonly encountered problems include severe lethargy and fatigue, cognitive problems, constipation, cold intolerance, and depression in the young, and ataxia, ambulation problems, falls, and cardiac and renal problems in the elderly. The severity of symptoms often translates to an inability to perform normal activities [3–6]. Additionally, there is a need to provoke a threshold level of hypothyroidism before the patient can be treated with radioiodine ablation (TSH typically >25–30 mU/L), which in practice can take anywhere from 3 to 8 weeks to achieve after surgery. This variability causes scheduling problems for hospital administrators, particularly where access to radio-protective beds is limited.
Alternatively, exogenous stimulation of TSH using recombinant human TSH (rhTSH) allows patients to commence thyroid hormone replacement therapy immediately after thyroidectomy, and therefore avoid hypothyroidism. Additionally, this offers hospitals and patients clarity with respect to scheduling radioiodine ablation. Investigators have shown that exogenous rhTSH can sufficiently stimulate 131I uptake in thyroid remnants to result in successful remnant ablation [7–10]. Additionally, a multicenter, randomized, controlled trial has demonstrated 100% successful ablation after 8 months of follow-up according to a predefined criterion (i.e., no visible thyroid bed activity or <0.1% 131I uptake), regardless of stimulation method [11,12]. A secondary criterion for successful ablation (a stimulated serum thyroglobulin level of <2 ng/ml 8 months after ablation) favored the euthyroid group (96 vs. 86%) [11]. Furthermore, the same study demonstrated considerable quality-of-life benefits associated with exogenous rhTSH stimulation, with a significant difference in the change between baseline and ablation between study arms in five of the eight domains measured (P < 0.05) [11,12].
In addition to an improvement in quality of life, exogenous stimulation results in faster clearance of 131I from the body after radioiodine ablation, presumably by avoiding the renal impairment associated with hypothyroidism [12–14]. This has translated to a shorter time required in the radio-protective ward in several hospitals, and less overall radiation exposure to the patient [15,16].
Exogenous stimulation with rhTSH, however, is associated with an additional acquisition cost relative to endogenous stimulation. To contextualize the increased cost, an economic analysis was performed capturing all costs accruing in the period between thyroidectomy and recovery from ablation, as well as the differences in quality of life associated with its use. In doing so, the value for money can be accurately examined.
The current model, an adaptation of an earlier German-based model [17], relates specifically to the Canadian setting, capturing local management of thyroid cancer patients. In addition, some Canadian institutions allow radioiodine ablation with up to 150 mCi to be performed as an outpatient service, provided the patient meets certain criteria [18]. This is notably different to the radiation regulations applicable in the previous model [17], and therefore this scenario is also investigated here.
Methods
Model Structure
Economic modeling was undertaken to demonstrate the value of rhTSH before ablation in terms of the extra costs relative to patient benefits. These benefits were expressed in units of quality-adjusted life-years (QALYs) using “utility weights” from 0 (death) to 1 (perfect health) derived from SF-36 data, a method common in health economics. By using a common metric, the value for money of rhTSH can be compared against other health technologies. A societal approach, rather than that of a single payer, ensured that all relevant costs and outcomes were captured.
A Markov model was used to compare the expected costs and outcomes of patients with low-risk thyroid cancer who would nevertheless require radioiodine ablation, and who are prepared with exogenous stimulation (with rhTSH), against those prepared with endogenous stimulation (with hypothyroidism resulting from the withdrawal or withholding of thyroid hormone). Markov modeling is a popular methodology in health economics because of its ability to follow patients as they move between health states over time.
The model was run for a total of 17 cycles of 1 week in length. The 17-week model duration is a departure from the previous German version [17] which took a lifetime perspective and encompassed the theoretical advantage of a reduction in secondary malignancies, a potential benefit of radioablation with rhTSH because of less total body radiation exposure. This simplification was motivated by the lack of influence the secondary malignancies had on the cost-effectiveness results [17]. Here, a shorter-term focus reduces uncertainty associated with long-term extrapolation, while still capturing all cost and outcome differences between thyroidectomy and recovery from ablation.
The model relates only to the treatment of low-risk, radioablation-requiring, thyroid cancer patients similar to those in the pivotal trial, which included patients with a T2, N0/N1, M0 or a T1, N1, M0 classification [12]. Patients with distant metastases detected any time up to, and including, the post-ablation scan were excluded from the model.
Four discreet health states were modeled in each of the two arms (Table 1). Following thyroidectomy, patients receive either exogenous rhTSH (Thyrogen, Genzyme Corporation, Cambridge MA) and remain euthyroid, or undergo a period of hypothyroidism induced by withholding TSH to raise their serum TSH. All patients receive thyroid replacement therapy following ablation. A schematic of the model is presented in Figure 1.
| Health state | Description |
|---|---|
| Preablation | Patient has received successful thyroidectomy and is undergoing a waiting period before ablation (1 week in the euthyroid arm, and between 2 and 7 weeks in the hypothyroid arm) |
| Ablation | Patient undergoes radioiodine ablation procedure (1 week comprising hospitalization and brief recovery period) |
| Postablation | A recovery period following ablation in which utility weights remain lower than complete recovery. Postablation is divided into two periods. The first period has a lower utility than the second, so as to capture a gradual recovery to the well health state (two sequential 4-week periods) |
| Well | Patient has recovered from thyroidectomy and ablation (7 weeks in the euthyroid arm, and between 1 and 6 weeks in the hypothyroid arm) |

Simplified schematic of the model structure.
A half-cycle correction was appropriately omitted on the basis that the model structure is such that individuals cannot move between health states during either the first or last cycle of the model.
Costs and outcomes were accrued at the end of each cycle, depending on the health state of the individual at that point in time. For simplicity, neither costs nor outcomes were subject to discounting, as the model duration is less than 1 year.
The analysis of the model included a number of sensitivity analyses aimed at testing the impact of key assumptions/areas of potential uncertainty, and to better understand the main drivers of the results. Note that the inputs required to reasonably conduct a probabilistic sensitivity analysis were not available for use.
The model as a whole was subject to internal review by a health economist not directly involved in the project. All inputs and calculations were checked. No clinical validation process was required because of clinical success being assumed in both arms of the model. Nonetheless, the Markov trace was examined to ensure logical movement of the cohort from one health state to another in each arm over the total duration of the model.
Clinical Inputs
Clinical inputs were sourced primarily from the pivotal multicenter, randomized, controlled trial [11,12] supplemented with information from the published literature.
The time spent in the model was quality adjusted using utility weights. Utility weights for the pre- and postablation health states that differ between the arms of the model were obtained from the pivotal controlled clinical trial. The SF-36 trial data [12] were transformed into utility weights using the SF-6D method [19] (Fig. 2). Table 2 lists the various utility weights, while Figure 2 presents a visual summary of the utility weights associated with each of the health states.
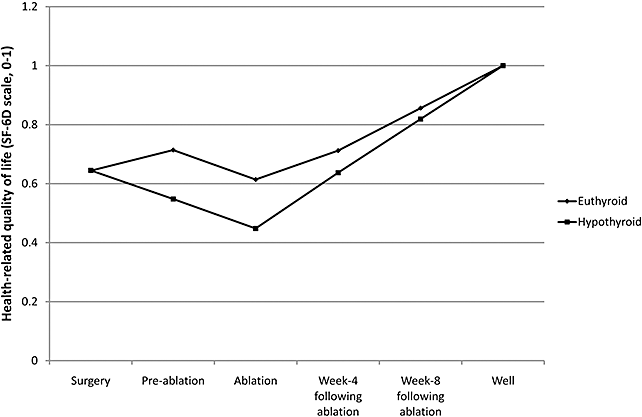
Health-related quality of life (SF-6D) over time.
| Input | Exogenously stimulated (rhTSH) arm | Endogenously stimulated (hypothyroid) arm | Notes | Source |
|---|---|---|---|---|
| Thyroid cancer treatment | ||||
| Radioiodine ablation success rate | 100% | 100% | <0.1% Uptake in thyroid bed at 8 months post-ablation | Pacini et al. [12] |
| Utility weights (annualized) | ||||
| Preablation | 0.714 | 0.548 | SF-6D data derived from week 4 SF-36 data | Pacini et al. [12] |
| Ablation | 0.614 | 0.448 | Preablation—0.1 | Assumption |
| 0–4 weeks postablation | 0.712 | 0.637 | SF-6D results derived from 1-month postablation SF-36 data from pivotal trial | Data on file (Genzyme Corporation) |
| 4–8 weeks postablation | 0.856 | 0.819 | Average of “0–4 weeks postablation” and “well” | Assumption |
| Well | 1.00 | 1.00 | Conventional value attributed to perfect health | Convention |
- rhTSH, recombinant human thyroid-stimulating hormone; SF-36, short-form 36; SF-6D, short-form 6 dimension.
Quality-of-life differences between the two stimulation methods are well supported by the pivotal trial data [12]. In the rhTSH-stimulated group, the SF-36 score increased from baseline in seven of the eight domains by the time of ablation; in the hypothyroid group, however, the SF-36 scores declined in seven of the eight domains. The change from baseline was significantly different between groups in five of the eight domains (P < 0.05). This implies that hypothyroid patients felt worse weeks later (before ablation) than they had immediately after surgery—a finding in keeping with clinical observation. Similar results were previously reported for rhTSH in the diagnostic setting, with a significant difference in all eight domains of the SF-36 (P < 0.0001) [20].
As described previously, clinical success of ablation was unrelated to preparation method. The model assumed successful ablation in all patients, according to the predefined criterion of the pivotal trial [12]. As in previous modeling [17], there was assumed to be no difference in longer-term recurrence rates. This has since been confirmed in long-term follow-up of the patients who took part in the pivotal randomized, controlled trial [21,22].
Potential adverse events were not included in the economic model. Although a number of common adverse events such as headache, fatigue, vomiting, and dizziness do occur in clinical practice, these are usually transient and have little if any impact on either quality of life or on cost. Rarely, rhTSH may cause a serious problem such as swelling of an unrecognized brain metastasis, although this can usually be prevented by proper screening because patients with distant metastasis are not eligible for rhTSH use.
Resource Use and Cost Inputs
Canadian resource use and unit costs were sourced with a societal perspective (see Table 3). The most up-to-date costs were sourced in all instances, with all costs as published in either 2007 or 2008. It is not possible, however, to state with certainty the cost year of the analysis as the information to do so was not consistently available from these sources.
| Resource | Unit cost ($) | Remarks | Source |
|---|---|---|---|
| Ablative dose of radioiodine (131I) to treat thyroid cancer (post-thyroidectomy) | 1424.00 | OCCI, August 2007 | |
| Whole-body scan using radioiodine | 297.25 | Inclusive of an associated $50.50 physician fee | London Health Science Centre, August 2007 |
| One day of hospital (inpatient) for patients receiving radioiodine ablation | 1000.00 | London Health Science Centre, August 2007 | |
| Visit to specialist (radiation oncologist)—initial assessment | 83.25 | OHIP Schedule of Physician Services, April 2008 | |
| Visit to specialist (radiation oncologist)—medical-specific reassessment | 45.90 | OHIP Schedule of Physician Services, April 2008 | |
| Visit to general practitioner—general assessment | 61.00 | OHIP Schedule of Physician Services, April 2008 | |
| Visit to general practitioner—general reassessment | 30.70 | OHIP Schedule of Physician Services, April 2008 | |
| TSH quantation | 14.48 | OHIP Schedule of Benefits for Laboratory Services, April 2008 | |
| Serum thyroglobulin count | 20.68 | OHIP Schedule of Benefits for Laboratory Services, April 2008 | |
| Tg antibody test | 15.00 | Pak Cheung Chan, chief biochemist, Sunnybrook Health Science Centre, personal communication | |
| Average daily wage | 146.92 | Statistics Canada, June 2007 | |
| Daily T4 drug cost (100 µg) | 0.03 | Cost of Eltroxin used | ODB formulary, August 2007 |
| rhTSH (two ampoules of Thyrogen®) | 1324.00 | ODB formulary, August 2007 |
- A further cost incurred by patients is a low-iodine diet, although this has been excluded on the basis that it is equal in both arms.
- 131I, iodine-131; OCCI, Ontario Case Costing Initiative database; ODB, Ontario Drug Benefit; OHIP, Ontario Health Insurance Plan; rhTSH, recombinant human thyroid-stimulating hormone; T4, thyroxine; Tg, thyroglobulin; TSH, thyroid-stimulating hormone.
Note that the cost of the low-iodine diet recommended before radioiodine ablation was not included as it is common to both arms and does not impact on the results.
The model captured the difference in time spent in the radio-protective ward on the basis of faster clearance of 131I from the body after radioiodine ablation following exogenous stimulation [12–14,23]. A survey was conducted for this study among clinicians practicing in Canada (n = 24, treating 1884 patients) to estimate the time spent in radio-protective conditions by endogenously stimulated patients. The most common lengths of stay in the radio-protective ward in the survey were 1 day (28.45%), 2 days (40.78%), and 3 days (26.50%), with an average of 2.05 days across all survey respondents when the results were weighted by the number of patients treated by each respondent. On the basis of the 35% lower dose of radiation to the blood in exogenously stimulated patients [12], the model assumed the duration of hospitalization was, on average, one-third less in that arm of the model (1.37 vs. 2.05 days). Although there are no data to specifically support this assumption, it is in keeping with estimates reported elsewhere [15,16]. Furthermore, there is no expectation that the Canadian experience would be any different as patients in Canada are typically discharged soon after the period of isolation is lifted for financial reasons. In institutions permitting radioiodine ablation to be performed on an outpatient basis, this cost offset will not apply. On this basis, a sensitivity analysis was performed to investigate the cost-effectiveness in such a situation.
The same survey was used, again by calculating a weighted average, to determine the current interval between thyroidectomy and ablation for endogenously stimulated patients in Canada, as the pivotal trial could not be relied upon to give a reliable estimate for the Canadian perspective. The model used these survey results to account for variation between patients (Table 4). The temporary use of triiodothyronine (T3) during this pre-ablation period was not included in the economic model as this was used for greater than 50% of treated patients by only 6 of the 24 clinicians in the survey. Furthermore, the pivotal clinical trial did not represent the situation where T3 was used. With respect to exogenously stimulated patients, radioiodine ablation was assumed to occur closer to the time of thyroidectomy. This assumption was based on clinical opinion that ablation would occur earlier because: 1) time to elevate TSH levels endogenously is not required when exogenous stimulation is provided; and 2) timely ablation of any cancer remnants to reduce the risk of spread or recurrence is an important clinical aim, although this is controversial with respect to low-risk patients with well-differentiated thyroid cancer. Nonetheless, at least a week is typically required for adequate recovery from thyroidectomy.
| Duration of time between thyroidectomy and ablation | Proportion of patients estimated in clinician survey (%) | Remarks |
|---|---|---|
| Less than 3 weeks | 0.90 | Assumed to be 2 weeks for all patients |
| 3 weeks | 6.48 | |
| 4 weeks | 12.50 | |
| 5 weeks | 10.85 | |
| 6 weeks | 21.15 | |
| Greater than 6 weeks | 48.12 | Assumed to be 7 weeks for all patients |
A number of investigators reported reduced productivity and increased absenteeism in the hypothyroid state compared with the euthyroid state [3–6]. Similar to the earlier model [17], and based on the literature [12], a 50% reduction in the number of work days lost for exogenously stimulated patients relative to endogenously stimulated patients was applied. The difference is particularly important, as thyroid cancer typically affects people in the prime of their productive work lives [24]. The model adopted a friction cost method [25] for calculating the impact this has on the economy's productivity by assuming that only 80% of productivity is lost with absence from work with the remainder made up upon return to work or by coworkers. This assumption is based on Koopmanschap et al. [26], which estimated that, in the case of short-term absences from work, a 10% decrease in labor time leads to an 8% fall in production. This is tested in the sensitivity analyses. Because a societal perspective is adopted, the model acknowledges the productive output of all individuals by valuing the productive time lost of all, regardless of employment status. This was achieved by using the average Canadian wage rate as a proxy for the productivity loss. That is, the average daily wage is multiplied by 80%, without adjustment for workforce participation and unemployment rates, to capture the magnitude of the loss. It is acknowledged, however, that the inclusion of productivity loss in the model risks double-counting a portion of the productivity loss, because the SF-36 includes two dimensions related to the impacts upon professional life. Unfortunately, the extent of any double-counting is unknown and cannot be accounted for. It should be noted, however, that the friction cost method ensures the impact of double-counting is minimized relative to other methods, such as the human capital approach, because it generates a lower estimate of the loss of productivity.
The rhTSH cost used in the model was based on a daily intramuscular injection dose of 0.9 mg over a 2-day period before radioiodine ablation. There is no requirement for monitoring following an rhTSH injection. All other costs associated with administration were included in the costs applied to each health state, as shown in Table 5.
| Resource | Unit cost ($) | Resources consumed, exogenously stimulated (rhTSH) arm | Resources consumed, endogenously stimulated (hypothyroid) arm | Cost per health state, exogenously stimulated (rhTSH) arm ($) | Cost per health state, endogenously stimulated (hypothyroid) arm ($) |
|---|---|---|---|---|---|
| Preablation | |||||
| T4 thyroid hormone replacement therapy | 0.03 | 7 | — | 0.23 | — |
| TSH test | 14.48 | 1 | 1 | 14.48 | 14.48 |
| Tg test | 20.68 | 1 | 1 | 20.68 | 20.68 |
| Tg antibody test | 15.00 | 1 | 1 | 15.00 | 15.00 |
| Specialist visit—initial consultation | 83.25 | 1 | 1 | 83.25 | 83.25 |
| Specialist visit—repeat consultation | 45.90 | 1 | 1 | 45.90 | 45.90 |
| GP visit—general assessment | 61.00 | 1 | — | 61.00 | — |
| GP visit—general reassessment | 30.70 | 1 | — | 30.70 | — |
| Productivity loss | 117.54 | 5.5 | 11 | 646.47 | 1292.94 |
| rhTSH | 1324.00 | 1 | — | 1324.00 | — |
| Total cost of preablation | 2241.71 | 1472.25 | |||
| Ablation | |||||
| 131I ablation | 1424.00 | 1 | 1 | 1424.00 | 1424.00 |
| T4 thyroid hormone replacement therapy | 0.03 | 7 | — | 0.23 | — |
| Hospitalization in radio-protective ward | 1000.00 | 1.37 | 2.05 | 1366.67 | 2050.00 |
| Specialist visit—repeat consultation | 45.90 | 2 | 2 | 91.80 | 91.80 |
| Whole-body scan | 297.25 | 1 | 1 | 297.25 | 297.25 |
| Total cost of ablation | 3179.95 | 3863.05 | |||
| Post-ablation | |||||
| T4 thyroid hormone replacement therapy | 0.03 | 56 | 56 | 1.84 | 1.84 |
| Specialist visit—repeat consultation | 45.90 | 1 | 1 | 45.90 | 45.90 |
| Total cost of postablation | 47.74 | 47.74 | |||
| Well | |||||
| T4 thyroid hormone replacement therapy | 0.03 | 14.77 | 49 | 1.61 | 0.48 |
| Total cost of “well” | 1.61 | 0.48 | |||
| Total cost | 5471.00 | 5383.52 | |||
- 131I, iodine-131; GP, general practitioner; rhTSH, recombinant human thyroid-stimulating hormone; T4, thyroxine; Tg, thyroglobulin; TSH, thyroid-stimulating hormone.
Results
The results of the economic model are presented in Table 6, with the base case relating to Canada in toto, and the primary sensitivity analysis relating to the specific situation where radioiodine is administered as an outpatient service. The remaining deterministic one-way and two-way sensitivity analyses are presented in Figure 3.
| Parameter | Exogenously stimulated (rhTSH) arm | Endogenously stimulated (hypothyroid) arm | Incremental difference |
|---|---|---|---|
| Canada-wide: | |||
| Average cost incurred, per patient ($) | 5,471 | 5,384 | 87 |
| Average total QALY over course of model, per patient | 0.2808 | 0.2232 | 0.0576 |
| ICER | $1,520 per QALY gained | ||
| Where ablation performed as outpatient, so no time spent in radio-protective ward: | |||
| Average cost incurred, per patient ($) | 4,104 | 3,334 | 771 |
| Average total QALY over course of model, per patient | 0.2808 | 0.2232 | 0.0576 |
| ICER | $13,391 per QALY gained |
- ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; rhTSH, recombinant human thyroid-stimulating hormone.
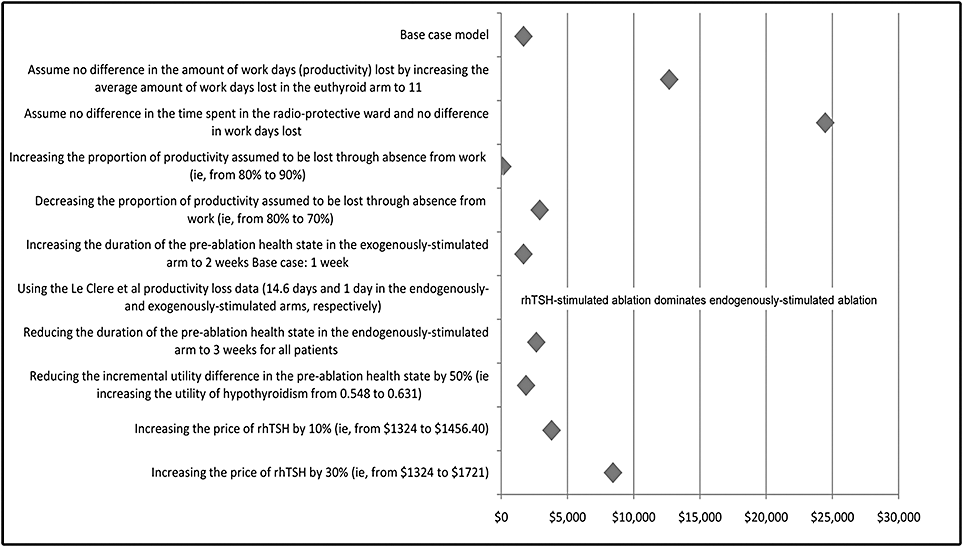
Other sensitivity analysis.
Note that a one-way deterministic sensitivity analysis aimed at examining the impact of a change to the number of productive days lost because of ablation shows rhTSH preparation dominating exogenously prepared stimulation. By using alternative values from LeClere et al. [4] (1 day in the rhTSH arm, and 14.6 days in the endogenously stimulated arm), rhTSH preparation is shown to save an average of $854.59 per patient. This result is discussed in greater details below.
Discussion
The economic model demonstrates that the additional health benefits of using rhTSH in preparation for radioiodine ablation are obtained with minimal incremental cost to society ($87 per patient). This incremental cost is modest when considered relative to the total cost of the initial surgical and radio-ablative treatment of thyroid cancer. Cost offsets associated with use of rhTSH, including earlier discharge from radio-protective care and a reduction in the number of productive days lost because of symptoms of hypothyroidism, drive the low cost. The modest net cost of exogenous stimulation must be considered in the context of the health benefit it provides. Avoiding hypothyroidism by using exogenous stimulation results in a substantial improvement in patient quality of life, translating to an incremental benefit of 0.0576 QALY over a 17-week period (or one-third of a year). The incremental cost and benefit generate an incremental cost-effectiveness ratio (ICER) of $1520/QALY. In Canada, new health-care interventions are almost universally accepted if the cost/QALY is less than $20,000, and are also considered to be fundable if the cost/QALY lies between $20,000 and $100,000 [27]. The use of rhTSH, therefore, represents a highly cost-effective technology (i.e., the additional benefits to the patient and society are procured for an acceptable net cost).
The sensitivity analyses demonstrate the base case result and conclusions drawn are robust. One-way sensitivity analyses indicate the model is most sensitive to the difference in time spent in the radio-protective ward. The primary sensitivity analysis indicates an ICER of $13,391 when patients receive their radioiodine as outpatients, that is, with no difference in the time spent under radio-protective conditions, although patients are usually isolated at home for 48 hours. In this situation, the ICER remains below the $20,000 threshold [27]. The model is also sensitive, albeit less so, to the difference in the number of productive days lost. Similarly, reducing the length of the pre-ablation health state to 3 weeks for all patients in the endogenously stimulated arm results in only a modest increase in the ICER ($2678). This may be more indicative of the result in those institutions that temporarily use T3 to shorten the period of hypothyroidism, albeit without the inclusion of the T3 cost, which would further reduce the ICER. A two-way sensitivity analysis considered the simultaneous impact of entirely removing both the difference in time spent in the radio-protective ward and productive days lost. This generated an ICER of less than $25,000/QALY. Even under these extreme assumptions, therefore, exogenous stimulation using rhTSH remains acceptable value for money [27].
In addition to the health benefits to the patient, the productivity benefits are also important to broader society, and warrant further discussion. Data from the diagnostic follow-up setting are useful in that they are free of the influence surgery or ablation per se may have on productive capacity, that is, they are likely because of hypothyroidism alone. Estimates of work days lost in the diagnostic setting range from 11 to 13 days [4–6]. Of these, one study [4] reporting paired data for a subgroup of patients who received both stimulation methods, showed a mean of 14.6 days lost in the hypothyroid group, and 1.0 day for the rhTSH group (P < 0.0001). There are no comparative published data available in the ablation setting, although the duration and intensity of hypothyroidism are similar to the diagnostic setting. The pivotal trial similarly fails to provide insight, instead reporting that 15.6% of those rendered hypothyroid experienced a marked reduction in their ability to perform normal activities compared with 8.6% in the rhTSH arm [12]. This differential formed the basis of the 50% difference applied to the Luster et al. [5] data in the current model. The paired data [4] suggest, however, that the model may underestimate the difference and generate an overly conservative estimate. For instance, some hypothyroid patients may not return to work at all in the period between thyroidectomy and ablation. This would further improve the value of rhTSH.
The modeled economic evaluation relied upon translation of SF-36 trial data to utilities using the SF-6D method [19]. There is a potential bias in using this method because of the “floor effect,” which restricts possible utility estimates such that they cannot lie below 0.3. In this case, however, this is unlikely to be problematic given that the utility weights of the health states included in the model are well above this threshold. It could be reasonably expected, therefore, that the “floor effect” has not had an impact on the estimates used. Unfortunately, no alternative utility estimates, such as EQ-5D for example, were available to test the potential impact of the SF-36 data on the final cost-effectiveness results. It would not be expected, however, that estimates derived via an alternative methodology would alter the conclusions drawn.
The model presented the incremental cost-effectiveness associated with exogenously stimulated preparation for ablation using rhTSH in the context of Canada. The method represents a departure from the earlier, longer-term model [17], as extrapolation of the model to account for a theoretical reduction in secondary malignancies had no impact on the conclusions drawn. The model captured all the measurable benefits of rhTSH in the ablative setting nonetheless. Unquantifiable benefits offered by the greater flexibility of ablation scheduling offered by rhTSH and possibly a lessening of acute radiation effects on salivary and lacrimal function resulting from the lower radiation exposure with rhTSH are not included in the model. Additionally, some of the more salient clinical benefits previously reported [17], for which more data are required, remain outside the scope of the model. Additional to these, the model could benefit from future research on the relative merits of rhTSH with respect to productivity loss in the ablative setting and the duration of time spent in the radio-protective ward. Although these were estimated in the best way possible, controlled, head-to-head data would be ideal. It is not expected, however, that inclusion or improvement of any of these factors would alter the conclusion of rhTSH's value for money in Canada.
Source of financial support: Genzyme Corporation provided funding to Health Technology Analysts for the development of the economic model and the application of this model to the Canadian setting. None of the authors have a financial interest in Genzyme Corporation. Dr. Silverberg has received speaker's fees from Genzyme in the past, and has received funds for participating in an Advisory Board.




