Cost-Effectiveness of Preventing Hip Fractures by Hip Protectors in Elderly Institutionalized Residents in Germany
ABSTRACT
Objective: To determine the long-term cost-effectiveness of hip protector use in the prevention of hip fractures in elderly institutionalized residents in Germany compared to no prevention.
Methods: A lifetime Markov decision model was developed using published data on costs and health outcomes. A societal and statutory health insurance perspective was adopted.
Results: From a societal/statutory health insurance perspective, use of hip protectors yields savings of 315 EURO/257 EURO and a gain of 0.13 quality-adjusted life years per person over lifetime.
Conclusion: Hip protector use in elderly institutionalized residents in Germany is highly cost-effective.
Introduction
Hip fractures are a common consequence of falls in the older population [1]. A hip fracture is a fracture of the femoral neck or below (intertrochanteric hip fracture). With a current incidence of 125,000 (152/100,000 inhabitants) cases per year, hip fractures are one of the most common causes of death in the elderly in Germany [2]. In addition, hip fractures have an enormous economic impact. In Germany, costs related to hip fractures are estimated to be 2.8 billion EURO per year [3]. Because of demographic changes and the growing number of elderly expenditures are expected to increase in the future.
Efforts to reduce the number of hip fractures by prevention are therefore important. Strategies to prevent hip fractures include muscle strengthening and balance training, drug regimes to treat osteoporosis, and the use of external hip protectors [4–8]. Given that almost 90% of hip fractures are caused by falls [9], hip protectors can have an important role in preventing hip fractures. Because the incidence of hip fractures is especially high in nursing home facilities [10], their use in this population seems particularly appealing. In Germany, there are currently about 225,000 people above the age of 80 years who live in an institutional setting [11]. The German statutory health insurance currently does not pay for hip protectors on a regular basis. To be covered, hip protectors need to demonstrate cost-effectiveness.
So far, a number of economic evaluations on hip protector use in elderly populations living in institutions have been conducted internationally [12–17]. Nevertheless, none of these studies considers that patients who are noncompliant may be urged to forward their protectors to other residents so that protectors can provide benefit to someone else. Furthermore, previous international studies do not capture all societal costs including costs of revision surgeries and unrelated future costs during added years of life. Reasons are a limited time horizon (up to 18 months) and a narrow perspective (Medicare). Finally, previous international studies do not include implementation costs, i.e., costs of educating nurses. Without decision modeling, however, it is impossible to say what the net cost impact of including these factors is.
The only German evaluation was conducted alongside a randomized controlled trial (RCT) in nursing homes [18]. Although this study includes implementation costs, it is also limited by the assumption that in case of noncompliance, hip protectors are not used by someone else. Further, it does not consider evidence for protector efficacy or effectiveness from other trials [19] and also does not consider long-term costs of hip fractures because of a time horizon of 18 months.
Given the above limitations of previous cost-effectiveness analyses, the goal of this article was to determine the long-term costs and cost-effectiveness of hip protectors in elderly institutionalized residents living in German nursing homes. Note that not all nursing homes are the same. Some provide basic services (custodial services), although others resemble medical units in hospitals. We built a Markov decision model which uses published data and extends a model we published on the long-term costs and effects of treating hip fractures [20].
Methods
Overview and Model Design
The target population for the study consists of male and female persons without hip fracture or treatment thereof who live in institutional settings at the age of 81 years, which is the average age of elderly residents in Germany [11]. Using our model, we compared the clinical and economic outcomes of using hip protectors to no prevention. Health outcomes are measured in terms of quality-adjusted life years (QALYs). Economic outcomes were set in relation to clinical outcomes by dividing the incremental costs of one strategy compared to other by the incremental QALYs gained (cost-effectiveness or cost-utility analysis). The time horizon is limited to 17 years because the probability of survival beyond 17 years or the age of 99 years is less than 0.001%. According to Sonnenberg and Beck [21], this is an acceptable stopping rule for a Markov model.
The study's analysis takes a societal viewpoint because this perspective is the most comprehensive one requiring the consideration of all direct and indirect costs (i.e., productivity losses) regardless of whether payments are made by insurance, patients (copayments), or any other party. In addition, a societal viewpoint is the perspective recommended by the US Panel on Cost-Effectiveness in Health and Medicine [22]. We considered costs of prevention, treatment, rehabilitation, and long-term care. It must be noted, however, that in this study, indirect costs are not included in the societal analysis because of the patients' starting age of 81 years.
The economic evaluation is also performed from the perspective of the statutory health insurance, which insures 89% of the German population. The statutory health insurance pays hospitals a flat rate per case using diagnosis-related groups (DRGs). DRGs are a classification of hospital case types into groups expected to have similar hospital resource use [23]. Nevertheless, whereas the statutory health insurance does not pay for hospital infrastructure (building and equipment maintenance) and nursing care, the analysis from the societal perspective considers these costs.
In addition to the cost-effectiveness analyses from both perspectives, the study includes a budget impact analysis from the perspective of the statutory health insurance. This analysis determines the total annual economic impact of hip protector use. It considers the compliance rate of hip protectors as well as the age distribution of nursing home inhabitants (age categories: <75, 75–80, 80–84, 85–89, 90–94, and >95 years) [24]. For the base-case analysis, both costs and effects are discounted at an annual rate of 3% [22].
To model clinical and economic outcomes, the study uses a Markov decision analysis model [25]. We chose a cycle time of 6 months for the health states defined by the Markov model, because all transition probabilities gathered from the literature refer to duration of at least 6 months. Our Markov model contains the following three health states: nursing home, fracture (with and without rehabilitation), and death (Fig. 1). The transition probabilities in our model are derived from published studies. A half-cycle correction is used to allow for transition events occurring midway through each 6-month cycle. We converted cumulative transition probabilities (i.e., mortality rates and fracture risks without hip protector) into biannual transition probabilities (reflecting the 6-month cycle length) using the formulas in the appendix.
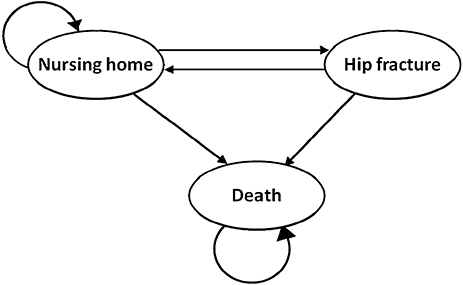
Markov model that estimates clinical and economic outcomes of hip fracture prevention. Ellipses indicate health states, although arrows show transitions.
The Markov model uses a cohort simulation. Two cohorts with 100,000 hypothetical patients each (one cohort for hip protectors and no prevention, respectively) at the age of 81 years entered the model and transited through it simultaneously. After 34 cycles (equivalent to 17 years prevention/no prevention), the simulation ended as nearly the whole cohort then had entered the state “death.” All modeling in the study was performed in Microsoft Excel 2000 (Microsoft Corporation, Redmond, WA, USA).
Sensitivity Analysis
To address uncertainty around the mean incremental cost-effectiveness ratio, we conducted univariate sensitivity analyses. When possible, we ran the analysis using the upper and lower boundaries of the 95% confidence interval of the mean. When the limits of the 95% confidence interval were unavailable, we used the limits of a plausible range (see Table 1 for uncertainty ranges). To assess how a simultaneous change of several variables affected the cost-effectiveness ratio, we performed a Monte Carlo simulation, which is a multivariate sensitivity analysis. For variables that are probabilities (mortality rates, complication rates, and transition probabilities), we assumed a beta distribution when there were only two outcomes (yes, no); when there were more than two outcomes, we assumed a Dirichlet distribution [42].
| Input variables | Base-case (sensitivity range) | Reference/comment |
|---|---|---|
| Mortality rate | Data are by age (1-year age groups) | [26] (German mortality statistics) |
| Relative risk of death (in nursing homes) | 1.16 (1.10–1.20) | [27] |
| Fracture risk without hip protector | Data are by age (5-year age groups) | [28,29] |
| Relative risk of fracture with hip protector | 0.77 (0.62–0.97) | [30] |
| In-hospital mortality after fracture | HP: 5.8%; IF: 5.6% (FNF)/5.2% (IHF) | [2] |
| Complication rate after fracture | HP: 16.1%; IF: 16.8% | [31] |
| Mortality rate 6 months after fracture | 24.3% (18.6–31.1%) | [32] |
| Probability of transfer to rehabilitation after fracture | 40% (32–48%) | [33] |
| Compliance with hip protectors | 0.56 | [34] |
| Cost data | ||
| Inpatient care | HP: 7252 euro; IF: 5628 euro | [35] |
| Complication costs per fracture | 500 euro | [36] |
| Capital costs of hospitals per day | 60 euro per day | [37] |
| Cost of transportation | 318 euro | [18] |
| Rehabilitation care | 2753 euro | [37] |
| Long-term care (nursing home) | 837 euro per month | [38] |
| Health-care costs | 1450 euro per 6 months | [39] |
| Hip protector costs | ||
| • Implementation | 146 euro /person | [18] |
| • Materials | societal perspective: 28 euro per 6 months; insurer's perspective: 32.50 euro per 6 months | [supplier] |
| Health-related quality of life: | ||
| Without need of care (77%) | ||
| • Without fracture | 0.84 (0.80–0.88) | [40] |
| • Hip fracture | HP: 0.73 (0.69–0.77); IF: 0.60 (0.56–0.64) | [40] |
| In need of care (23%) | ||
| • Without fracture | 0.35 (0.30–0.40) | [40,41] |
| • Hip fracture | 0.21 (0.15–0.27) (HP/IF) | [40,41] |
| Death | 0 | |
- FNF, femoral neck fracture; HP, hip replacement; IF, internal fixation; IHF, intertrochanteric hip fracture.
Given that the interpretation of negative cost-effectiveness ratios is ambiguous, we transformed cost-effectiveness ratios into net monetary benefits (NMBs) [43]. The decision rule we used was to adopt the intervention in question if the NMB was greater than 0. Given that the appropriate value of λ was unknown, λ was varied from 0 euro to 100,000 euro. We generated a cost-effectiveness acceptability curve based on the distribution of NMB for each λ[43].
Data
Data used in the model are shown in Table 1 and explained below.
Effectiveness.
To identify meta-analyses on the effectiveness of hip protectors in institutionalized elderly, we used the following search strategy in PubMed (1966–April 2007): nursing OR institutional AND hip protectors AND hip fractures AND (Meta-Analysis[ptyp]), where hip fractures and meta-analysis are MeSH terms and nursing, institutional, and hip protectors are free search terms.
Two relevant meta-analyses of RCTs were detected [30,44]. Both apply rigorous quality criteria to evaluate the validity of RCTs. In our analysis, we used the more conservative result by the Cochrane review of Parker et al. [30] who calculated a relative risk of 0.77 (95% CI 0.62–0.97). In this meta-analysis, 12 out of 14 included trials received similar care in both arms other than hip protector use (e.g., physical training and drug regimes). Hence, chance of a systematic bias is small.
Costs.
The costs of hip protectors vary considerably depending on type and quality. We used a price of 112 euro for four pairs of hip protectors, which presented at the time of the study the lower limit of the range. At a given time one pair is worn, one pair is washed, and two pairs are spare. Hence, protection continues during laundry. From the perspective of the statutory health insurance, we also included value-added tax. From a societal viewpoint, value-added tax was neglected because it does not represent opportunity costs. Based on information by the manufacturer, protectors have a lifetime of 2 years and may not be usable thereafter because of wear and tear. Hence, for each compliant individual, we assumed four pairs of hip protectors every 2 years, which results in an annual consumption rate of two hip protectors.
As stated in the introduction, the cost-effectiveness analysis considers the fact that in the case of noncompliance, hip protectors are used by someone else in the nursing home. Suppose an individual stops wearing her protectors after 1 year. For the remaining lifetime of 1 year, protectors are used by another individual. Although individual compliance is only 50%, protectors are used 100% of the time. Note, however, that we used efficacy data from clinical trials, in which protectors have not been forwarded to other individuals. Applying this set of data to our model results in an underestimation of the benefit. To avoid this underestimation, we can either increase the benefit (by multiplying the relative risk reduction with the relative compliance increase) or reduce protector costs proportional to the benefit reduction (i.e., multiply costs of hip protectors by the rate of compliance). We used the latter approach. It ensures equivalence of the numerator and denominator of the cost-effectiveness ratio in terms of compliance. In a sensitivity analysis, we checked how not forwarding protectors affects results.
Besides, the costs for implementation were taken into account. They are based on a study by Meyer et al. [18], who determined—for a period of 6 months—average costs of €146 for training material, compensation of nursing staff (attendance of training, implementation of the program), and compensation of training staff (including travel expenses). The time required for nursing staff to put on the garments (at best 5 minutes) was neglected [15].
Nursing home costs cover room and board as well as assistance for bathing, cooking, dressing, eating, grooming, and personal hygiene. Health-care costs are not included and come in addition.
Costs of hip-fracture surgery were calculated as a weighted average of the DRGs of hip replacement and internal fixation. In femoral neck fractures 80% of patients receive hip replacement, whereas in intertrochanteric hip fractures only 6% do [2]. After surgery, 40% of patients are transferred to rehabilitation [32]. Hence, 60% of patients do not incur rehabilitation costs. For revision hip fracture surgery, we assumed the same follow-up costs as for the primary operation. All costs were adjusted to 2004 euros.
Health-related quality of life.
All health-related quality-of-life data were taken from two surveys [40,41] using the EQ-5D questionnaire. The first survey [40] included 90 patients with a hip fracture at a mean age of 80 years. Patients lived independently, i.e., were not institutionalized and had no severe cognitive dysfunction. They were interviewed concerning their prefacture health status. In our model, data from these patients were applied to residents without need of daily care (see Table 1). The other survey [41] investigated 60 patients with a hip fracture at a mean age of 84 years (52% were institutionalized). Again, patients were interviewed concerning their prefacture health status. In our model, data from these patients were applied to residents with need of daily care (see Table 1). Weights to calculate EQ-5D index scores were derived from the time trade-off method in a large UK population survey [45].
Results
Base-case Analysis
Table 2 presents the results of the base-case analysis from the societal viewpoint. Wearing hip protectors leads to savings of €315 and a gain of 0.13 QALYs per subject. Therefore, hip-protector use dominates no prevention. Results were similar from the perspective of the statutory health insurance: using hip protector leads to savings of €257 per subject.
| Lifetime costs(discounted) | Life expectancy(discounted) | QALYs(discounted) | Incremental cost-effectiveness ratio | |
|---|---|---|---|---|
| No prevention | 45,101 euro | 5.72 years | 2.99 | Hip protector dominates no prevention |
| Hip protector | 44,786 euro | 5.83 years | 3.12 |
- QALYs, quality-adjusted life years.
The budget impact analysis, which takes the perspective of the statutory health insurance and considers the compliance rate of hip protectors as well as the age distribution of nursing home inhabitants, shows annual savings of approximately €12.9 million in Germany.
Ancillary Analyses
The base-case analysis comparing hip protectors to no prevention was robust to sensitivity analysis. Table 3 shows the results of the univariate sensitivity analysis plus changes in the discount rate from the societal perspective. The relative risk of fracture with hip protectors is the variable with the largest impact on the cost-effectiveness ratio. Hip-protector use leads to savings unless protector effectiveness is extremely low or mortality rate at 6 months after fracture is extremely high.
| Incremental costs | Incremental QALYs | Incremental cost-effectiveness ratio | |
|---|---|---|---|
| Discount rate of costs and effects | |||
| 0% | <0 euro(−341 euro) | 0.15 | <0 euro(−2270 euro) |
| 3% (base-case) | <0 euro(−315 euro) | 0.13 | <0 euro(−2420 euro) |
| 5% | <0 euro(−274 euro) | 0.12 | <0 euro(−2280 euro) |
| 7% | <0 euro(−235 euro) | 0.11 | <0 euro(−2140 euro) |
| Relative risk of death without hip protector | |||
| Lower bound | <0 euro(−426 euro) | 0.09 | <0 euro(−4730 euro) |
| Higher bound | <0 euro(−153 euro) | 0.16 | <0 euro(−960 euro) |
| Fracture risk without hip protector | |||
| Lower bound | <0 euro(−80 euro) | 0.07 | <0 euro(−1140 euro) |
| Higher bound | <0 euro(−575 euro) | 0.18 | <0 euro(−3190 euro) |
| Relative risk of fracture with hip protector | |||
| Lower bound | <0 euro(−577 euro) | 0.17 | <0 euro(−3400 euro) |
| Higher bound | 323 euro | 0.008 | 40,380 euro |
| Mortality rate 6 months after fracture | |||
| Lower bound | <0 euro (−347 euro) | 0.11 | <0 euro(−3160 euro) |
| Higher bound | 645 euro | 0.16 | 4030 euro |
| Probability of transfer to rehabilitation after fracture | |||
| Lower bound | <0 euro(−295 euro) | 0.13 | <0 euro(−2270 euro) |
| Higher bound | <0 euro(−329 euro) | 0.13 | <0 euro(−2530 euro) |
| Health-related quality of life with hip fracture | |||
| Lower bound | <0 euro(−315 euro) | 0.15 | <0 euro(−2100 euro) |
| Higher bound | <0 euro(−315 euro) | 0.12 | <0 euro(−2630 euro) |
| Health-related quality of life without hip fracture | |||
| Lower bound | <0 euro(−315 euro) | 0.11 | <0 euro(−2860 euro) |
| Higher bound | <0 euro(−315 euro) | 0.16 | <0 euro(−1970 euro) |
| Hip protector sharing | |||
| No sharing | <0 euro(−154 euro) | 0.13 | <0 euro(−1185 euro) |
- “Lower bound” and “higher bound” refer to the limits of the 95% confidence interval.
- QALYs, quality-adjusted life years.
A threshold sensitivity analysis from an insurer's perspective shows that at a hip protector price of €49 over 6 months (base-case value: €32.50), the breakeven point is reached. This means that unless the loss rate because of protector loss or spoilage is higher than 54% within the lifetime of 2 years, protectors still save money from an insurer's viewpoint. Similarly, unless the lifetime of protectors is lower than 54% of the expected lifetime of 2 years (i.e., 1.1 years), protectors remain cost-saving.
Figure 2 shows the cost-effectiveness acceptability curve from a societal viewpoint. The probability of savings is 99%. At a willingness to pay of €3680 per QALY, hip protectors have a 100% probability of being cost-effective.
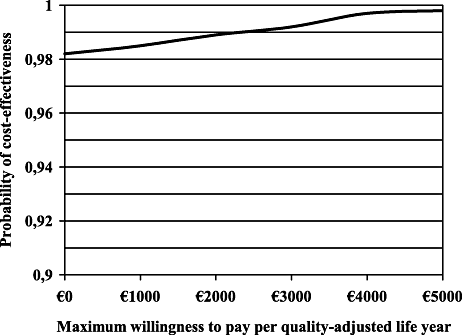
Cost-effectiveness acceptability curve showing the probability that hip fracture use is cost-effective.
Discussion
This modeling study shows that hip protectors not only increase life expectancy, but may also lead to cost savings in the long run, both from the perspective of society and the statutory health insurance. The probability of savings is 99% and only assuming extremely low hip-protector effectiveness or high mortality rate 6 months after fracture they are not realized. Previous studies conducted in Canada and the USA show similar results. They suggest that hip protectors are cost-effective or even cost-saving, particularly in high-risk elderly living in nursing homes [12–17]. The only German evaluation [18] shows a small cost increase of €42 per person, but this study was subject to the limitations described in the introduction. In contrast, preventing fractures by osteoporosis screening and bisphosphonate treatment has a small potential for savings, as suggested by many international cost-effectiveness models. Nevertheless, results vary considerably, ranging from savings in women above 80 years [46] to a cost-effectiveness ratio of $72,877 per QALY in 65-year-old women [47]. Furthermore, many models include only short-term costs of hip fractures. In Germany, a screen-and-treat strategy in 80-year-old women does not save costs, but is highly cost-effective (€1397 per QALY) [48].
Modeling studies, by nature, tend to contain weaknesses because of constraints of resources, time, and information availability. Our results are rather conservative for several reasons. First, we assumed that patients who had a fracture returned to the baseline risk of nonfractured persons 6 months after fracture. This underestimates the follow-up costs of hip fractures and the savings potential. Patients who have had a fracture might be at higher risk for another fracture and long-term complications. Second, hip-fracture incidence may be slightly underestimated, thus reducing again the prevention and savings potential. The reason is that our incidence data are from the general population and thus include elderly who receive drug regimes to treat osteoporosis. The resulting bias is likely to be small, however, as no more than 2% of the German population receives osteoporosis drugs including calcium, vitamin D, and bisphosphonates [49]. And third, the model did not take into consideration that an increase in life expectancy postpones the expensive last year of life, which becomes cheaper with age [50]. Unfortunately, this factor could not be taken into consideration because of the lack of data on the costs of survivors and decedents among hip fracture patients.
There is also the possibility that hip protectors may be less cost-effective than suggested by the base-case analysis. For example, it was assumed that wearing a hip protector has no negative impact on health-related quality of life. On the other hand, it is likely that patients who have a reduced quality of life because of protector use are not compliant. Thus, any reduction of quality of life may be already captured by the rate of noncompliance. Further, patients who are willing to wear protectors might be those who are willing to engage in other preventive activities such as exercise. In this patient group, protectors might be less effective because patients have a lower risk of falling and experiencing a subsequent fracture.
The additional assumptions made by the model are described as follows. First, although German data were preferred as inputs to our model, a few input variables were unavailable, thus had to be drawn from surveys outside Germany (e.g., long-term mortality rates and quality-of-life data) and may not reflect exact modes of health-care delivery in Germany. Second, the budget impact analysis assumes that patients at different ages have the same hip replacement/internal fixation surgery mix. This assumption seems unproblematic, however, because the costs and outcomes of hip replacement and internal fixation surgery differ only slightly. Third, health-related quality-of-life data were partly taken from noninstitutionalized elderly, thus leading to an upward bias. The difference between both arms may be unaffected, however, because the upward bias affects both arms and hence may cancel out. And forth, the approach of converting cumulative transition probabilities into biannual transition probabilities assumes that biannual transition probabilities stay constant for a certain period of time. The bias might be small, however, because again both arms are affected similarly (thus canceling out part of the bias).
In summary, some uncertainty around the cost-effectiveness of hip protector for preventing fractures in Germany remains. We do not know to what degree reasons for a rather conservative estimate offset those for a rather optimistic estimate. The fact, however, that the probability of savings is close to 100% makes it likely that hip protectors are truly cost-effective.
We exclusively investigated the effects of external hip protector use in elderly institutionalized residents. For the present, the results of hip protector use in populations of different ages and fracture risks remain open.




