Host range expansion by Rhopalomyia yomogicola (Diptera: Cecidomyiidae) from a native to an alien species of Artemisia (Asteraceae) in Japan
Abstract
In 2001, subconical galls were found on the leaves of an alien Artemisia species (Asteraceae) in Ibusuki City, Kagoshima Prefecture, Japan. These galls were quite similar to those induced by Rhopalomyia yomogicola (Diptera: Cecidomyiidae) on Artemisia princeps, Artemisia montana, and Artemisia japonica in Japan. The morphological features of the pupal head and molecular sequencing data indicated that the gall midge from the alien Artemisia was identical to R. yomogicola. Usually, galling insects do not expand readily their host range to alien plants, but R. yomogicola is considered to have expanded its host range to the alien Artemisia by its multivoltine life history trait and oligophagous habit across two different botanical sections of the genus Artemisia. Adult abdominal tergites and sternites and immature stages of R. yomogicola are described for the first time and detailed biological information is presented.
INTRODUCTION
Several hundred species of alien plants (Angiospermae), called “recent arrivals” (Maekawa 1973), have naturalized in Japan since the 1850s when the Japanese government opened the country to foreign trade after a long-term isolation of about 250 years (Washitani & Morimoto 1993). Since the 1950s, various alien plants, including some congeneric with Japanese species, have been brought into Japan to spray their seeds on slopes to prevent landslip after road construction (Sasaki 2002). These plants include species of Lespedeza (Fabaceae), Sicyos (Cucurbitaceae), Artemisia, Chrysanthemum, Ambrosia (Asteraceae), and other genera of Poaceae. The spreading of alien plants has been causing serious problems in Japan in recent years (e.g. Murakami & Washitani 2002).
Yukawa and Uechi (1999) pointed out that galling insects seldom expand their host range to alien plants because of the difficulty in establishing themselves on newly encountered plants due to difficulties in gall induction, a delicate event during the course of cell division, tissue differentiation, shoot extension, and other processes of plant growth. For example, Japanese gallers utilize only 0.7% of 607 alien Angiospermae species as hosts, which is distinctly lower than the 13.1% of 4162 domestic Angiospermae used (Yukawa & Uechi 1999). Nevertheless, in October 2001 we found several subconical midge galls on leaves of an alien Artemisia species on the roadside of a newly constructed area in Ibusuki City, Kagoshima Prefecture, Japan. These galls were quite similar to those induced by a domestic gall midge, Rhopalomyia yomogicola (Matsumura) (Diptera: Cecidomyiidae), on native Artemisia princeps Pampanini, Artemisia montana (Nakai) Pampanini, and Artemisia japonica Thunberg to Japan (Yukawa 1971; Yukawa & Masuda 1996). Because gall shape is generally species-specific in gall midges (e.g. Yukawa & Rohfritsch 2005), the subconical leaf galls on Japanese and alien Artemisia species are likely to be induced by the same gall midge species, R. yomogicola. To confirm whether the domestic R. yomogicola has expanded its host range to alien Artemisia species, we compare the gall midge from the alien Artemisia to R. yomogicola on the basis of morphological features and molecular sequencing data. Concurrently, we describe adult abdominal tergites and sternites, mature larva, and pupa of R. yomogicola for the first time and present information on its host range, gall, parasitoids, distribution, and life history.
MATERIALS AND METHODS
Collection and preservation of specimens, and morphological study
In October 2001, several subconical leaf galls (Fig. 1) similar to those induced by R. yomogicola (Fig. 2) were collected from an alien species of Artemisia growing on the roadside of a newly constructed area (31°16′20′′N, 130°37′48′′E) in Nishikata, Ibusuki City, Kagoshima Prefecture, Japan (Fig. 3). Three pupae were obtained from these galls. One of them was stored in 99.5% acetone for DNA analysis and two were kept in 75% ethanol for morphological study. The alien plant had leaves that were quite different in shape from those of Artemisia in Japan and was later identified as a species of Artemisia by examining flowers in late November (Mitsuru Hotta, pers. comm., 2001). We suspect that the alien Artemisia is a Chinese species, but species identification is difficult at present without the help of Chinese plant taxonomists.

Subconical leaf gall on Artemisia species. (1) A gall induced by Rhopalomyia sp. on an alien Artemisia species; (2) A gall induced by Rhopalomyia yomogicola on Artemisia princeps.
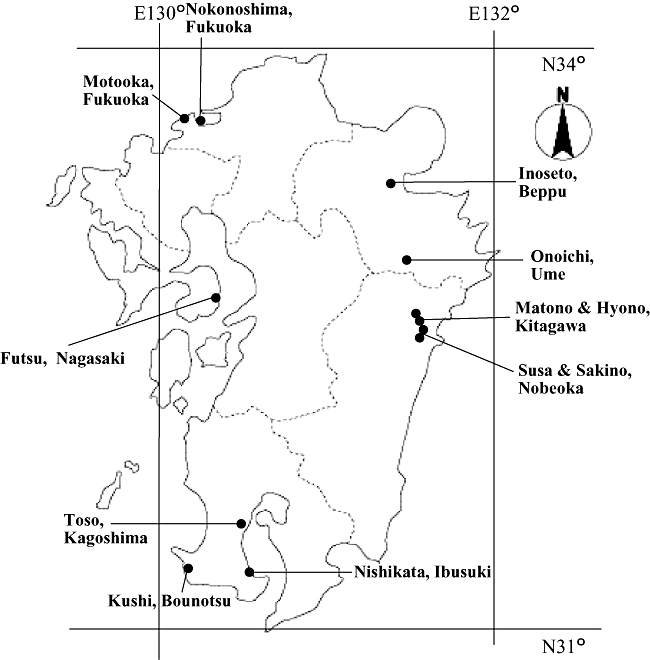
Map of Kyushu, Japan, showing places where specimens of Rhopalomyia sp. on an alien Artemisia and Rhopalomyia yomogicola on Artemisia princeps were collected.
In October 2001, some R. yomogicola galls on A. princeps were collected from Kushi (31°19′53′′N, 130°12′29′′E), Bounotsu, Minamisatsuma City, Kagoshima Prefecture (Fig. 3), which is connected with Nishikata, Ibusuki City by land, and about 42.6 km distant from Nishikata. Two full-grown larvae of R. yomogicola were obtained from these galls and stored in 99.5% acetone for DNA analysis. In addition, mature and immature stages of R. yomogicola were obtained from leaf galls on A. princeps that had been collected from Tate (38°19′57′′N, 140°59′40′′E), Rifu Town, Miyagi Prefecture, Honshu; Nishime (28°19′58′′N, 129°58′22′′E), Kikai Island about 370 km south of Kagoshima City, and various localities in Fukuoka, Oita, Miyazaki, and Kagoshima Prefectures, Kyushu (Fig. 3). Some of them were used for morphological study and remaining specimens were submitted to DNA analysis (Table 1). We also collected R. yomogicola galls on A. montana from Oyahuru (43°11′11′′N, 141°20′19′′E), Ishikari City, Hokkaido.
| Gall midge | Host plant | Collection site (collector) | Collection date | Sample no. | N | Accession no. |
|---|---|---|---|---|---|---|
| Rhopalomyia yomogicola | Artemisia montana | Oyafuru, Ishikari, Hokkaido, Japan (JY, MN) | 30 July 2002 | N163, N167, N168 | 2P, 1L | Ab299108–Ab299110 |
| Rhopalomyia yomogicola | Artemisia princeps | Tate, Rifu, Miyagi, Japan (KG) | 15 Oct 2001 | N59-61 | 3L | Ab299111–Ab299113 |
| Rhopalomyia yomogicola | Artemisia princeps | Nokonoshima, Fukuoka, Japan (MN, NU) | 4 May 2001 | N35-36, N215-218, N220 | 7A | Ab299114–Ab299120 |
| Rhopalomyia yomogicola | Artemisia princeps | Inoseto, Beppu, Oita, Japan (JY, MN) | 28 Aug 2001 | N236-237 | 2L | Ab299121–Ab299122 |
| Rhopalomyia yomogicola | Artemisia princeps | Onoichi, Ume, Oita, Japan (JY, MN, MT) | 15 Nov 2001 | N264 | 1L | Ab299123 |
| Rhopalomyia yomogicola | Artemisia princeps | Matono, Kitagawa, Miyazaki, Japan (JY, MN, MT) | 15 Nov 2001 | N254-255 | 2L | Ab299124–Ab299125 |
| Rhopalomyia yomogicola | Artemisia princeps | Hyono, Kitagawa, Miyazaki, Japan (JY, MN, MT) | 15 Nov 2001 | N256-257 | 2L | Ab299126–Ab299127 |
| Rhopalomyia yomogicola | Artemisia princeps | Susa, Nobeoka, Miyazaki, Japan (JY, MN, MT) | 15 Nov 2001 | N258-261 | 4L | Ab299128–Ab299131 |
| Rhopalomyia yomogicola | Artemisia princeps | Kushi, Bounotsu, Minamisatsuma, Kagoshima, Japan (JY, MN) | 13 Oct 2001 | N62-63 | 2L | Ab299132–Ab299133 |
| Rhopalomyia sp. | Artemisia sp. | Nishikata, Ibusuki, Kagoshima, Japan (JY, MN) | 12 Oct 2001 | N34 | 1P | Ab299134 |
| Outgroup | ||||||
| Asphondylia yushimai | Glycine max | Chikushino, Fukuoka, Japan (NU) | 24 Jul 2000 | 1A | AB164447 | |
| Contarin okadai | Citrus iyo | Tachibana, Yamaguchi, Japan (YH) | 20 Apr 2002 | 1L | AB105485 | |
| Rhopalomyia longitubifex | Artemisia princeps | Matono, Kitagawa, Miyazaki, Japan (JY, MN, MT) | 15 Nov 2001 | 2L | AB162392 | |
- JY, Junichi Yukawa; KG, Katsuo Goukon; MN, Machiko Nohara; MT, Makoto Tokuda; NU, Nami Uechi; YH, Yoshimitsu Higashiura.
- L, mature larva; P, pupa; A, adult (female).
Specimens that had been obtained from the galls on the alien and domestic Artemisia and stored in 70–75% ethanol were mounted on slides for microscopic study in Canada balsam, based on techniques outlined in Gagné (1989). The pupal head was compared between specimens from the two different species of Artemisia, because pupae of Rhopalomyia gall midges are distinctive and useful for species identification (e.g. Ganaha et al. 2004; Ganaha et al. 2007). The slide-mounted specimens examined in this study are kept in the collection of the Entomological Laboratory, Faculty of Agriculture, Kyushu University, Fukuoka, Japan.
Molecular phylogenetic analysis
For every individual analyzed, total DNA was extracted from the whole body with the Dneasy tissue kit (Qiagen, Tokyo, Japan) following the manufacturer's instructions. A region of the cytochrome oxidase subunit I (COI) gene fragment of mtDNA was amplified, purified, sequenced, and electrophoresed following the methods described by Yukawa et al. (2003). See Ganaha et al. (2007) for the primers used for the amplification. The sequence data reported in this study are deposited in the DNA Data Bank of Japan, the European Molecular Biology Laboratory, and the GenBank nucleotide sequence databases with the accession numbers shown in Table 1. The following are outgroup taxa: Asphondylia yushimai Yukawa and Uechi that infests pods of Glycine max (Linnaeus) Merril (Fabaceae) (accession no. AB164447), Contarinia okadai (Miyoshi) collected from blossoms of Citrus iyo Hortorum ex Tanaka (Rutaceae); (accession no. AB105485), a congeneric species Rhopalomyia longitubifex (Shinji) that induces axillary bud galls on Artemisia species (accession no. AB162392) was also included (Table 1).
The sequence data were analyzed with the neighbor-joining (NJ) method using the software package phylip Version 3.66 (Felsenstein 2006). Evolutionary distances were calculated by Kimura's two-parameter method (Kimura 1980). The resulting trees were subjected to bootstrap analysis (Efron 1982; Felsenstein 1985) with 1000 replications for the NJ cladogram.
RESULTS
Morphological comparison of pupal frontal area
Adults and mature larvae could not be obtained from the alien Artemisia to compare with those of R. yomogicola, but fortunately three pupae were taken out of the galls on the alien Artemisia. Two of them were examined, but we could not find any differences between R. yomogicola and the gall midge from the alien Artemisia in pupal characteristics such as antennal horn, cephalic pair of setae, lower and lateral facial papillae, and prothoracic spiracle (Figs 4,5). These features of R. yomogicola are described below on this occasion because the species was previously known only from adults (Yukawa 1971).

Pupal frontal area. (4) Rhopalomyia sp. on an alien Artemisia species; (5) Rhopalomyia yomogicola on Artemisia princeps. Scale lines: 0.1 mm.
Rhopalomyia yomogicola (Matsumura) (4 and 5, 6 and 7)

Rhopalomyia yomogicola. (6) Papillae on larval prothorax, ventral view; (7) Terminal segment of mature larva, ventral view. Scale lines: 0.1 mm.
Asynapta yomogicola Matsumura, 1931: 403.
Diarthromyia yomogicola (Matsumura 1931): Yukawa (1971): 99 (as Diathronomyia).
Rhopalomyia yomogicola (Matsumura 1931): Gagné (1975): 483; Yukawa and Masuda (1996): 307; Gagné (2004): 250.
Rhopalomyia foliorum (Loew 1850): Shinji (1938): 120 (as R. foliorum Kieffer); Shinji (1939a); Shinji (1939b); Shinji (1944) (misidentification).
Rhopalomyia florum (Kieffer 1890): Shinji (1944): 356 (misidentification).
Male and female: See Yukawa (1971) for adult morphological features other than abdominal tergites and sternites. Male abdomen: abdominal tergites I to VI rectangular, wider than long, with caudal rows of setae and a pair of anterior trichoid sensilla; tergite VII narrower than the preceding, the vestiture similarly arranged; tergite VIII not sclerotized, with a few caudal setae and a pair of anterior trichoid sensilla; abdominal sternites II to VII rectangular, wider than long, with middle and caudal rows of setae, and a pair of anterior trichoid sensilla; sternite VIII narrower than sternite VII, middle and caudal rows of setae, and without a pair of anterior trichoid sensilla. Female abdomen: abdominal tergites I to VII as in male; tergite VIII narrower and longer than tergite VII, bare, and with a pair of anterior trichoid sensilla; abdominal sternites II to V as in male; sternites VI to VII quadrate, the vestiture similarly arranged; sternite VIII narrower and longer than sternite VII, not sclerotized, with only a few caudal setae.
Mature larva. Reddish orange; second antennal segment 16.3–18.8 μm long; cervical papillae without seta; six dorsal papillae present on prothoracic segment; eight dorsal papillae present on meso- and metathorax and first through seventh abdominal segments, each with seta, 13.8–18.8 μm long; two dorsal papillae of eighth abdominal segment each with 15.0–16.3 μm long seta; two pleural papillae each with 12.5–21.3 μm long seta; stigma present on prothorax and first through eighth abdominal segments; sternal spatula absent (Fig. 6); lateral papillae reduced to two setose papillae on each side (Fig. 6); two sternal papillae present on each thoracic segment without seta; inner pleural papillae on all thoracic segments with 12.5–17.5 μm long seta; two anterior ventral papillae without seta; the pair of posterior ventral papillae each with 6.3–12.5 µm long seta on first to seventh abdominal segments; two ventral papillae of eighth abdominal segment each with 13.8–15.0 µm long seta; three pairs of terminal papillae each with seta 8.8–15.0 μm long (Fig. 7); two anal papillae without setae.
Pupa. Base of antenna relatively long, well sclerotized, apically pointed (Figs 4,5); cephalic pair of setae elongate, 147.5–218.8 μm in length; frons smooth; one pair of lower facial papillae each with seta, which is 3.8–7.5 μm long; one of three pairs of lateral facial papillae each with 2.5–5.0 μm long seta; prothoracic spiracle 56.3–85.0 μm in length, rounded apically; abdominal spiracles short, rounded apically, present on second to sixth abdominal segments.
Specimens examined: From A. princes: two males and four females (on slide, Cecid. nos. A314–A319), galls collected by M. Shoubu on 9 December 2003 from Nishime, Kikai Town, Kagoshima Prefecture, reared by T. Ganaha on 17 December 2003; four larvae (on slide, Cecid. nos. A320–A323), galls collected by J. Yukawa on 1 November 1971 from Toso, Kagoshima City, Kagoshima Prefecture; five larvae (on slide, Cecid. nos. A324–A328), galls collected by J. Yukawa and T. Ganaha on 4 November 2004 from Sakino, Nobeoka City, Miyazaki Prefecture; four pupae (on slide, Cecid. nos. A329–A332), galls collected by M. Nohara on 12 May 2000 from Futsu Town, Nagasaki Prefecture; one pupa (on slide, Cecid. No. A333), a gall collected by J. Yukawa and M. Nohara on 31 August 2001 from Inoseto, Beppu City, Oita Prefecture; one pupa (on slide, Cecid. No. A334), a gall collected by M. Shoubu on 9 December 2003 from Nishime, Kikai Town, Kagoshima Prefecture; two pupae (on slide, Cecid. nos. A335–A336), galls collected by J. Yukawa and T. Ganaha on 2 August 2004 from Motooka, Fukuoka City, Fukuoka Prefecture. From alien Artemisia: two pupae (on slide, Cecid. nos. A337–A338), galls collected by J. Yukawa and M. Nohara on 12 October 2001 from Nishikata, Ibusuki City, Kagoshima Prefecture.
Remarks: Shinji (1938) ignored the original description of R. yomogicola (Matsumura 1931) and identified this species as Rhopalomyia foliorum (Loew 1850) (incorrectly as R. foliorum Kieffer). He used this name in his subsequent papers (Shinji 1939a,b). Later, Shinji (1944) identified this species as Rhopalomyia florum (Kieffer 1890) but used the name R. foliorum in the explanation of its photograph. Rhopalomyia foliorum induces suboval leaf galls on Artemisia vulgaris Linnaeus in Europe (see figs 18–20 of Plate 22 in Rübsaamen & Hedicke 1926), which are quite different in shape from the subconical leaf galls of R. yomogicola on Artemisia species in Japan. Morphologically R. yomogicola can be distinguished from R. foliorum as follows: in R. yomogicola, two ventral papillae on larval eighth abdominal segment 13.8–15.0 μm in length; base of pupal antennal sheath relatively long, well sclerotized, apically pointed; pupal prothoracic spiracle 56.3–85.0 μm in length; in R. foliorum, two ventral papillae on larval eighth abdominal segment 7.5–8.8 μm in length; base of pupal antennal sheath undeveloped, gently rounded apically; pupal prothoracic spiracle 30.0–40.0 μm in length.
As pointed out by Yukawa (1971), R. florum is certainly different from R. yomogicola, because larvae of R. florum live in the misshapen capitula and flowers on A. vulgaris in Europe (Kieffer 1890; Barnes 1949), while R. yomogicola has been reared only from conical leaf galls on Japanese Artemisia (Yukawa & Masuda 1996).
Biological information
Host plants. Artemisia princeps Pampanini, A. montana (Nakai) Pampanini, and A. japonica Thunberg (Asteraceae) are known to be host plants of R. yomogicola (e.g. Shinji 1944; Yukawa & Masuda 1996). The first two species belong to the section Artemisia and the last one to the section Dracunculus of the genus Artemisia (Kitamura et al. 1970). Current molecular sequencing data revealed that A. princeps and A. montana are included in the host range of R. yomogicola, but unfortunately specimens of R. yomogicola from A. japonica were not available for molecular phylogenetic analysis.
Gall. Subconical swellings are induced usually on the upper surface of leaves. Galls are green to yellowish green in the beginning, later turn to pinkish red, and are covered with fine whitish hairs. Gall height is 1.6–8.0 mm and maximum diameter is 1.6–3.5 mm. Each gall consists of a single larval chamber occupied by one gall midge larva or pupa.
Parasitoids in Japan. Platygastrid and pteromalid wasps have been reared from the galls (Yukawa & Masuda 1996).
Distribution. Japan (Hokkaido, Honshu, Shikoku, Kyushu, Sadogashima Island, Tanegashima Island, Yakushima Island, and the Nansei Islands) and the Korean Peninsula (Yukawa & Masuda 1996).
Life history. Collecting and dissecting data indicate that this species is multivoltine with overlapping generations, and overwinters as mature larvae in the galls (Yukawa 1971; Yukawa & Masuda 1996).
Molecular phylogenetic analysis
The length of the amplified mitochondrial COI gene fragment was 439 bp. Monophyly of the clade including all R. yomogicola populations on A. princeps and A. montana from different localities in Japan and the gall midge obtained from the alien Artemisia species was supported by a high bootstrap value (Fig. 8). The sequencing data of the gall midge from the alien Artemisia completely coincided with those of R. yomogicola from Bounotsu. Some other haplotypes were found in R. yomogicola populations on different host plants and from different localities in Japan. The maximum genetic distance was 0.055, which was recorded between individuals from Onoichi, Ume Town (N 264) and Hyono, Kitagawa Town (N 256). The number of different nucleotides and deduced amino acid residues between them were 23 (5.24% of 439 bp) and 2 (1.37% of 146), respectively.
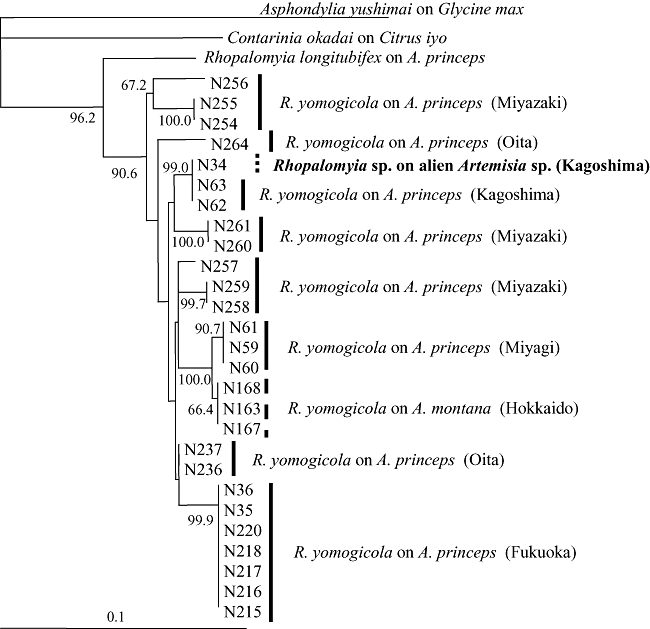
Neighbor-joining tree based on 439 bp of the mtDNA cytochrome oxidase subunit I gene for Rhopalomyia sp. on an alien Artemisia species and Rhopalomyia yomogicola on Artemisia princeps and Artemisia Montana in Japan. Bootstrap values are indicated for nodes gaining more than 66% support (1000 replications). Contarinia okadai and Asphondylia yushimai were used as outgroup species.
DISCUSSION
Morphological features (Figs 4,5) and sequencing data (Fig. 8) indicate that the gall midge that induces subconical leaf galls on the alien Artemisia species is identical with R. yomogicola that is responsible for similar leaf galls on A. princeps and A. montana in Japan. This means that R. yomogicola seems to have expanded its host range quite recently from A. princeps to the alien Artemisia, which has been spreading after introduction to Japan to aid against landslip on slopes.
As mentioned in the introduction, gallers do not expand readily their host range to alien plants because of their galling habit (Yukawa & Uechi 1999). In addition, the oviposition seasons, particularly of such short-lived insects as gall midges, must synchronize well with the host plant phenology, for otherwise they cannot induce galls even on their appropriate host plant species (Yukawa 2000). Therefore, the host range expansion to the alien plant by R. yomogicola seems to be relatively unusual for gall midges, but it could be realized by its oligophagous habit and multivoltine life history pattern. Rhopalomyia yomogicola induces galls on A. princeps, A. montana, and A. japonica (Yukawa & Masuda 1996) across two different botanical sections, Artemisia and Dracunculus, of the genus Artemisia (Kitamura et al. 1970). Thus, R. yomogicola may have a potential for galling on a wide range of congeneric plant species. In addition, R. yomogicola is a multivoltine species and would have frequent opportunities to synchronize with Artemisia species than univoltine gall midges, because Artemisia species produce new shoots continuously during growing seasons.
ACKNOWLEDGMENTS
We thank Dr R. J. Gagné (Systematic Entomology Laboratory, Plant Science Institute, USDA, USA) reviewing the manuscript of this paper. We are grateful to Emeritus Prof. M. Hotta (Kagoshima University) who identified the alien Artemisia and to Dr M. Tokuda, Ms M. Shoubu, and Dr K. Goukon for their help in collecting Rhopalomyia gall midges. Our thanks are also due to Prof. O. Tadauchi, Dr S. Kamitani, and Mr D. Yamaguchi (Kyushu University) for their support in various ways. This study was partly supported by the Foundation for Riverfront Improvement and Restoration, Tokyo, Japan to J. Y. This study was also supported partly by the Research Fellowship of the Japanese Society for the Promotion of Sciences for Young Scientists to N. U. This is a contribution from the Entomological Laboratory, Faculty of Agriculture, Kyushu University, Fukuoka (series 6, no. 31).




