Establishment of four pairs of canine mammary tumour cell lines derived from primary and metastatic origin and their E-cadherin expression
Abstract
Four new pairs of canine mammary carcinoma cell lines derived from both primary and metastatic lesions were established. The cells were cultured in RPMI-1640 with 10% fetal bovine serum and they showed stable growth for more than 120 passages. Using these cell lines, the expression of E-cadherin was measured by flow cytometry and the function of E-cadherin was evaluated by cell aggregation assay and results from the primary and metastatic lesions were compared statistically. E-cadherin was strongly expressed in all of the cell lines, without a notable difference between cells of primary and metastatic origin. In the cell aggregation assay, the function of E-cadherin was significantly weaker in the cells of primary origin (p < 0.05), as compared with cells of metastatic origin. The present results suggest that a reduction in E-cadherin function may be implicated in the invasive and metastatic potential of canine mammary tumour cells; however, further study will be needed to clarify E-cadherin function in the context of the metastasis of canine mammary carcinoma.
Introduction
Canine mammary tumours are one of the most common neoplastic diseases among female dogs, and more than 50% of these tumours are malignant.1,2 In malignant mammary tumours, lung metastasis is the major cause of death. Mammary cancer in dogs and breast cancer in women are known to share many clinicopathological features, including age-dependent occurrence, prevalence of adenocarcinomas, pattern and frequency of metastasis, hormonal dependency, frequency of hormonal receptors and tendency towards multicentric disease.3–6 Therefore, canine mammary tumours are considered to be an appropriate and valid model of human breast cancer.7–10
Molecular studies regarding the mechanisms of metastasis have recently indicated the importance of adhesion molecules in the process of metastasis. Tumor metastasis is known to take place via a complex series of sequential steps that are influenced by various properties of tumour cells, including several adhesion molecules.11,12 The initial step of cancer invasion and metastasis requires dissociation of cancer cells from the primary site.13–16 Cadherin is a Ca2+-dependent, homotypic cell–cell adhesion molecule that plays an important role in the organization and maintenance of tissue structure. E-cadherin is the major cadherin molecule expressed by epithelial cells.17,18 Recent studies of a variety of human and canine cancers have revealed that a reduction in the expression of E-cadherin may initiate the release of cancer cells from their adhesive constraints, allowing them to migrate away from the primary tumour site and to metastasize.14,15,19–28 No in vitro studies comparing E-cadherin level or function in tumour cells derived from primary and metastatic lesions in the same patient have been conducted to date.
In the present study, we established eight canine mammary tumour cell lines originating from the primary and metastatic lesions from four dogs with mammary cancer. Using these established cell lines, we analysed the expression and the function of E-cadherin. The results obtained with primary- and metastasis-derived cell lines were compared in order to investigate a correlation of E-cadherin levels with tumour metastasis in cases of canine mammary carcinoma.
Materials and methods
Tissue samples and cell culture
Tissue samples were obtained from four dogs with canine mammary carcinoma (CMC) admitted to the Veterinary Hospital of the University of Tokyo. Breed, age, gender, tissue source, histological tissue type and the clinical staging of these dogs are summarized in Table 1. The primary tumour cells were collected from a primary lesion in the mammary gland, and the metastatic cells were collected from an enlarged regional lymph node or by thoracocentesis in one dog with thoracic metastasis. Tissue samples were placed in 50-mL tubes with phosphate buffer solution (PBS) supplemented with 0.2 mg mL−1 gentamicin sulphate (Sigma Chemical Co., St Louis, MO, USA). The samples were kept overnight at 4 °C, and were then digested with collagenase (a mixed solution of DNase and pronase; Sigma Chemical Co.). The digested cells were washed and resuspended with RPMI-1640 (Nissui Pharmaceutical Co., Tokyo, Japan) supplemented with 20% fetal bovine serum (Equitech-Bio Inc., Ingram, TX, USA), 0.01 mg mL−1l-glutamine (Nissui Pharmaceutical Co.), fungizone (Gibco BRL, Grand Island, NY, USA) and 5 mg L−1 gentamicin sulphate (Sigma Chemical Co.). The cells were then incubated at 37 °C in a humidified atmosphere of 5% CO2.
| Patients | Age (years) | Gender | Name of cell line | Histopathological view | Clinical stage TMN | Source of the tumour cells | Method of collection |
|---|---|---|---|---|---|---|---|
| Mixed | 12 | Female | CHMp | Inflammatory AC | T4N1(+)M1 | Primary mass | Surgery |
| CHMm | Solid, sample | Stage IV | Pleural effusion | Thoracocentesis | |||
| Shih Tzu | 10 | Female | CIPp | AC | T1cN1(+)M1 | Primary mass | Surgery |
| CIPm | Tubular, solid | Stage IV | Metastatic RLN | Surgery | |||
| Mixed | 12 | Female | CTBp | Inflammatory AC | T4N1(+)M0 | Primary mass | Surgery |
| CTBm | Tubular, solid | Stage IV | Metastatic RLN | Surgery | |||
| Maltese | 11 | Female | CHMp | AC | T1cN1(+)M0 | Primary mass | Surgery |
| CNMm | Tubular | Stage II | Metastatic RLN | Surgery |
- AC, adenocarcinoma, RLC, regional lymph node.
After the 10th passage, when cell growth appeared to be stable with typical features of epithelial cells based on growth patterns on plastic dishes and morphology on light microscopy, the concentration of FBS in the culture medium was decreased from 20 to 10%. The growth of culture cells was stably maintained without fibroblast cells contamination. While the cell passage number used in the assays described below varied from as few as 20 to as many as 120, cells from the same dog with the same approximate number of passages were used for both primary and metastatic lines.
Light and electron microscopy
For light microscopic observation, cells grown on coverslips within a flask were fixed in methanol and stained with Giemsa solution and Sudan III. For electron microscopic examination, cultured cells were centrifuged and fixed with 1.25% glutaraldehyde in 0.1 M cacodylate buffer and embedded in 1.5% agarose in the same buffer. Then cells were minced and postfixated with 2% osmium tetroxide in s-collidine buffer. After fixation, the cells were dehydrated and embedded in a propylene oxide-Quetol sequence. Ultrathin sections (80 nm) were stained with uranyl acetate and lead citrate, and the sections were examined with an electron microscope (JEM-1200EX) (JEOL LTD, Nippon Denshi Tokyo, Japan).
Immunohistochemistry
An indirect immunofluorescence assay was performed in order to detect the expression of keratin and vimentin in these cell lines. The cells, grown on coverslips, were fixed in 2% paraformaldehyde in PBS for 15 min and permeabilized with 0.5% Triton-X-100 (Sigma Chemical Co.) in PBS. The cells were then stained with primary antibodies, i.e. rabbit polyclonal anti-keratin (DACO, Carpinteria, CA, USA) or goat anti-vimentin (ICN Biomedical, Inc., Costa Mesa, OH, USA), for 1 h at 25 °C. The cells were then stained with fluorescein-conjugated anti-rabbit IgG (ICN Biomedical, Inc.) or FITC–anti-goat IgG (Cappel, Aurora, OH, USA) as a secondary antibody for 30 min at 25 °C. The cells were examined under a fluorescence microscope.
Doubling times
Cells were plated in 24-well plates (Sumitomo Bakelite, Tokyo, Japan) at a concentration of 1 × 105 mL−1 and were cultured in RPMI-1640 supplemented with 10% FBS. The number of viable cells was determined every 24 h using trypan blue staining, and the doubling time for the logarithmic phase was calculated.
Transplantation into nude mice
Five-week-old female BALB/c nu/nu mice (Nippon SLC, Hamamatsu, Japan) were irradiated with X-rays at a dose of 4 Gy. Three days irradiation, a suspension of 1 × 107 cells in 0.3 mL PBS was transplanted subcutaneously into the latero-ventral area of nude mice (n = 3 for each cell line). All mice were euthanized when the tumours reached a diameter of 3 cm, or 12 months after transplantation in cases when no palpable tumour mass was detected. The tumours and organs (the swollen lymph nodes, lung, liver, spleen, kidney and heart) were removed and fixed in 10% neutral-buffered formalin, and were stained with haematoxylin and eosin for histopathologic examination.
Expression of E-cadherin
Cells were trypsinized by two different methods for the differential removal of E-cadherin or its fusion molecules.17 The cells were rinsed three times with PBS without Ca2+ or Mg2+, and were then incubated for 30 min at 37 °C with collagenase 0.1 U mL−1. Monolayers were then incubated for 15 min with 0.01% trypsin + 0.02% EDTA in 0.85 g NaCl L−1, a method used for degrading E-cadherin (TE treatment). The detached cells were then seeded for 24 h in normal culture medium in order to enable attachment and recuperation, in order to ensure that the cells used in the experiment expressed newly synthesized E-cadherin. The next day, the monolayers were rinsed three times with Hank's buffer without Ca2+ or Mg2+ (HCMF) and were then incubated 30 min at 37 °C with collagenase (0.1 U mL−1 HCMF + 0.04 mM CaCl2 + 1 g glucose L−1), followed by incubation with trypsin (0.05%) in HCMF + 0.04 mM CaCl2 + 1 g glucose L−1 for 15 min at 37 °C, a method known to maintain the activity of E-cadherin (TC treatment). Finally, the trypsin was neutralized with soybean trypsin inhibitor (Sigma) at a level of 0.1% in HCMF + 1 g glucose L−1. The cells were fixed in acetone + ethanol (7 : 3) for 2 h at 4 °C. Then, the cells were rinsed with PBS without Ca2+ or Mg2+, but were supplemented with 0.5% BSA + 0.01% NaN3 by three cycles of centrifugation (190 g, 5 min). Clotted cells were strained off using 70-µm nylon mesh (NBC Industry, Tokyo, Japan). In order to avoid the non-specific binding of antibodies, the fixed cells were pretreated with 0.5% BSA + PBS(–) for 30 min at 4 °C. Then, the cells were incubated with a primary antibody (human monoclonal antibody for E-cadherin; 1 : 400; Transduction Laboratories, Lexington, KY, USA) for 24 h at 4 °C. The cells were once again rinsed and were incubated with FITC-labelled antimouse IgG antibody (1 : 100; Cappel, Aurora, OH, USA) as a second antibody for 1 h at 4 °C. After rinsing the cells in PBS, fluorescence was measured by FACS analysis. Cells collected by TE treatment were used for negative control.
Cell aggregation assay
Cells were collected according to the method described above. Detached cells were centrifuged and suspended at a concentration of 105 cells mL in the following medium: HCMF medium + 1 mg BSA mL−1 + 40 ng mL−1 Dnase mL−1 + 40 mM Hepes + 1 g glucose L−1 containing either 1 mM EDTA (to block E-cadherin function) or 1 mM CaCl2 (to preserve E-cadherin function). Cells collected by TE treatment were used for negative control. Twelve replicates from each cell line were assayed. All of these procedures were performed on ice. The suspensions were incubated on a gyratory shaker (0.07 g) for 1 h at 37 °C in a 24-chamber flask. Flasks, pipettes, and tubes were pretreated overnight in 1% albumin kept at 4 °C. The number of particles was then counted. The sum of single cells (S60′) and aggregated clots (A60′) was divided by the cell number initially observed (S0′). The equation 1–[(S60′ + A60′)/S0′] was used to obtain the index of aggregation.
Statistical analysis
The data were statistically analysed using the Student's t-test. The level of significance was set at p < 0.05.
Results
Establishment of cell lines
Four pairs of canine mammary adenocarcinoma cell lines, derived from primary and metastatic tumours, were successfully established and characterized. All were derived from intact females. These cell lines, designated as CHMp, CHMm, CIPp, CIPm, CTBp, CTBm, CNMp and CNMm (Table 1), showed stable proliferation without fibroblasts contamination. The current passages, 3–4 years after initiation of the starting culture, range between the 90th and the 150th passage. Among these cell lines, CHMp, CIPp, CTBp and CNMp were established from primary lesions, whereas CHMm, CIPm, CTBm and CNMm, respectively, were established from metastatic lesions in the same four dogs.
Light and electron microscopic findings
Light microscopy revealed that the cells were epithelioid and round- to spindle-shaped. The cells had large nuclei and frequently two or more nucleoli (Fig. 1). There were multiple vacuoles in the cytoplasm, which were stained black by Sudan III. In the culture, cells formed tightly packed monolayer colonies, and often formed adenose organoid structures. Ultrastructural studies revealed an epithelium-like cell morphology with large irregular nuclear outlines, some lipid accumulation and vacuole structures, numerous free ribosomes, mitochondria, endoplasmic reticulum and intermediate filaments. The cell surface exhibited numerous microvilli (Fig. 2).

Light microscopy of cell lines (Giemsa stain 1500×). Each cell line shows round- to spindle-shaped cells. The cells have large nuclei and frequently two or more nucleoli. Multiple vacuoles in the cytoplasm stained black by Sudan III.
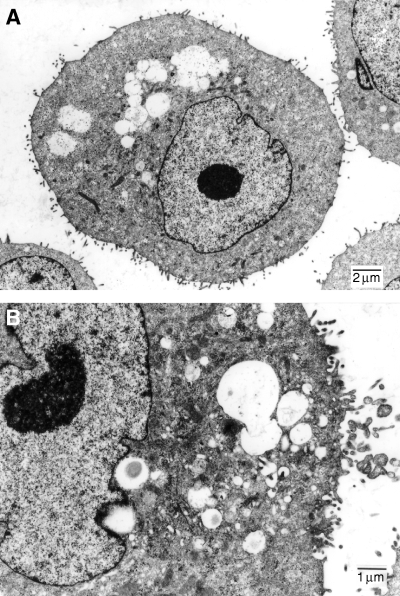
Transmission electron micrograph of a CHMp cell (A, 3500×; B, 6000×). Ultrastructural studies revealed an epithelium-like cell morphology with large irregular nuclear outlines, some lipid accumulation and vacuole structures, numerous free ribosomes, mitochondria, endoplasmic reticulum and intermediate filaments. The cell surface exhibited numerous microvilli.
Immunohistochemistry
All of the cultured cells examined were double positively stained with anti-vimentin and anti-keratin monoclonal antibodies in individual cells (data not shown).
Doubling time
The doubling times of CHMp, CHMm, CIPp, CIPm, CTBp, CTBm, CNMp and CNMm were calculated to be 24.7, 23.1, 24.6, 20.7, 30.3, 25.8, 50.1 and 25.6 h, respectively.
Growth in nude mice
In all nude mice except those receiving CTBm and CNMm, tumour growth was detected at the transplanted sites. In particular, the tumours grew steadily in all nude mice transplanted with CIPp and CIPm until sacrifice at 12 weeks postinjection, and the tumour size reached a diameter of 3 cm. All CIP-transplanted mice showed metastasis to regional lymph nodes and the lung. Some mice transplanted with either cells of primary or metastatic origin showed systemic metastasis including the liver, spleen, kidney and/or heart. In all mice transplanted with CHMp, CHMm and CTBp that developed a tumour, metastasis developed in the axillary and inguinal lymph nodes and the lung. Histologically, these masses had features similar to those of their respective primary lesions. Mice transplanted with CTBm and CNMm cells did not develop tumours at the transplant site nor did metastasis develop within the 12-month observation period.
Expression of E-cadherin
All cells strongly expressed E-cadherin on the cell surface (Fig. 3). When fluorescent intensity of cells were compared, the primary cells appeared to show stronger expression of E-cadherin in the CHM, CIP and CNM lines; however, this difference was only statistically significant (p < 0.05) in CHM cell lines.
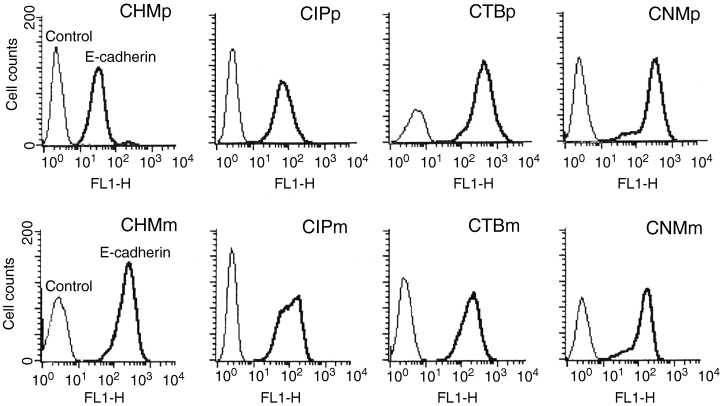
Flow cytometry of eight canine mammary tumour cell lines after being immunohistochemically stained with E-cadherin. The x-axis indicates immunofluorescence, and the y-axis indicates the number of cells. The first peak (thin line) represents the negative control, and the other peak (thick line) represents cells stained with immunofluorescent antibody.
Cell aggregation assay
In cell lines from all four dogs, the index of aggregation of cells derived from the primary lesion was significantly lower than that of cells derived from the metastatic lesion (p < 0.05) (Fig. 4).
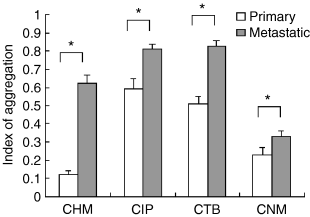
The results of an aggregation assay in canine mammary carcinoma cell lines. Cells of primary origin show a significantly lower aggregation index in all pairs of CMC cells (P < 0.05). The data were analysed using Student's t-test.
Discussion
Four pairs of CMC cell lines were successfully established from both primary and metastatic lesions following attempts from 50 different specimens. We could not establish CMC cell lines from other specimens, possibly because of fibroblasts contamination, bacterial contamination or unknown reasons. To date, other studies have established malignant CMC cell lines;29–34 however, plural cell pairs of primary and metastatic origin have not been reported thus far.
As described in the introduction, certain features of canine mammary tumours are similar to those in human breast cancer. Histologically the most of human breast cancers are adenocarcinoma, which are further subdivided to many subtypes. Because there is a decline rather than cessation of ovarian activity in ageing dogs, canine mammary tumours may be a better model for breast cancer in premenopausal women rather than in postmenopausal women.
The properties of the eight CMC cell lines established here were typical for cultured epithelial cells both in terms of growth pattern on plastic dishes, as well as with respect to cellular morphology. The patterns of growth of cells derived from primary and metastatic lesions in the medium used in this study were found to be similar. All of the cell lines formed a number of small colonies and multiplied with tight cell–cell adhesion. There was no obvious difference in morphology among the eight cell lines; however, the respective sizes of the cells did differ slightly. All four cell lines showed intense immunoreactivity to both keratin, a characteristic of epithelial cells,35 and vimentin, an intermediate filament of mesenchymal cells in vivo.36 Co-expression of cytokeratin and vimentin has been reported both in the human and canine mammary gland, as well as in associated tumours.30,37,38 In addition, ductal breast carcinomas expressing vimentin have been shown to be correlated with high tumour growth, as a result of dedifferentiation associated with loss of cell-to-cell contact.39,40 The cells established in this study showed rapid growth in a monolayer fashion. Doubling times of all CMC cells were similar except for CNMp cells, in which longer doubling time was recorded. This may be due to the earlier clinical stage in this particular patient. Successful transplantation of six of eight CMC cell lines into nude mice produced palpable masses. In addition, five of these produced metastasis to regional lymph nodes and the lungs. The histological findings of the lesions produced in the nude mice were quite similar to those of the original lesions. Thus, these established cell lines are thought to have maintained their original histological features and most likely possess tumorigenic properties.
Previous in vivo studies using various human cancer cell lines have indicated a correlation between E-cadherin expression and tumour metastasis;14,15,19–28 however, no comparison of E-cadherin function in cells of primary and metastatic origin has been reported to date. In the present series of experiments, all CMC cells strongly expressed E-cadherin. No correlation was observed with regard to the degree of expression of E-cadherin in cells of primary and metastatic origin; however, E-cadherin function was shown to differ significantly according to site of origin. This measurement utilized the calcium dependency of E-cadherin, therefore the result would not likely affect non-E-cadherin-mediated aggregation.
A significant reduction in the activity of E-cadherin was observed among all of the cells of primary origin, as compared with cells of metastatic origin from the same dog. This reduction in E-cadherin function may suggest that the neoplastic cells at the primary tumour site underwent a temporary loss and/or down-regulation of E-cadherin function, thus enabling the cells to easily migrate. After metastasizing, it is possible that such cells may recover E-cadherin function. Previous reports have indicated that a loss of E-cadherin expression may contribute to malignant progression in a variety of organs, whereby an invasion to adjacent tissues and metastasis are attributed to a loss of normal intercellular cohesion. However, this theory remains controversial, and contradictory results have also been reported.24–27,41,42 In canine mammary tumours, we suggest that reduction in the activity of E-cadherin is necessary for cells to migrate from the primary tumour site and to metastasize to distant sites.
The mechanism of intercellular adhesion is complicated, and has not yet been fully clarified. Intracellular molecules other than E-cadherin, such as α-, β- and γ-catenin, actin filaments, as well as tyrosine phosphorylation or mutation of these protein complexes have been considered in the regulation of cellular adhesion via cadherins.43–47 In tumour-bearing individuals, the in vivo extracellular conditions might also have been responsible for E-cadherin function and the metastasis of tumour cells.
A lack of vimentin/keratin immunohistochemical characterization in the original surgical specimens is a weakness of the current report. Additionally, as with any investigation based on immortalized cell lines, it is important to point out the possibility that such artificially manipulated cell lines do not recapitulate the behaviour of tumours in vivo.
In conclusion, four pairs of CMC cells of primary and metastatic origin were established and characterized. These cells could prove useful for future studies of human and canine mammary tumours. The cell lines of primary origin showed a reduction in E-cadherin function, as compared with that of metastatic origin. Our data suggest that CMC cells at the primary site may have reduced E-cadherin function that may suggest a malignant phenotype. Further study is needed to clarify the relationship between E-cadherin function and metastasis in canine mammary cancer.