Fungivory of Anatatha lignea, an interesting habit in Noctuidae (Lepidoptera)
Abstract
The larvae of Anatatha lignea (Butler) were found consuming the brown surface of synthetic logs on which the shiitake mushroom, Lentinula edodes (Berk.) Pegler, formed a thick mycelial coat. Fungivory has been rarely reported in the Noctuidae, and this is the first record of fungivory from the genus Anatatha. The female genitalia and the immature stages of A. lignea are illustrated and described for the first time. Its lectotype is designated and illustrated, and details of the species’ biology is given. The female genitalia of A. misae Sugi, a species closely related to A. lignea, are illustrated and described for the first time.
INTRODUCTION
Many beetles and flies feed on fungi, and sometimes cause damage to cultivated mushrooms, but fungivory is rare in the Lepidoptera. In his review of mycophagy in Lepidoptera of the world, Rawlins (1984) wrote “Mycophagous habits may be divided into two general types. Feeding on free fungi, especially sporophores, is called fungivory to distinguish it from feeding on the fungal hosts of algae (lichens), which is called lichenivory” (p. 1). As noted by Rawlins, lichenivory is often seen only in the largest lepidopteran suborder, Ditrysia, whereas obligate fungivory is rarer and only found in the Tineidae, Arrhenophanidae, Phyctiinae (Pyralidae), and Noctuidae. In the family Noctuidae, only a small number of fungivorous moths have been reported until now.
Yoshimatsu and Nakata (2003) reported that a fungivorous moth, Diomea cremata (Butler, 1878) (Noctuidae), has become a new pest of the shiitake mushroom, Lentinula edodes (Berk.) Pegler, which is cultivated on synthetic logs (mainly composed of sawdust). They noted that the larvae mainly consume the brown surface of synthetic logs. In Japan, a tineid moth, Morophagoides moriutii Robinson, 1986, is a notorious pest of shiitake mushrooms when cultivated using natural logs, but damage by this moth has never been noted on synthetic logs.
When we found larvae of another noctuid moth feeding on the synthetic logs used in shiitake cultivation, on the basis of superficial features, we considered Anatatha lignea (Butler, 1879), which is distributed in Japan, Korea and the Russian Far East. However, detailed comparison of the morphology of the last instar larvae of our material with that of “A. lignea” given in Nakamura (1989) revealed that they are different species.
Anatatha misae Sugi, 1982, a species closely related to A. lignea, is also distributed in Japan and Korea (Kononenko et al. 1998). Detailed morphological comparison of these two species, however, has not yet been carried out, which prevents us from identifying them. To establish their comparative features, the type specimens of both species were examined. In this paper, fungivory is reported for the first time from the genus Anatatha, which currently comprises seven species (Poole 1989; Kononenko et al. 1998).
MATERIALS AND METHODS
The larvae were collected from an un-air-conditioned shiitake cultivation house at Hiyoshi-mura, Ehime Prefecture, on 2 August and 6 October 2000. They were brought to a laboratory alive with the synthetic logs and were reared until they emerged as adults. The dried specimens of adults, pupae, pupal exuviae and cocoons, and the larvae preserved in 70% ethanol were used for the descriptions.
Except for the lectotype and paralectotypes of A. lignea deposited in the Natural History Museum, London, England (NHM), all specimens used in this study are deposited in the National Institute for Agro-Environmental Sciences, Tsukuba, Japan (NIAES).
RESULTS
Description
Anatatha lignea (Butler) (1–6-20–23)

Adults and labels of Anatatha spp. 1 A. lignea, lectotype; 2 labels of the lectotype of A. lignea; 3 A. lignea, male; 4 A. lignea, female; 5 A. misae, male paratype; and 6 A. misae, female. Arrow indicates the underside.
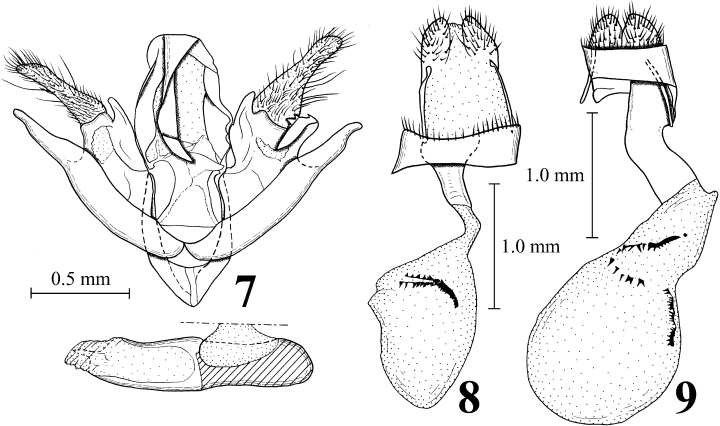
Male and female genitalia of Anatatha spp. 7 Male genitalia of A. lignea; 8 female genitalia of A. lignea; and 9 female genitalia of A. misae.
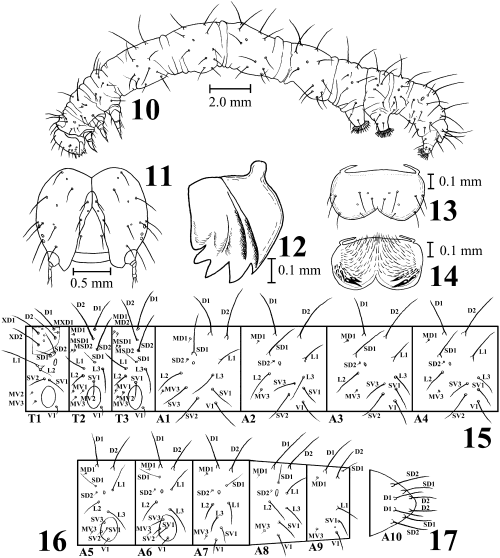
Last instar larva of Anatatha lignea. 10 Larva in left lateral view; 11 head in frontal view; 12 right mandible in inner view; 13 labrum in dorsal view; 14 labrum in ventral view; 15 setal map of thorax and abdominal segments I–IV; 16 setal map of abdominal segments V–IX; and 17 anal plate.
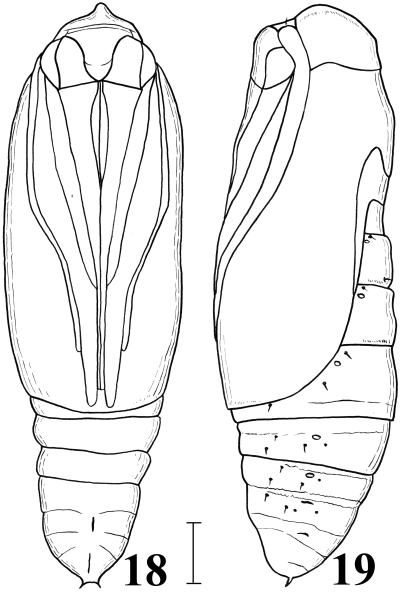
Female pupa of Anatatha lignea. 18 Ventral view; 19 left lateral view. Scale line: 1 mm.
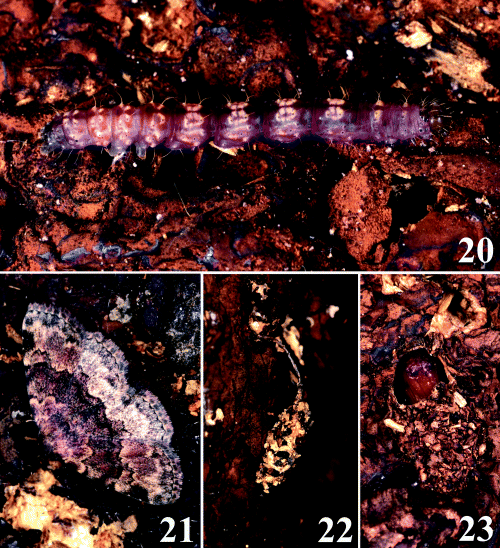
Anatatha lignea on the surface of synthetic logs used to grow shiitake mushrooms. 20 Last instar larva; 21 resting posture of adult; 22 cocoon, summer type; and 23 cocoon, winter type, upper portion of cocoon removed.
-
Bleptina lignea Butler, 1879: 64, pl. 56, fig. 14.
-
Anatatha lignea: Sugi (1961): 101 (♂), fig. 4; Sugi (1982): 882 (♂), pl. 218: 40, 377: 9; Poole (1989): 81.
Male genitalia. As shown in Fig. 7 and Sugi (1961). Right and left valvae asymmetric.
Female genitalia. As shown in Fig. 8. Ductus bursae short, narrow and sclerotized. Corpus bursae egg-shaped, membranous, with signa represented by a left spiny plate and dorsal and ventral series of spines connected with the spiny plate.
Last instar larva (10–17, 20–23). Body length approximately 25 mm. Head brown with broad dark brown lines along adfrontal sutures, and with long white setae. Mandible with five outer teeth and internal ridges, but no internal tooth. Body slender, purplish brown or dark brown, sometimes with several white rings or spots in each segment. Dorsal and subdorsal lines blackish brown, but sometimes indistinct. Thoracic legs brown. Cervical shield brown. On prothorax, setae SD1 and SD2 on a common pinaculum and setae L1 and L2 also on a common pinaculum. Abdominal segments I–IV long. Abdominal segment I with seta D1 posterodorsad to spiracle and with seta SV3 anterodorsad to seta SV2. Abdominal segments III and IV lacking in legs. Abdominal segments V and VI with well-developed legs. Crochets lateroseries and almost uniordinal. Abdominal setae long and white, almost always forming chalaza in D1 and D2, and sometimes forming chalaza in SD1 and L1. Spiracle black. Pinaculum usually dark brown. Anal plates brown.
Pupa (18,19). Body length about 10 mm. Frons crested at the tip. Abdominal segment X with a pair of tiny spines at the caudal end.
Lectotype (1–6). ♀, here designated, Yokohama, “Japan 77-9,” F. M. Jonas, “Bleptina lignea Butler Type,”“Noctuidae General prep. no. 19074 ♀.”
Specimens examined. Adults: 2♀ (paralectotypes, here designated), Yokohama, “Japan 77-9,” F. M. Jonas, “Bleptina lignea Butler”; 1♀, Nikamaki, Niigata, 9.viii.1970, R. Sato; 1♂, Mount Akiba, Niigata, 8.viii.1961, R. Sato; 1♂, Sugigawa, Niigata, 22.vi.1962, R. Sato; 1♂, Ohira, Tochigi, 5.viii.1960, I. Ochiai; 1♂, Aoyama, Tokyo, 25.vii.1957, H. Matsuura; 3♂1♀, Nuno, Asa-cho, Asakita-ku, Hiroshima City, Y. Yamate (1♂, 16.vi.1992; 1♂, 6.vii.1992; 1♂, 20.vii.1992; 1♀, 1.viii.1992); 1♂, Shikoku, T. Kondani Collection; 3♂2♀, Kamiita, Chichinokawa-shimo, Hiyoshi-mura, Ehime, larvae collected 2.viii.2000, Y. Nakata (adult emergence: 1♂, 16.viii.2000; 1♀, 17.viii.2000; 1♀, 21.viii.2000; 2♂, 5.ix.2000); 7♂4♀, same locality and collector, immature stages collected 6.x.2000 (adult emergence: 1♀, 30.xi.2000; 3♂, 1.v.2001; 1♂, 7.v.2001; 1♀, 14.v.2001; 1♀, 18.v.2001; 2♂, 24.v.2001; 1♂, 27.vi.2001; 1♀, 30.v.2001); 1♂2♀, same locality and collector, immature stages collected 6.x.2000 and kept at 20–23°C after 24.xi.2000 (adult emergence: 1♀, 24.iv.2001; 1♂1♀, 1.v.2001); 1♂, Yoshii, Fukuoka, 28.v.1957, N. Gyotoku. [Last instar larvae] 3 exs., viii.2000, Chichinokawa-shimo, Hiyoshi-mura, Ehime. [Pupae and pupal exuviae] 4 exs., viii.2000, Chichinokawa-shimo, Hiyoshi-mura, Ehime.
Remarks. Butler (1879) clearly noted that the specimen(s) of A. lignea he examined were collected in Yokohama by Jonas, but he did not refer to the sexes and the number of the specimens. Dr J. D. Holloway and Mr M. R. Honey of the NHM informed us that the NHM houses three female syntypes of A. lignea, and they sent us photographs of the female genitalia of one of the syntypes. When Dr K. Ueda of the Kitakyushu Museum of Natural History and Human History, Kitakyushu, Japan (KMNH) visited the NHM, he kindly took photographs of these three specimens and all labels attached to them (Fig. 2). Through our study it became clear that adults of A. lignea and its close relative A. misae (Figs 5,6) are superficially distinguishable: while the basal half the hindwing is fuscous in A. lignea, it is gray and more or less paler than the ground color in A. misae. The two paralectotypes have external characters indicating that they are conspecific with the lectotype, although their genitalia have not been examined. Examination of adult external characters and female genitalia showed that our own materials are conspecific with the lectotype of A. lignea. Female genitalia of A. lignea (Fig. 8) differ from those of A. misae (Fig. 9) as follows: signa represented by a left spiny plate and dorsal and ventral series of spines connected with the spiny plate (in A. misae, represented by a dorsal spiny plate in posterior part of corpus bursae, a ventral spiny plate at the middle of the corpus bursae and a series of spines connected to the two plates) and ductus bursae narrow in dorsal view (wide in A. misae). The main differences in morphology of the last instar larva of the “A. lignea” of Nakamura (1989) and that collected in the present study are as follows (the characteristics given by Nakamura are inserted in parentheses): on the prothorax, SD1 and SD2 are located on a common pinaculum and L1 and L2 are also located on a common pinaculum (no pinaculum is developed around SD1, SD2, L1 and L2); on the abdominal segments I–IV, the distance between D1 and D2 is always shorter (longer) than that between SD1 and L1; on the abdominal segments I–IV, the SV group is trisetose (bisetose); on the abdominal segments VII and VIII, SV2 is absent (present); the number of outer mandibular teeth is five (four); the M3 labral setae are short and just reach the ventral margin of the labrum (apparently beyond the ventral margin of the labrum).
Biology
Damage to the shiitake mushroom. The larvae of A. lignea usually consume the thick brown mycelial coat formed on the surface of synthetic logs. In damaged areas, the yellowish-white inside of the logs is exposed, which later becomes darker and indistinct as in the case of Diomea cremata (Yoshimatsu & Nakata 2003; fig. 1A). The larvae sometimes feed on old, unharvested, withered fruit bodies left on the logs, but they never feed on fresh fruit bodies growing on the logs. When the mycelial coat formed on the surface of the synthetic logs is damaged severely, the amount of fruit bodies harvested will decrease markedly.
Bionomics. In the shiitake mushroom cultivation house, many newly emerged adults and a small number of mature larvae were observed on 2 August, whereas on 6 October many larvae and no adults were present. The larvae collected in August made cocoons and pupated, and adult moths emerged in late August through to September The larvae in October made cocoons in late November, pupated soon afterwards, then hibernated, and new adults emerged in April through to June of the next year. It is likely that the adult moths emerge twice per year. There were two types of cocoons: those observed in the summer, which had a long stem arising from the dorsal portion (Fig. 22), and those made in November in which larvae hibernated, which were attached directly to the surface of the synthetic logs without a stem (Fig. 23). In both cases, mature larvae cut off the surface of the synthetic logs piece by piece, and utilized these pieces to make cocoons.
DISCUSSION
The larvae that Nakamura (1989) successfully reared on dead leaves are different from the larvae in the present study in many respects, as mentioned above. However, the specimens used by Nakamura (1989) are presently unavailable, and their specific affinities are not clear. The nutritive content of most fungi is comparable to or greater than that of higher plants, and gaining access to these nutrients is not a problem for general herbivores, as noted by Rawlins (1984). Therefore, many beetles and flies utilize fungi as host substrates. Rawlins (1984) also noted that:
. . . it is clear that the lichenivorous and fungivorous lineages of higher Ditrysia are relative derived assemblages whose morphology relies on characters other than diet (e.g. Herminiinae or Ophiderinae of the Noctuidae). These relationships suggest that the mycophagy has arisen repeatedly, although perhaps in similar, gradualistic manner. The restriction of mycophagy entirely to the suborder Ditrysia implies that such habits are apomorphic not only in Lepidoptera but in Ditrysia as well.
The discovery of a fungivorous habit in A. lignea is important in supporting Rawlin’s hypothesis that such habits have arisen independently several times in the Lepidoptera.
ACKNOWLEDGMENTS
We thank Mr M. R. Honey and Dr J. D. Holloway of the NHM for giving us information on the types of Anatatha lignea and fungivorous moths. Dr K. Ueda of the KMNH kindly provided us with photographs of all syntypes of A. lignea when he visited the NHM. We are grateful to Mr S. Sugi, Tokyo, Dr R. Sato, Niigata, and Mr I. Sagara, Hiroshima, for the donation of their valuable specimens to the NIAES, Tsukuba. Thanks are also due to Dr M. Nakamura for kindly providing information on his collection of A. lignea.




