Impact of Body Mass Index on the Incidence of Cardiometabolic Risk Factors in Ambulatory Care Settings over 5 Years or More
Current address: Endo Pharmaceuticals, Chadds Ford, PA, USA
ABSTRACT
Objective: This study was performed to evaluate the association of body mass index (BMI) with the incidence of cardiometabolic risk factors in ambulatory care electronic medical records (EMRs) over 5 years or more.
Design: A retrospective cohort of normal versus obese patients.
Subjects: Subjects ≥18 years were identified between 1996 and 2005.
Measurements: Patients were categorized as either normal weight (18 kg/m2 < BMI ≤ 27 kg/m2) or obese (BMI > 27 kg/m2) based on baseline BMI (measured 395 days or more after first EMR activity). Outcomes included development, at least 180 days after the first BMI reading date, of four cardiometabolic risk factors (elevated triglycerides, low high-density lipoprotein cholesterol [HDL-C], hypertension, or type 2 diabetes) determined from ICD-9 code, prescribed drug, or biometric reading. Logistic regression estimated the odds of developing cardiometabolic risk factors, alone and combined for normal versus obese patients forward for at least 5 years.
Results: Seventy-one percent were female, mean age was 43.5 years, and 37.6% had a baseline BMI > 27 kg/m2. Comparing obese versus normal weight patients, adjusted odds ratios for the incidence of elevated triglycerides, hypertension, diabetes, and low HDL-C were 2.1 (95% confidence interval [95% CI] 1.9–2.3), 2.2 (95% CI 2.1–2.4), 2.3 (95% CI 2.0–2.7), and 2.2 (95% CI 2.0–2.4), respectively. Adjusted odds ratios of developing one and all four new risk factors were 1.9 (95% CI 1.8–2.1) and 7.9 (95% CI 5.9–10.5), respectively.
Conclusion: Obese patients are approximately twice as likely to develop cardiometabolic risk factors compared with those having normal weight over 5 or more years.
Introduction
Obesity is associated with insulin resistance, and the combination of obesity and reduced glucose tolerance is an important underlying risk factor to the development of dyslipidemia [1]. A recent analysis of the Medical Expenditure Panel Survey (MEPS) found that diabetes and dyslipidemia occur much more frequently in obese patients than in nonobese patients. Relative to nonobese patients, the prevalence of diabetes plus dyslipidemia was two and a half times higher in obese patients aged 70–79 (prevalence ratio = 2.54), and almost seven times higher in obese subjects aged 20–29 (prevalence ratio = 6.94) [2]. Obesity and weight gain are also associated with the development of hypertension. Among adult women, weight at age 18 and weight gain after that age were positively associated with development of hypertension (P-value for trend <0.001) [3].
Several other studies have demonstrated the association between obesity and development of other components of cardiometabolic risk [4–8]. Nevertheless, most studies report cross-sectional data on coexistence of cardiometabolic risk factors with weight. Further, most of these studies have been conducted in small populations or as a randomized controlled trial, and are not representative of the United States as a whole. Therefore, there is a need to investigate the effect of existing obesity on the incidence of cardiometabolic risk factors over time using a large national database in a real-world setting.
The primary aim of this study was to investigate the relationship between body mass index (BMI), measured at a baseline, and the subsequent development of cardiometabolic risk factors in broadly representative ambulatory care settings. Specifically, the study examined the progression toward cardiometabolic risk factors over a period of at least 5 to 8.5 years using a large, nationally representative electronic medical record (EMR) database.
Methods
Data Source
This study utilized data from the General Electric Centricity Electronic Medical Record (GE EMR) research database (GE Healthcare, Waukesha, WI). The GE EMR, a deidentified, Health Insurance Portability and Accountability Act compliant database, is comprised of longitudinal patient data and includes, but is not limited to, demographic information, vital signs, laboratory orders and results, medication list entries and prescribed medications, and diagnoses or problems.
Over 100 physician practice sites located in 35 states that participate in the Medical Quality Information Consortium submit GE Centricity EMR data. Consortium members represent a variety of ambulatory care practice types including solo practices, group practices, community clinics, academic medical centers, and large integrated delivery networks. At the time of the study (1996–2005), the GE EMR held data on 3.3 million patients contributed by over 5000 physicians. Data are centrally collected and undergo a quality control process to remove or correct invalid data.
An analysis of patients in the EMR database in 2005 indicated that the population is similar to the US population based on the 2005 US Census estimates, data from the National Health and Nutrition Examination Survey 2003–2004, and the National Health Interview Survey 2005 [9]. Nevertheless, the GE EMR has a higher representation of women, and patient data are available for only 35 of 50 US states with a higher representation of northeastern and midwestern states.
Study Population
Subjects with clinical activity were identified from the GE EMR database from 1996 to 2005. Subjects were included in the analysis if 18 years of age or older on or before the patient's first activity date with at least one BMI reading measured 395 days or more after the first activity date (defined as the date the patient first became active in the EMR for a specific office site indicating an interaction with the physician such as an office visit, a telephone consult, or a prescription order).
The first BMI reading recorded after 395 days of activity was defined as the baseline BMI and the study index date for the patient. A preindex BMI window of 395 days or more was included to allow for identification of baseline risk factors which may have only been recorded in the EMR on an annual basis. An additional 30-day period was added to a 1 year window to allow time for a prescription which had expired at the pharmacy to be reordered by the physician. To be included in the study, a subject must have had an EMR activity recorded at 5 years or more after the index date. Thus, all subjects were observed for a minimum of 5 years to ensure that the patient continued to be cared for by the EMR provider. Patients with an index BMI of less than 18 kg/m2 (underweight) were excluded, as were patients with any indication of elevated triglycerides, low high-density lipoprotein cholesterol (HDL-C), hypertension, or type 2 diabetes prior to index date.
Study Time Frame
The study period was from January 1996 through June of 2005. The observation window for each patient was 395 days before the index BMI date or the first activity date, whichever was greater, through 5 years post–index date or their last activity date, whichever was greater. Thus, all study subjects had between 5 and 8.5 years of GE EMR follow-up postbaseline period.
Study Variables
Study outcome variables were the incidence of one or more of four cardiometabolic risk factors, high triglycerides, low HDL-C, hypertension, or type 2 diabetes, as determined from clinical diagnosis, pharmacotherapy, or biometric reading. Patients were defined as having elevated triglycerides if they had a triglyceride reading equal to or greater than 150 mg/dl, were taking fibrates, or had an ICD-9 code of 272.1. Low HDL-C was identified if a patient had a HDL-C value of less than 40 mg/dl in men or less than 50 mg/dl in women, or was on niacin. Patients were considered to have hypertension if there were at least two recorded systolic BP readings equal to or greater than 130 mmHg or diastolic blood pressures equal to or greater than 85 mmHg within 395 days, or had been prescribed an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, beta-blocker, thiazide diuretic, vasodilator, or fixed dose antihypertensive combination, or had an ICD-9 code of 401.x. Patients were categorized as diabetic if they had a fasting blood glucose level equal to or above 126 mg/dl, or if the patient was treated with sulfonylureas, metformin, thiazolidinediones, meglitinides, alpha glucosidase inhibitors, or fixed dose antidiabetic combinations, or had an ICD-9 code for type 2 diabetes of 250.x0 or 250.x2. Biometric criteria for the selection of patients with hypertension, low HDL-C, and high triglycerides were based on the third report of the National Cholesterol Education Program Adult Treatment Panel (NCEP-ATP III) [10]. With the exception of blood pressures in which two elevated readings were required, the occurrence of a single out-of-range laboratory value, single diagnosis code, or one prescription drug order to treat the related condition was used to identify the presence of a risk factor.
Cardiometabolic risk factors were considered to be incident if they were documented more than 180 days after the index date and were not documented before the index date or within 180 days post–index date. Incident risk factors were identified both as individual risk factors and as the count of risk factors ranging from 0 to 4. In the case of availability of multiple measurements of a risk factor in the follow-up period, only the first indicator of a risk factor in the GE EMR data was considered.
The primary independent variable in this study was the baseline BMI, which was calculated from the patients' height and weight measurements, and categorized as either BMI ≤ 27 kg/m2 or BMI > 27 kg/m2. This cutoff acknowledged that weight-loss drugs are approved by the US Food and Drug Administration as adjuncts to diet and exercise in patients with a BMI of 30 kg/m2 or greater, or a BMI > 27 kg/m2 for patients with one or more concomitant risk factors, including hypertension, dyslipidemia, and type 2 diabetes [11]. Thus, in calculating the odds ratios (ORs) for the outcome measure, a BMI > 27 kg/m2 was used as the cutoff. Although the term “obesity” is generally used for those with a BMI greater than 30 kg/m2, for ease of communication we have termed the patients in this study with a baseline BMI > 27 kg/m2 as obese, recognizing that our terminology differs from the technical definition. Nevertheless, over half of the patients in the BMI > 27 kg/m2 group (56%) were technically obese with a baseline BMI > 30 kg/m2.
NCEP [10] and IDF [12] guidelines use waist circumference as a measure of central or abdominal obesity. Waist circumference is rarely available in clinical data, BMI and waist circumference are highly correlated and each independently contributes a significant risk for cardiometabolic risk factors and chronic diseases [7,8,13,14]. A recent publication examining waist circumference, BMI, and their association with cardiometabolic risk demonstrated that increased waist circumference and BMI were equally strong predictors of risk [15]. Thus, BMI, calculated from height and weight information in the EMR, was used as a proxy measure of obesity.
Additional independent variables that were collected included gender; age at index date (categorized as 18–30, 31–45, 46–64, and 65 years or older); insurance status (commercial, Medicare, Medicaid, self-pay, unknown); and geographic region (northeast, south, midwest, west).
Analysis
Population characteristics describing the data were evaluated. The number and proportion of patients who developed each cardiometabolic risk factor during the observation period were calculated overall and by baseline BMI. Four binary logistic regression models were utilized to estimate the adjusted odds of developing each of the four cardiometabolic risk factors post-index date by baseline BMI category. In an effort to isolate the impact of baseline BMI on the development of risk factors, we adjusted the models for potential confounding factors (age, gender, insurance status, and geographic region). Similarly, the odds of developing a specific number (0–4) of risk factors post–index date by BMI category were estimated using a multinomial logistic regression model, adjusting for the same independent variables as was done for the individual risk factor models. The general form of the model is the following:
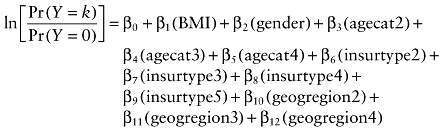
where ln is the natural log; Y is the outcome variable or specific risk factor, and takes on values of 0, 1, 2, 3, or 4; k is the outcome variable category and takes on value of 1 when the outcome is binary, and values of 1, 2, 3, or 4 when the outcome is polychotomous; Pr is the probability, BMI is body mass index and takes on value of 1 when BMI > 27 kg/m2, and 0 when BMI ≤ 27 kg/m2, with BMI ≤ 27 kg/m2 being the reference category; gender takes on value of 1 when male, with female as the reference category; agecat2, agecat3, and agecat4 are age categories of 31 to 45 years, 46 to 64 years, and 65 years or greater, respectively, with age category of 18 to 30 years being the reference; insurtype2, insurtype3, insurtype4, and insurtype5 represent Medicare, Medicaid, self-pay, and unknown categories, respectively, with commercial insurance being the reference category; geogregion2, geogregion3, and geogregion4 represent south, midwest, and west regions of the United States, respectively, with northeast being the reference category; and β, s are the parameters to be estimated.
All statistical tests were performed using Stata SE v. 9 (College Station, TX) and SAS v. 9 (SAS Institute, Cary, NC).
Results
Figure 1 illustrates identification of the study population. Of the initial GE data set of 3,301,897 patients, 3,216,092 were 18 years or older. Of these, 1,110,718 had a BMI measurement at 395 days or more after the first activity date. Applying a minimum 5-year activity after index date and baseline BMI greater than 18 kg/m2 resulted in 70,375 people. Of these, 18,372 subjects did not have any of the four cardiometabolic risk factors at baseline. Thus, the final study population for analysis consisted of 18,372 subjects, of whom 6,906 (37.6%) were obese (BMI > 27 kg/m2) and 11,466 (62.4%) had a normal weight (BMI ≤ 27 kg/m2) (Fig. 1).
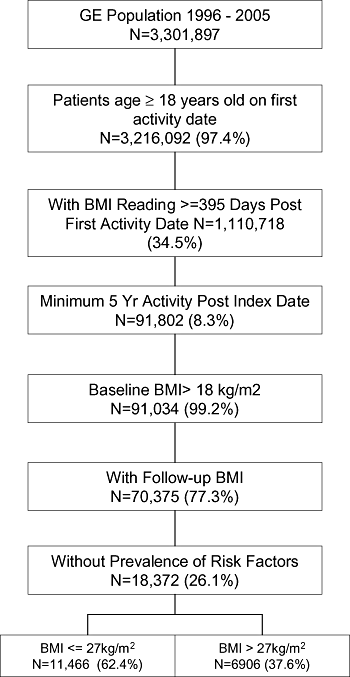
Study population identification. (Note: The percentages in each box of the flowchart are of the step above in the chart.)
Descriptive Results
Descriptive characteristics for the study population are shown in Table 1. Approximately 38% had a baseline BMI > 27 kg/m2. The study population was 29.3% male with a mean (±standard deviation) age of 43.5 (±14.9) years (Table 1). A total of 10.9% of the population was 65 years or older. The study population had a mean (SD) BMI of 26.6 kg/m2 (±5.9). The mean BMI (SD) of those with BMI < 27 kg/m2 and those with BMI > 27 kg/m2 was 23.2 kg/m2 (±2.2) and 32.3 kg/m2 (±5.6), respectively.
| Total | BMI ≤ 27 kg/m2 | BMI > 27 kg/m2 | ||||
|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | |
| N | 18,372 | 11,466 | 6,906 | |||
| Mean age (SD) (years) | 43.5 | (14.9) | 43.3 | (15.3) | 43.9 | (14.3) |
| 18–30 | 3,833 | (20.9) | 2,531 | (22.1) | 1,302 | (18.9) |
| 31–45 | 7,328 | (39.9) | 4,565 | (39.8) | 2,763 | (40.0) |
| 46–64 | 5,207 | (28.3) | 3,059 | (26.7) | 2,148 | (31.1) |
| 65+ | 2,004 | (10.9) | 1,311 | (11.4) | 693 | (10.0) |
| Gender | ||||||
| Male | 5,376 | (29.3) | 2,901 | (25.3) | 2,475 | (35.8) |
| Insurance type | ||||||
| Commercial | 10,516 | (57.2) | 6,445 | (56.2) | 4,071 | (58.9) |
| Medicaid | 2,207 | (12.0) | 1,373 | (12.0) | 834 | (12.1) |
| Medicare | 214 | (1.2) | 123 | (1.1) | 91 | (1.3) |
| Self-pay | 306 | (1.7) | 218 | (1.9) | 88 | (1.3) |
| Unknown | 5,129 | (27.9) | 3,307 | (28.8) | 1,822 | (26.4) |
| Region | ||||||
| Northeast | 5,377 | (29.3) | 3,280 | (28.6) | 2,097 | (30.4) |
| Southeast | 2,400 | (13.1) | 1,396 | (12.2) | 1,004 | (14.5) |
| Midwest | 8,211 | (44.7) | 5,178 | (45.2) | 3,033 | (43.9) |
| West | 2,384 | (13.0) | 1,612 | (14.1) | 772 | (11.2) |
- BMI, body mass index.
Table 2 provides information on the distribution of risk factors by BMI, demonstrating that a higher proportion of patients fell in the obese (BMI > 27 kg/m2) category when a specific risk factor developed as compared to when the risk factor did not develop.
| Total | Without risk factor | With risk factor | ||||
|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | |
| Elevated triglycerides | ||||||
| BMI ≤ 27 kg/m2 | 11,466 | (62.4) | 10,771 | (64.2) | 695 | (43.6) |
| BMI > 27 kg/m2 | 6,906 | (37.6) | 6,007 | (35.8) | 899 | (56.4) |
| Total | 18,372 | 16,778 | 1,594 | |||
| Low HDL-C | ||||||
| BMI ≤ 27 kg/m2 | 11,466 | (62.4) | 9,566 | (66.8) | 1,900 | (46.9) |
| BMI > 27 kg/m2 | 6,906 | (37.6) | 4,758 | (33.2) | 2,148 | (53.1) |
| Total | 18,372 | 14,324 | 4,048 | |||
| Hypertension | ||||||
| BMI ≤ 27 kg/m2 | 11,466 | (62.4) | 6,910 | (71.1) | 4,556 | (52.7) |
| BMI > 27 kg/m2 | 6,906 | (37.6) | 2,815 | (28.9) | 4,091 | (47.3) |
| Total | 18,372 | 9,725 | 8,647 | |||
| Type 2 diabetes | ||||||
| BMI ≤ 27 kg/m2 | 11,466 | (62.4) | 11,085 | (63.5) | 381 | (41.8) |
| BMI > 27 kg/m2 | 6,906 | (37.6) | 6,376 | (36.5) | 530 | (58.2) |
| Total | 18,372 | 17,461 | 911 | |||
- * Elevated triglycerides: triglyceride ≥ 150 mg/dl, use of fibrates, or ICD-9 code of 272.1. Low HDL-C: HDL-C < 40 mg/dl in men or <50 mg/dl in women, or use niacin. Hypertension: two blood pressure readings ≥130/85 mmHg, use of an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blockers, beta-blocker, thiazide diuretic, vasodilator, fixed-dose antihypertensive combination, or ICD-9 code of 401.x. Type 2 diabetes: fasting blood glucose level ≥126 mg/dl use of sulfonylureas, metformin, thiazolidinediones, meglitinides, alpha glucosidase inhibitors, fixed-dose antidiabetic combinations, or ICD-9 code 250.x0 or 250.x2.
- BMI, body mass index; HDL-C, high-density lipoprotein cholesterol.
Table 3 reports the distribution of count of risk factors (0–4) by BMI. The proportion of patients in the obese group (BMI > 27 kg/m2) increased as the count of incident risk factors increased. Approximately, 57% of patients developed at least one new risk factor; 38% developed one new risk factor, of whom 41% had a baseline BMI > 27 kg/m2; 13% developed two risk factors, of whom 52% had a baseline BMI > 27 kg/m2; 5% developed three risk factors, of whom 65% had a baseline BMI > 27 kg/m2; and 1% developed four new risk factors, of whom 82% had a baseline BMI > 27 kg/m2.
| Total | BMI ≤ 27 kg/m2 | BMI > 27 kg/m2 | ||||
|---|---|---|---|---|---|---|
| N | % of total | n | % with risk factor* | n | % with risk factor* | |
| 18,372 | 11,466 | 6,906 | ||||
| Count of risk factors | ||||||
| 0 | 7,848 | 42.7 | 5,796 | 73.9 | 2,052 | 26.1 |
| 1 | 7,048 | 38.4 | 4,172 | 59.2 | 2,876 | 40.8 |
| 2 | 2,442 | 13.3 | 1,164 | 47.7 | 1,278 | 52.3 |
| 3 | 868 | 4.7 | 304 | 35.0 | 564 | 65.0 |
| 4 | 166 | 0.9 | 30 | 18.1 | 136 | 81.9 |
- * Shown as percent of total number of subjects with 0, 1, 2, 3, or 4 risk factors(s).
Regression Results
As stated earlier, we estimated a separate logistic regression model for each of the incident cardiometabolic risk factors, and one model for the count of incident risk factors. Tables 4–7 show that adjusted odds of developing a cardiometabolic risk factor were greater for those with baseline BMI > 27 kg/m2 relative to those with baseline BMI ≤ 27 kg/m2 for each of the four risk factors examined, ranging from 2.1 (95% CI 1.9–2.3) for elevated triglycerides, 2.2 (95% CI 2.1–2.4) for hypertension, 2.2 (95% CI 2.0–2.4) for low HDL-C, and to 2.3 (95% CI 2.0–2.7) for diabetes. Further, male gender and age were significantly associated with an increased risk of developing all four risk factors.
| Variable | Unadjusted OR | 95% CI | Adjusted OR | 95% CI |
|---|---|---|---|---|
| Baseline BMI > 27 kg/m2 (ref ≤27 kg/m2) | 2.3* | (2.1, 2.6) | 2.1* | (1.9, 2.3) |
| Gender (ref = female) | 2.3* | (2.1, 2.6) | 2.0* | (1.8, 2.2) |
| Age (ref = 18–30) (years) | ||||
| 31–45 | 2.7* | (2.2, 3.3) | 2.6* | (2.1, 3.2) |
| 46–64 | 4.5* | (3.7, 5.5) | 4.1* | (3.4, 5.0) |
| 65+ | 3.8* | (3.0, 4.7) | 4.3* | (3.3, 5.6) |
| Insurance status (ref commercial) | ||||
| Medicare | 1.6* | (1.3, 1.8) | 1.2 | (1.0, 1.5) |
| Medicaid | 0.9 | (0.5, 1.6) | 1.1 | (0.6, 1.8) |
| Self-pay | 1.0 | (0.6, 1.5) | 1.2 | (0.7, 1.9) |
| Unknown | 1.6* | (1.5, 1.8) | 2.0* | (1.7, 2.2) |
| Geographic region (ref northeast) | ||||
| South | 1.0 | (0.8, 1.1) | 1.0 | (0.8, 1.2) |
| Midwest | 0.9 | (0.8, 1.0) | 0.7* | (0.6, 0.8) |
| West | 0.4* | (0.3, 0.5) | 0.3* | (0.2, 0.4) |
- * Significant at P < 0.05.
- The c-statistic for the adjusted model was 0.72 (95% CI = 0.71–0.73).
- BMI, body mass index; OR, odds ratio; CI, confidence interval.
| Variable | Unadjusted OR | 95% CI | Adjusted OR | 95% CI |
|---|---|---|---|---|
| Baseline BMI > 27 kg/m2 (ref ≤27 kg/m2) | 2.3* | (2.1, 2.4) | 2.2* | (2.0, 2.4) |
| Gender (ref = female) | 1.5* | (1.4, 1.6) | 1.3* | (1.2, 1.4) |
| Age (ref = 18–30) (years) | ||||
| 31–45 | 1.6* | (1.5, 1.8) | 1.6* | (1.4, 1.7) |
| 46–64 | 1.8* | (1.6, 2.0) | 1.6* | (1.4, 1.8) |
| 65+ | 1.7* | (1.4, 1.9) | 1.5* | (1.2, 1.7) |
| Insurance status (ref commercial) | ||||
| Medicare | 1.2* | (1.1, 1.3) | 1.2* | (1.0, 1.3) |
| Medicaid | 0.7 | (0.5, 1.0) | 0.8 | (0.6, 1.2) |
| Self-pay | 0.7* | (0.5, 1.0) | 0.7 | (0.5, 1.0) |
| Unknown | 0.9* | (0.8, 0.9) | 0.9 | (0.8, 1.0) |
| Geographic region (ref northeast) | ||||
| South | 0.5* | (0.5, 0.6) | 0.5* | (0.5, 0.6) |
| Midwest | 0.8* | (0.7, 0.8) | 0.8* | (0.7, 0.9) |
| West | 0.7* | (0.6, 0.7) | 0.6* | (0.6, 0.7) |
- * Significant at P < 0.05.
- The c-statistic for the adjusted model was 0.64 (95% CI = 0.63–0.65).
- HDL-C, high-density lipoprotein cholesterol; BMI, body mass index; OR, odds ratio; CI, confidence interval.
| Variable | Unadjusted OR | 95% CI | Adjusted OR | 95% CI |
|---|---|---|---|---|
| Baseline BMI > 27 kg/m2 (ref ≤27 kg/m2) | 2.2* | (2.1, 2.3) | 2.2* | (2.1, 2.4) |
| Gender (ref = female) | 1.8* | (1.7, 1.9) | 1.4* | (1.3, 1.5) |
| Age (ref = 18–30) (years) | ||||
| 31–45 | 1.4* | (1.2, 1.5) | 1.3* | (1.2, 1.4) |
| 46–64 | 2.5* | (2.3, 2.8) | 2.3* | (2.1, 2.6) |
| 65+ | 6.1* | (5.4, 6.9) | 4.9* | (4.2, 5.7) |
| Insurance status (ref commercial) | ||||
| Medicare | 3.0* | (2.7, 3.3) | 1.4* | (1.2, 1.6) |
| Medicaid | 1.2 | (1.0, 1.6) | 1.5* | (1.1, 2.0) |
| Self-pay | 0.8 | (0.6, 1.0) | 1.0 | (0.8, 1.2) |
| Unknown | 0.8* | (0.7, 0.8) | 0.8* | (0.7, 0.9) |
| Geographic region (ref northeast) | ||||
| South | 0.9* | (0.8, 1.0) | 1.0 | (0.9, 1.1) |
| Midwest | 0.8* | (0.7, 0.8) | 0.8* | (0.7, 0.9) |
| West | 1.3* | (1.2, 1.4) | 0.9 | (0.8, 1.0) |
- * Significant at P < 0.05.
- The c-statistic for the adjusted model was 0.70 (95% CI = 0.69–0.71).
- BMI, body mass index; OR, odds ratio; CI, confidence interval.
| Variable | Unadjusted OR | 95% CI | Adjusted OR | 95% CI |
|---|---|---|---|---|
| Baseline BMI > 27 kg/m2 (ref ≤27 kg/m2) | 2.4* | (2.1, 2.8) | 2.3* | (2.0, 2.7) |
| Gender (ref = female) | 2.1* | (1.9, 2.4) | 1.6* | (1.4, 1.9) |
| Age (ref = 18–30) (years) | ||||
| 31–45 | 1.1 | (0.9, 1.4) | 1.1 | (0.8, 1.3) |
| 46–64 | 2.0* | (1.6, 2.5) | 1.7* | (1.3, 2.1) |
| 65+ | 4.8* | (3.8, 6.1) | 3.5* | (2.6, 4.6) |
| Insurance status (ref commercial) | ||||
| Medicare | 3.3* | (2.8, 3.8) | 1.6* | (1.3, 2.0) |
| Medicaid | 1.0 | (0.5, 2.0) | 1.0 | (0.5, 2.1) |
| Self-pay | 0.9 | (0.5, 1.6) | 1.1 | (0.6, 2.1) |
| Unknown | 1.3 | (1.1, 1.5) | 1.3* | (1.1, 1.6) |
| Geographic region (ref northeast) | ||||
| South | 1.3* | (1.1, 1.6) | 1.4* | (1.1, 1.8) |
| Midwest | 1.0 | (0.9, 1.2) | 0.9 | (0.8, 1.1) |
| West | 1.5* | (1.2, 1.8) | 0.9 | (0.7, 1.2) |
- * Significant at P < 0.05.
- The c-statistic for the adjusted model was 0.72 (95% CI = 0.70–0.74).
- BMI, body mass index; OR, odds ratio; CI, confidence interval.
The adjusted odds of developing a specific number of risk factors was greater when baseline BMI was greater than 27 kg/m2 relative to when BMI was less than or equal to 27 kg/m2, with the odds increasing with each additional number of new risk factors (Table 8). The odds of developing one new risk factor were 1.9 (95% CI 1.8–2.1) for baseline BMI > 27 kg/m2 relative to baseline BMI ≤ 27 kg/m2, increasing to 7.9 (95% CI 5.9–10.5) for developing four new risk factors.
| Variable | One risk factor | Two risk factors | Three risk factors | Four risk factors | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Baseline BMI > 27 kg/m2 (ref ≤27 kg/m2) | 1.9* | (1.8, 2.1) | 3.1* | (2.8, 3.4) | 4.9* | (4.3, 5.7) | 7.9* | (5.9, 10.5) |
| Gender (ref = female) | 1.4* | (1.3, 1.5) | 1.8* | (1.6, 2.0) | 2.1* | (1.8, 2.4) | 2.9* | (2.3, 3.8) |
| Age (ref = 18–30) (years) | ||||||||
| 31–45 | 1.3* | (1.2, 1.4) | 1.8* | (1.5, 2.0) | 2.9* | (2.2, 3.8) | 2.2* | (1.3, 3.7) |
| 46–64 | 2.0* | (1.8, 2.2) | 3.1* | (2.6, 3.6) | 6.1* | (4.6, 8.0) | 6.2* | (3.8, 10.2) |
| 65+ | 3.4* | (2.8, 4.0) | 7.4* | (5.9, 9.1) | 14.3* | (10.1, 20.2) | 15.5* | (8.5, 28.4) |
| Insurance status (ref commercial) | ||||||||
| Medicare | 1.4* | (1.2, 1.6) | 1.6* | (1.4, 2.0) | 1.7* | (1.3, 2.2) | 2.3* | (1.5, 3.5) |
| Medicaid | 1.3 | (0.9, 1.7) | 0.8 | (0.5, 1.4) | 1.6 | (0.8, 3.1) | 3.0* | (1.0, 8.5) |
| Self-pay | 1.0 | (0.8, 1.3) | 0.9 | (0.6, 1.3) | 0.6 | (0.3, 1.4) | 1.4 | (0.4, 4.5) |
| Unknown | 0.7* | (0.7, 0.8) | 0.9 | (0.8, 1.1) | 1.3* | (1.1, 1.5) | 2.0* | (1.4, 2.7) |
| Geographic region (ref northeast) | ||||||||
| South | 0.9 | (0.8, 1.0) | 0.7* | (0.6, 0.8) | 0.7* | (0.6, 0.9) | 0.7 | (0.4, 1.0) |
| Midwest | 0.8* | (0.7, 0.8) | 0.7* | (0.6, 0.7) | 0.6* | (0.5, 0.7) | 0.7* | (0.5, 0.9) |
| West | 1.0 | (0.9, 1.1) | 0.8* | (0.7, 0.9) | 0.4* | (0.3, 0.5) | 0.2* | (0.1, 0.3) |
- * Significant at P < 0.05.
- † Multinomial logistic regression.
- BMI, body mass index; OR, odds ratio; CI, confidence interval.
Analysis Using BMI of 30 kg/m2 as Cutoff
We also conducted analyses by recategorizing the study cohort by baseline BMI. Specifically, we categorized those with baseline BMI ≤ 30 kg/m2 as “normal weight,” and those with baseline BMI > 30 kg/m2 as “obese.” We performed these analyses to confirm that our main results using a cutoff of BMI of 27 kg/m2 hold true when using the standard definition of obesity, BMI > 30 kg/m2, as a cutoff point. We found the results in the analyses using BMI of 30 kg/m2 as cutoff to be similar to those using BMI of 27 kg/m2 as cutoff. All of the point estimates for both individual cardiometabolic risk factor and count of risk factor models were statistically significant. Adjusted odds ratios for individual risk factors ranged from 2.8 for diabetes to 2.1 for elevated triglycerides. Further, adjusted odds ratios for the count of risk factor model ranged from 2.1 for one risk factor to 8.5 for four risk factors. These results provide a further validation of our main results reported earlier.
Discussion
This retrospective cohort analysis of 18,372 geographically diverse ambulatory adult subjects assessed the association between baseline BMI and subsequent development of four other cardiometabolic risk factors—elevated triglycerides, low HDL-C, hypertension, and type 2 diabetes—over at least 5 years of clinical follow-up in an ambulatory setting.
This study found that baseline BMI > 27 kg/m2 was associated with the subsequent development of all examined risk factors, with adjusted odds increasing 110% for elevated triglycerides, 130% for diabetes, and 120% for low HDL-C and hypertension (P < 0.05 for all). Similarly, baseline BMI > 27 kg/m2 was associated with an increase in the number of new risk factors that developed over time. Thus, patients with baseline BMI > 27 kg/m2 were more likely to have a single or multiple incident risk factors over the follow-up period than were patients with baseline BMI ≤ 27 kg/m2. The likelihood of developing multiple risk factors, relative to developing no risk factors, increased dramatically for BMI > 27 kg/m2, with the risk rising with the number of incident risk factors, thereby magnifying their overall cardiometabolic risk.
The association between an above normal body weight and the incidence of cardiovascular risk factors has been demonstrated in several cohort studies. An analysis based on the Framingham Heart Study found the risk of developing hypertension was two and a half times higher (relative risk [RR] 2.63; 95% CI 2.20–3.15) for obese women (BMI ≥ 30 kg/m2), and more than two times higher (RR 2.23; 95% CI 1.75–2.84) for obese men than individuals of normal weight; the risk of developing type 2 diabetes was 36% higher in obese women (RR 1.36; 95% CI 1.03–1.78), and 85% higher in obese men (RR 1.85; 95% CI 1.31–2.26) [16]. Similarly, the San Antonio Heart Study found an association between BMI and the development of type 2 diabetes [17], with each standard deviation increase in weight with increasing odds of developing type 2 diabetes by 51% in women (OR 1.51; 95% CI 1.21–1.90) and 69% in men (OR 1.69; 95% CI 1.07–2.65). A recent study by Ghandehari et al. studied the association of obesity (BMI) and abdominal obesity (waist circumference) with cardiometabolic risk factor burden among adult Americans [18]. Mean levels of low-density lipoprotein cholesterol, systolic and diastolic blood pressure, fasting glucose, and C-reactive protein increased, and HDL-C decreased (P < 0.001) as BMI and waist circumference increased [18]. Of those with high waist circumference, 25–35% had ≥3 cardiometabolic risk factors.
This study is unique in that it was specifically designed to evaluate the development of cardiometabolic risk using a large longitudinal, nationally representative EMR database receiving treatment in an ambulatory care setting. This study demonstrates a significant association of subsequent development of new cardiometabolic risk factors among patients with baseline BMI of >27 kg/m2. This is the same cutoff used by NIH guidelines for treatment with weight loss agents in addition to diet and physical activity in overweight patients with other cardiovascular risk factors [11]. Results were similar in post hoc analyses when the study cohorts were recategorized with baseline BMI ≤ 30 kg/m2 as “normal weight,” and those with baseline BMI > 30 kg/m2 as “obese.”
In contrast to much of the related literature that evaluates BMI or weight and the prevalence of additional risk factors, this study was conducted in routine community practice settings drawn from a major EMR research database. In addition, this is one of a small number of studies that evaluated the association between BMI at a given point in time and the development of new cardiometabolic risk factors over a relatively long period of time (5–8.5 years). The focus on an ambulatory care setting provides insight for primary care physicians about the likelihood of their overweight and obese patients developing other cardiometabolic risk factors.
The results of this study are also important from a policy perspective. Investment in shorter-term weight loss strategies may delay or avoid the development of risk factors associated with greater costs for a health plan. From a societal perspective, it is apparent that obesity may lead to increase in cardiometabolic risk over time. These complications not only impact national direct costs, but also impact indirect costs associated with quality of life and productivity.
Limitations
This study has a number of limitations. First, the use of an EMR database may contain errors of omission and commission, which is a ubiquitous challenge when conducting real-world studies based on secondary data. First, patient race was missing in almost two-thirds of the study population, thus it was not included as an independent variable. In addition, having multiple measures indicating the presence of a risk factor or to confirm BMI to address concerns of coding errors was not required in this study. Nevertheless, coding errors that could lead to misclassification are likely to be random, and thus not expected to bias study findings.
Lack of documentation of diagnosis and other clinical data may be of greater concern. Thus, multiple data elements were utilized to identify the presence of cardiometabolic risk factors including diagnosis codes, prescription drug orders, and laboratory values in an effort to reduce false negatives. Similarly, BMI is not recorded for every patient in the EMR, thus for this study only those patients with BMI readings were included. This inclusion requirement may have introduced a bias as physicians may be more likely to measure height so that a BMI can be calculated in patients who are obese compared with normal-weight patients. Further, the possibility that patients with high BMI are more likely to receive lab tests and thus are more likely to be “diagnosed” may also have introduced a bias increasing the likelihood of capturing incidence of risk factors for the obese group. Thus, because of clinical data capture and reporting biases, this study may overestimate the association between obesity and the development of cardiometabolic risk factors.
The core outcome variable in this study was obesity, as determined by BMI. Although other indicators such as waist circumference and waist–hip ratio have been shown to be equivalent [15] or better predictors than BMI of cardiovascular risk factors [18], they are generally not accessible in insurance claims or medical record databases. Likewise, physicians are less likely to measure, and patients are less likely to associate an increase in waist circumference with increased cardiometabolic risk [19], and therefore information based on BMI may be more clinically relevant.
Finally, the minimum study period of 5 years may seem limited in terms of assessing the risk of a population developing cardiometabolic risk factors. We used a 5-year minimum as it exacted a balance between the amount of time patients were followed in the EMR and in obtaining a sufficient number of patients to evaluate study outcomes.
Conclusions
The study highlighted that obese patients are more likely to develop cardiometabolic risk factors than those with normal weight. Specifically, baseline overweight or obesity (BMI > 27 kg/m2) was found to be significantly associated with subsequent development of individual, as well as multiple cardiometabolic risk factors in a real-life ambulatory care setting. Future research should examine the impact of prevention of obesity and reduction of weight and obesity on development of cardiometabolic risk factors. A further extension of research is to explore the extent of effects of decreases in cardiometabolic risk factors on reduction in cardiovascular disease-related morbidity and mortality. If this link is established, then weight loss may reduce adverse clinical events and associated health-care costs.
Source of financial support: This research was funded in part by an unrestricted research grant by Sanofi-Aventis.




