The role of ribavirin in direct acting antiviral drug regimens for chronic hepatitis C
Abstract
Despite years of clinical use and extensive research efforts, the mechanism of action of ribavirin (RBV) is not well understood. Although it has only a mild, transient antiviral effect on HCV replication when administered as monotherapy, when combined with interferon, RBV improves sustained virological response (SVR) rates by approximately 25–30%. Proposed mechanisms of action for RBV against HCV include (1) a direct effect against the HCV RNA dependent RNA polymerase; (2) induction of misincorporation of nucleotides leading to lethal mutagenesis; (3) depletion of intracellular pools via inhibition of inosine monophosphate dehydrogenase; (4) alteration in the cytokine balance between a Th2 profile (anti-inflammatory) to a Th1 profile (pro-inflammatory); and (5) potentiating the effect of interferon via up-regulation of genes involved in interferon signalling. Given the lack of a clear understanding of RBV mechanism of action, it has been challenging to confidently position this drug with new direct antiviral agents (DAA). However, early clinical studies provide strong evidence for a benefit of RBV in combination with DAAs for both IFN containing and sparing regimens. The addition of RBV reduces viral breakthroughs and/or relapses, at least when drugs with low to moderate genetic barriers to resistance are paired together. This is particularly true in patients harbouring HCV subtype 1a. Ongoing studies are now addressing the utility of RBV in nucleoside containing DAA regimens, which offer both potent antiviral activity as well as a high genetic barrier to resistance. It is remarkable that the age-old question of the role of RBV in the future of HCV therapy remains as real today as it was two decades ago.
Abbreviations
-
- BOC
-
- boceprevir
-
- DAA
-
- direct acting antivirals
-
- HCV
-
- hepatitis C virus
-
- IFN
-
- interferon
-
- PEG-IFN
-
- pegylated interferon
-
- RBV
-
- ribavirin
-
- SOC
-
- standard of care
-
- SVR
-
- sustained viral response
-
- TVR
-
- teleprevir
In 1994, Brillanti et al. demonstrated that ribavirin, a purine-nucleotide analogue, associated with interferon-α gave promising results 1. Ribavirin (RBV), has become a critical component of successful hepatitis C virus (HCV) treatment with interferon-based therapy 2, 3. Despite only a mild, transient antiviral effect on HCV replication when administered as monotherapy 4, when combined with interferon, RBV improves sustained virological response (SVR) rates by approximately 25–30%. The improvement in SVR rates with the addition of RBV has been largely attributed to the prevention of relapse after treatment is completed. RBV has been shown to prevent relapse by significantly accelerating the second slope of viral-RNA decrease in patients who have a response to the antiviral effect of peginterferon (PEG-IFN) alfa 5. However, despite years of clinical use and extensive research efforts, the mechanism of action of RBV is not well understood. Proposed mechanisms of action for RBV against HCV include (1) a direct effect against the HCV RNA dependent RNA polymerase 6; (2) induction of misincorporation of nucleotides leading to lethal mutagenesis 7; (3) depletion of intracellular pools via inhibition of inosine monophosphate dehydrogenase; (4) alteration in the cytokine balance between a Th2 profile (anti-inflammatory) to a Th1 profile (pro-inflammatory); and (5) potentiating the effect of IFN via up-regulation of genes involved in IFN signalling 8-11. It has been shown that in patients who fail treatment, several interferon-stimulated genes are upregulated before treatment 9.
Benefit of ribavirin in interferon and direct acting antivirals regimens
Given the lack of a clear understanding of the mechanism of action of RBV and its minimal direct antiviral activity, it has been challenging to confidently position this drug with new direct antiviral agents (DAA). However, early clinical studies provide strong evidence for a benefit of RBV in certain DAA drug regimens. The first DAA study to demonstrate the potential impact of RBV involved RG1626, a nucleoside inhibitor 12. A phase 2 study evaluated the safety and efficacy of RG1626 in combination with PEG-IFN, with or without RBV, vs. standard of care (SOC) in treatment-naïve patients with chronic HCV genotype 1 infection. A marked increase in antiviral activity was found in patients who received RBV in combination with RG1626 and PEG-IFN (Table 1). As the effects of RBV alone on HCV RNA levels were minimal (< 0.5 log10 IU/ml over a 4-week period), the additional reduction in mean HCV RNA level at week 4 by 1.6 log10 IU/ml (observed when comparing the TRIPLE 1500 and DUAL 1500 arms) was suggestive of a synergistic effect in these patients. There was no evident pharmacokinetic interaction between RG1626 and RBV leading to increased RG1626 plasma levels that could explain the observed synergy.
| Treatment arm | Mean reduction in HCV RNA level from baseline log10 (median; range) IU/ml | |||
|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | |
| DUAL 1500 | 2.4 (2.1; 0.5–4.0) | 3.1 (3.1; 0.0–5.5) | 3.9 (4.2; + 0.1–6.4) | 3.6 (4.2; 0.1–6.3) |
| TRIPLE 1500 | 3.3 (3.0; 1.2–5.2) | 4.6 (4.7; 2.5–6.6) | 5.1 (5.2; 3.0–6.6) | 5.2 (5.3; 3.1–6.6) |
| SOC | 1.1 (0.8; + 0.2–3.8); | 1.6 (1.1; 0.1–3.8); | 2.1 (1.7; + 0.1–5.4); | 2.4 (2.5; 0.2–5.2); |
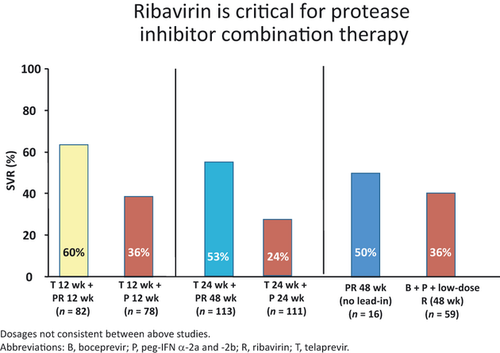
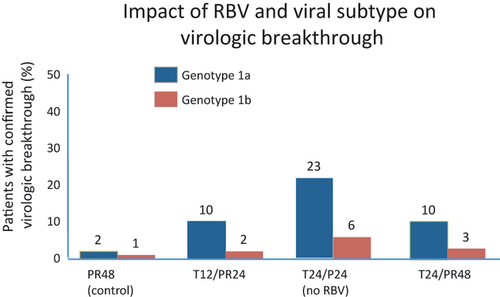
In vitro data have also suggested that RBV has an additive antiviral activity when combined with protease inhibitors and synergistic effect in combination with IFN and a protease inhibitor 13. Three recent large phase 2 clinical trials were designed to help evaluate the relative impact of RBV in combination with PEG-IFN and protease inhibitors. PROVE II and III had arms randomized with the presence/absence of RBV and SPRINT-1 evaluated the utility of low dose RBV. In the PROVE II trial, a shorter duration of treatment was explored for the protease inhibitor telaprevir (TVR) with treatment groups receiving triple therapy (TVR + PEG-IFN/RBV) for only 12 weeks (with and without RBV) compared with an additional 12 weeks of SOC. SVR was 46% in the control group, compared with 36% in the non-RBV group (P = 0.20), 60% in the 12 week triple therapy TVR group (P = 0.12) and 69% in the 24 week triple therapy TVR group (P = 0.004). Relapse rates were highest in the non-RBV treated group (48%) compared with the control group (22%), 12 week triple therapy group (30%) and 24 week triple therapy group (14%) 14. RBV reduced the risk of selecting for telaprevir resistance and virological breakthrough. PROVE III evaluated genotype 1 chronic HCV patients who were non-responders, partial responders, or relapsers following a prior course of at least 12 weeks of PEG-IFN plus RBV 15. Participants were assigned to four treatment arms receiving TVR plus PEG-IFN, with or without RBV. A higher breakthrough rate (32%), higher relapse rate (53%), and lower SVR rate (24%) was observed in the T24P24 group compared with the T12PR24 group and the T24PR48 group, again demonstrating improved outcomes when RBV was used in combination with TVR and PEG-IFN.
HCV SPRINT-1 study was a phase 2 study where HCV genotype 1 patients were randomly assigned to receive different combinations of PEG-IFN, RBV (400–1400 mg/day) and boceprevir (BOC) (BOC, 800 mg TID) 16. The treatment regimens included a control group treated with 48 weeks of SOC compared with five BOC treatment regimens (4 weeks of PEG-IFN/RBV lead-in followed by triple therapy for 24 or 44 weeks; triple therapy for 28 or 48 weeks; triple therapy, but with low dose RBV for 48 weeks). The following SVR rates were reported: control group-38%; triple therapy 28 weeks-55%; triple therapy 48 weeks-67%; lead-in group 28 weeks-56%; lead-in group 48 weeks-75%; low dose RBV group-36%. The use of low-dose RBV in combination with PEG-IFN and BOC, while reducing haematological toxic effects, did not improve SVR rates compared with control. Again, the highest reported viral breakthrough was seen in low dose RBV group 16. In summary, patients that did not receive RBV in the PROVE trials and those with low dose RBV in the SPRINT-1 trial had increased viral breakthrough, higher relapse rates, and lower SVR (see Figs 1 and 2). Together, this data strongly indicates that standard-dose RBV is required to optimize response to first generation protease inhibitors via a reduction in the development of resistance and breakthrough. Likely mechanisms include a weak direct antiviral activity, lethal mutagenesis, along with induction of interferon sensitive genes.
Benefit of ribavirin in interferon-free regimens
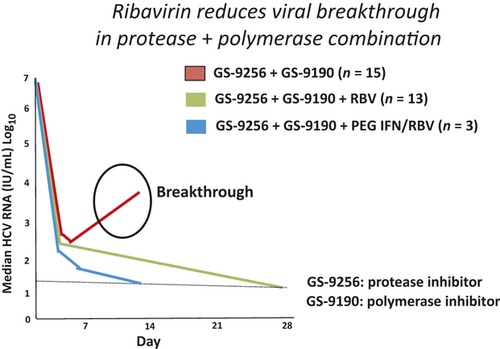
With the recent proof of concept that select patients can be cured with IFN-free DAA combinations 17, 18, there is great interest in also evaluating the necessity of RBV in the absence of IFN-containing regimens. Phase 2 clinical studies have shown that the efficacy of an IFN-free combination therapy involving a protease and a polymerase inhibitor can be strongly enhanced by adding RBV 19, 20. One of the most dramatic examples for a beneficial effect of RBV comes from a trial evaluating GS-9256 (protease inhibitor) plus a NS5B polymerase inhibitor GS9190 (tegobuvir), with or without RBV in a group of treatment-naïve genotype 1 patients 21. In this study design, 16 patients were randomly assigned to receive dual therapy with the two direct-acting agents alone (75 mg GS-9256 and 40 mg GS-9190, both twice-daily), 15 received triple therapy with these drugs plus 1000–1200 mg/day weight-adjusted RBV, and 15 were to be treated with quadruple therapy using the same three drugs plus 180 μg/week PEG-IFN α-2a for up to 28 days. Median maximum HCV viral load declines at day 28 were −4.1 log10 in the two drug group and −5.1 log10 in the three drug group, indicating that RBV enhanced the antiviral activity of GS-9256 plus GS-9190, even without IFN. Here too, some participants experienced virological breakthrough (see Fig. 3). Further analysis showed that most patients with virological breakthrough had HCV isolates with NS3 plus NS5B resistance mutations. At the time of analysis, all 14 participants who had taken the four drug regimen of GS-9256/GS-9190 plus standard therapy for 28 days achieved RVR (HCV RNA < 25 IU/ml) without viral breakthrough.
The ZENITH study is investigating the safety and antiviral activity of combination treatment with two doses of VX–222 (non–competitive inhibitor of the HCV NS5B polymerase) and a fixed dose of TVR administered with and without PEG-IFN and/or RBV for 12 weeks in treatment-naïve patients with genotype 1 chronic HCV infection 22. Both dual regimens (without RBV) were terminated after the arms met prespecified criteria for discontinuation because of a viral breakthrough rate ≥ 25%. Of note, HCV subtype 1a was present in 11/12 patients who experienced viral breakthroughs. No patient in either quad arm experienced viral breakthrough. This study was amended to add a new arm of IFN-free that added RBV to TVR and VX-222 in an attempt to prevent virological breakthrough. Clearly, the early results from ZENITH support a role for RBV in DAA combinations, especially when low genetic barrier agents are combined.
At this time, it seems that the addition of RBV reduces viral breakthroughs and/or relapses, at least when protease inhibitors are combined with non-nucleoside polymerase inhibitors. This is particularly true in patients harbouring HCV subtype 1a. However, as drug development progresses, and agents with more potent antiviral activity emerge, the addition of RBV may not be necessary. For example, all DAA except nucleos(t)ide analogues uniformly show rapid selection of resistance mutations in most patients when given as monotherapy. It is noteworthy that resistance mutations to nucleos(t)ide inhibitors have been selected in vitro, but as yet not shown in vivo, most likely reflecting their dramatic impact on viral replication. In the NUCLEAR study, PSI-7977 (pyrimidine) and PSI-938 (purine) were given alone or in combination for 14 days to 32 HCV genotype 1 patients without RBV 23. Mean viral load decline was 5 log10 in the combination arms and none of the patients selected drug resistance mutations. If the benefit of RBV in DAA regimens is the prevention of viral breakthrough from resistance, then the robustness of nucleos(t)ide analogues might be sufficient so that RBV will not be needed in IFN-sparing combination therapies. An ongoing study (QUANTUM) will evaluate the utility of RBV in an IFN-free regimen involving nucleoside inhibitors and provide data to help answer this question.
In summary, it appears that there is ample evidence to support the critical role of RBV in combination with DAA for both IFN containing and sparing regimens. Data suggest that, even in the absence of IFN, RBV accelerates the second slope of viral decline, prevents relapses and viral breakthrough, which may translate into increased SVR rates, through molecular mechanisms that remain to be defined. Thus, most DAA regimens to date have included RBV 24. However, most of the available data has been generated with first generation DAA which have a relative low-moderate genetic barrier to resistance. As RBV increases the risk of anaemia (RBV monotherapy leads to a mean drop in haemoglobin of approximately 2 g/dl over a 4 week period) 25, adds to the overall pill burden and includes a risk of drug-drug interactions there is still a desire to eliminate it from future DAA combinations. Ongoing studies are now addressing the utility of RBV in nucleoside containing DAA regimens, which offer both potent antiviral activity as well as a high genetic barrier to resistance. It is remarkable that the age-old question of the role of RBV in the future of HCV therapy remains as real today as it was two decades ago.
Conflicts of interest
DRN is supported in part by the NIH/NCRR Clinical and Translational Science Award to the University of Florida UL1 RR029890.
DRN and VC have received research support from Abbot, BMS, Genentech, Gilead, Merck, Pharmasset, Vertex. DRN has served as a consultant for Genentech, Merch Pharmasset and Vertex.




