Use of oseltamivir in the treatment of canine parvoviral enteritis
Previously presented in abstract form at the 13th IVECCS, 2007, New Orleans, LA.Funding provided by: The Barry and Savannah French-Poodle Memorial Fund.No financial conflict of interest exists.
Abstract
Objective – To determine if oseltamivir with standard therapy for canine parvoviral enteritis ameliorates disease morbidity, mortality, or both; to document significant adverse effects associated with its use.
Design – Prospective, randomized, blinded, placebo-controlled clinical trial.
Setting – University veterinary teaching hospital.
Animals – Thirty-five dogs.
Interventions – Standard therapy was administered to all dogs. Treatment dogs also received oseltamivir, while control dogs received an equivalent volume of placebo.
Measurements and Main Results – Dogs were monitored daily according to a clinical scoring system, physical parameters, and diagnostic evaluations. Dogs in the treatment group gained a significant percentage of weight during hospitalization (mean, +2.6%; SD, 7.1%) versus the control dogs (mean, −4.5%; SD, 6.9%) (P=0.006). Treatment dogs did not have any significant changes in their white blood cell (WBC) count, while control dogs experienced a significant drop in their WBC counts during their initial stay. In addition, it did not appear that oseltamivir use was associated with any major adverse clinical effects.
Conclusions – While a clear advantage to the use of oseltamivir was not established, a significant weight loss during hospitalization, as well as a significant decrease in WBC count were documented in the control group. No major adverse effects were identified that could be associated with oseltamivir administration. Based on these results, the true role of oseltamivir in the treatment of parvoviral enteritis remains speculative, although it is believed that further investigation is warranted.
Introduction
Canine parvovirus (CPV) is a single-stranded DNA virus that was first discovered in 1978.1 It is a hardy, highly contagious virus that remains a cause of significant disease in young dogs. It is estimated that over 1 million dogs are affected each year in the United States2 despite the availability of an effective vaccine.
CPV infects and replicates in rapidly dividing cells, most notably the lymphoid organs, latter myeloid progenitor cells in the bone marrow, and intestinal epithelial cells. Replication results in cell destruction, causing a clinical disease characterized by severe vomiting, hemorrhagic diarrhea, dehydration, and neutropenia. This disease is almost universally fatal without treatment, with reported survival rates of only 9% in an experimental model.3 Treatment increases this figure significantly, with reported survival rates ranging from 64% to 95%.2,4–10 Care given in specialized care settings has been associated with increased survival rates (96–100%)2,8 compared with nonspecialized care settings (67–75%).2,7 It is postulated that this increase may be due to the 24-hour care available, as well as the likelihood that treatments such as plasma or colloid therapy are more likely to be used in such settings.2
Therapy for CPV enteritis is supportive and aimed at controlling the clinical signs of disease. Multiple-directed therapies have been studied, such as human recombinant granulocyte colony stimulating factor,8,9,11 equine antiendotoxin,7,12 recombinant bactericidal/permeability-increasing protein,2 and interferon ω13,14 with variable or disappointing results. Early enteral nutrition (EEN) has been the only modality to date to show promise for a shortened recovery time and decreased disease morbidity associated with CPV enteritis.10 However, the need remains for a therapeutic agent that will help ameliorate the morbidity and mortality of this disease, and in doing so also decrease the cost of treatment. While the survival rate with aggressive treatment is very good, financial constraints often result in suboptimal treatment with lower survival, or even euthanasia. This fact demonstrates the clear need for a directed therapy to make the appropriate treatment for CPV enteritis more financially feasible for owners or shelters.
Oseltamivir is a neuraminidase (NA) inhibitor originally designed to treat human influenza virus.15 It has recently also shown efficacy in the treatment of avian influenza.16,17 Oseltamivir inhibits the viral NA enzyme and thus prevents the cleavage of sialic acid residues. This cleavage is necessary for liberating newly formed virons from the host cell, as well as for preventing the aggregation of viral particles. Both the viron liberation and decreased aggregation are mechanisms necessary for the spread and dissemination of the virus throughout the host in order to further infection. The NA enzyme is also needed for the virus to cleave the sialic acid residues in mucin to allow for penetration of this protective layer and infection of respiratory epithelial tissue.
Anecdotal reports of oseltamivir use in veterinary medicine claim that it is associated with a less severe form of disease and a quicker recovery when administered to dogs with CPV enteritis. However, unlike the influenza virus, CPV does not rely on NA for effective replication. Therefore, any beneficial effects that may be present would not be due to a direct antiviral action. Human studies have shown a significant decrease in the development of bacterial infections secondary to influenza when oseltamivir is used.15,18–23 This effect is believed to be due to a decrease in bacterial permeation through the mucin layer of the respiratory epithelial cells, because NA is necessary for this process to occur. It is postulated that oseltamivir could also have a similar inhibitory effect on bacterial permeation through the mucin layer of the gut epithelial cells. This inhibition would decrease bacterial translocation, resulting in a potentially lower incidence of endotoxemia, sepsis, systemic inflammatory response, and eventual multisystem failure, which is thought to be the main mechanism of mortality of CPV enteritis. Thus, oseltamivir could have an effect on the disease progression of CPV enteritis in a nonviral-dependent manner. In one report, 90% of dogs that died from CPV enteritis had Escherichia coli cultured from their livers and lungs.24 Acute respiratory distress syndrome-like changes in the lungs of these dogs on histopathologic examination were also present, demonstrating the potential for organ failure secondary to the systemic inflammation associated with this disease.24
The aim of this study was to investigate the efficacy and adverse effects of oseltamivir when added to the standard treatment of CPV enteritis. Our hypothesis was that oseltamivir would ameliorate the disease morbidity and mortality associated with naturally occurring CPV enteritis, thereby decreasing hospitalization time, as well as the need for colloid and other adjunctive therapies.
Materials and Methods
Study design
The present study was a prospective, randomized, blinded, placebo-controlled clinical trial of the effects of oseltamivir in dogs with naturally occurring parvoviral enteritis. Client-owned dogs and dogs from the local animal shelter with CPV enteritis were recruited between April 2005 and August 2006. A financial incentive was offered to the owner or agent to allow their dog to participate in the study. Inclusion criteria for enrollment included a positive CPV fecal antigen test,a presence of appropriate clinical signs (vomiting, diarrhea, lethargy, and anorexia), and lack of any treatment before enrollment. Informed consent was received from all owners or agents before any treatment at the Auburn University Small Animal Teaching Hospital. The study protocol was reviewed and approved by the Animal Care and Use Committee of Auburn University.
Following enrollment in the study, dogs were assigned to either the treatment or the control group randomly in a block of 20 and then blocks of 10 dogs (equal numbers of dogs enrolled in each arm in each block). Group assignment was selected based on the blind drawing of a folded piece of paper designated for either treatment or control. Assignment was allocated by hospital personnel not directly involved in the clinical aspects of the trial. Group assignments were uncovered to the investigators only at the conclusion of the study.
Standard treatment
A treatment protocol was designed to standardize therapy between the 2 groups with the exception of oseltamivir administration to the treatment group and a placebo to the control group. No deviations were made from this protocol for any of the patients. An intravenous (IV) catheter was placed in all dogs, either a peripheral cephalic catheter or central venous catheter in a jugular vein. Balanced electrolyte solutionb, c was administered IV at an initial rate to replace an estimated dehydration deficit over a minimum of 2 hours and a maximum of 4 hours. If the dog was assessed as being in shock, the crystalloid solution was bolused at a rate of 10–30 mL/kg over approximately 20 minutes. Boluses were repeated until physical parameter evaluation by the attending clinician indicated that shock had resolved, and the remaining fluid deficit estimated to replace dehydration was administered as above. No patient required the administration of colloidal solutions to treat a state of shock. Assessment of hydration and perfusion status, as well as fluid rate choice for all dogs was performed by a single investigator (M.R.S.). After rehydration, the hydration status was reassessed via physical parameters and the fluid rate adjusted as deemed appropriate. Fluids were supplemented with potassium chloride,d dextrose,e or both, as needed based on laboratory results.
Ampicillinf (22 mg/kg, IV, q 8 h), enrofloxacing (5 mg/kg, IV, q 12 h), and metoclopromideh (1–2 mg/kg/d, IV infusion) were administered to all patients. If vomiting persisted at a rate of more than twice per 12-hour period despite a maximum rate of metoclopramide, chlorpromazinei (0.5 mg/kg, SQ, q 8 h) was added. Pyrantel pamoatej (10 mg/kg, PO) was administered to all dogs within the first 3 days of arrival to eradicate intestinal parasites. If the total protein (TP) measured using a refractometer fell below 35 g/L (3.5 g/dL), hetastarchk was infused at a rate of 10–20 mL/kg/d. If anemia, defined as a packed cell volume (PCV)≤20%, was present, a blood transfusion was administered. The blood product administered depended on availability at that time, but was either packed red blood cells (10 mL/kg, IV, over 4 h) or a fresh whole blood transfusion (10–20 mL/kg, IV, over 4 h). If vomiting episodes were <4 per 12-hour period, water and a bland dietl were offered. If either appeared to induce nausea by their presentation, they were immediately removed from the animal. Voluntary eating was allowed through mild vomiting episodes (<4 per 12-h period). Food was not force fed or tube fed to any dog.
Discontinuation of IV fluids was at the discretion of a single investigator (M.R.S.). This occurred primarily when the animal was no longer vomiting and consistently eating and drinking sufficiently to maintain hydration. Once fluids were discontinued, all patients were monitored for at least 12 hours before discharge from the hospital in order to ensure no relapse of clinical signs. The day of discharge was assigned as the day when it was deemed the dog was healthy enough to be discharged. In those animals that did not survive, the day of death was considered an endpoint equivalent to the day of discharge for the purpose of data analysis. Postmortem exams were performed on all nonsurviving dogs.
Oseltamivir treatment
Dogs assigned to the treatment group received oseltamivirm (2 mg/kg, PO, q 12 h). An equivalent volume of a placebo, consisting of a standard suspension agent with color additive, was administered to dogs in the control group. Prior experience with oseltamivir by the authors has shown that dogs often react to its taste and frequently vomit shortly after administration. Dilution of the oseltamivir with water (1:1) before administration seemed to decrease these reactions; therefore, in this study, both oseltamivir and placebo were diluted (1:1) with water to lessen the risk of adverse reaction to the oseltamivir. In addition, for dogs receiving chlorpromazine for protracted vomiting, an attempt was made to dose this medication 30–60 minutes before administration of the oseltamivir or placebo to minimize the risk of vomiting. Given its propensity for gastric adverse effects (vomiting, nausea),15 significant adverse effects associated with the administration of oseltamivir, despite these precautions, was recorded using in the clinical scoring system used.
Monitoring/data acquisition
Historical data, including previous vaccination against CPV and the duration of clinical signs as noted by the owner or caretaker (rounded to the closest 12-h period of time), were obtained when this information was available at study entry. Signalment for each dog was also recorded, as was its estimated percentage of dehydration at entry. The estimated extracellular fluid deficit was derived from a combination of clinical characteristics such as skin turgor, mucous membrane color and capillary refill time, pulse quality and heart rate, and ocular position (ie, sunken or not). Laboratory values, including PCV, TP, lactate, and urine specific gravity when a voided sample was available, were also taken into consideration. Baseline diagnostic testing, consisting of a CBC, PCV, TP measured using a refractometer, serum electrolyte concentrations (sodium, potassium, and chloride), and blood glucose concentration, was evaluated for all dogs at entry. These values were monitored daily for all dogs. With the exception of the CBC data, these values were not analyzed statistically but were monitored for guiding treatment decisions only. For white blood cell (WBC) values, the following variables were evaluated: initial total WBC, neutrophil (NEUT), and lymphocyte (LYMPH) counts; the absolute nadir value and the day of hospitalization (with day of presentation being Day 1) on which the nadir occurred for each of the WBC, NEUT, and LYMPH counts; and the number of days for which the counts for each value were considered to show a clinically relevant decrease. These decreases were defined by a WBC <3.0 × 109/L, NEUT <2.0 × 109/L, and LYMPH <1.0 × 109/L. In addition, the WBC count change from presentation (Day 1) to Days 2, 3, 4, and 5 was analyzed for each group. The WBC data in conjunction with clinical scores on presentation and Day 4 were also analyzed. Day 4 was chosen for this comparison to represent a period in time that should have encompassed NEUT and clinical recovery for most dogs based on the expected timeline of disease progression as described previously.11 Body weight was recorded twice daily, with the same scale used to monitor each dog during its stay for consistency. In addition, vital parameters (heart rate, respiratory rate, rectal temperature, mucous membrane color, and capillary refill time) were assessed a minimum of twice daily, as were hydration status and mentation. Days on which dogs demonstrated systemic inflammatory response syndrome (SIRS) criteria were also calculated as a percentage of days of their total stay (days with positive SIRS criteria/total days in hospital × 100%). SIRS was defined as the presence of at least 2 of the following 4 criteria: (1) temperature >39.2°C (102.5°F) or <37.8°C (100.0°F), (2) heart rate >140/min, (3) respiratory rate >40/min, or (4) total WBC count >19.0 × 109/L or <6.0 × 109/L. These criteria were extrapolated from a recent study of dogs with CPV enteritis,25 with the exception of a modification of respiratory rate parameters from >20/min to >40/min. This modification was done in an effort to increase the specificity of the criteria, as it was noted that most dogs in this study had respiratory rates in the range of 21–36/min.
Clinical scoring system
A previously published clinical scoring system10 was used to evaluate 4 clinical attributes of each patient: attitude, appetite, vomiting, and feces. A score of 0 represented a clinically normal parameter, with increasing severity of signs as the score increased up to a maximum of 3 for each variable (Table 1). Scores were assigned twice daily, to encompass the previous 12-hour period, and were assigned by the same investigator for all dogs (M.R.S.). The clinical scores for each of the 4 categories were totaled for each dog per day, with a possible category score ranging from 0 (normal) to 6 (most severely affected) per 24-hour period. In addition, a cumulative score consisting of a total of all 4 categories was calculated for each dog per day, with a possible score ranging from 0 (normal) to 24 (most severely affected). Occurrence of adverse events associated with medication administration was a subjective observation based on the timing of vomiting or increased nausea in the immediate period following drug/placebo dosing. Other indicators of possible adverse reactions to oseltamivir administration could also be indicated by the routine monitoring of other standard clinicopathologic data and physical exam characteristics throughout the study.
| Score | Attitude | Appetite | Vomiting | Feces |
|---|---|---|---|---|
| 0 | Normal | Normal | Absent | Well formed or absent |
| 1 | Mild to moderate depression | Voluntarily eats small amounts | Mild; once per 12 hours | Soft or pasty feces |
| 2 | Severe depression | No interest in food | Moderate; 2–5 times per 12 hours | Watery diarrhea, nonbloody |
| 3 | Collapsed or moribund | Not offered | Severe; >6 times per 12 hours | Watery, bloody diarrhea |
- Scores for each category were assigned to each dog twice daily to encompass the previous 12-hour period.
Statistical Analysis
The Shapiro-Wilks test was used to evaluate the distribution of continuous variables. Continuous variables not normally distributed are described as median (minimum, maximum) and normally distributed continuous variables are described as mean (standard deviation). The Wilcoxon rank sum test was used to test for differences in not normally distributed continuous variables, while the t test was used for normally distributed variables. Categorical variables were described using percentage and the Fisher's exact test was used to test for differences between the treatment and control groups. For all comparisons, a P<0.05 was considered significant. All statistical analyses were performed using a statistical software program.n
Results
A total of 35 dogs were enrolled: 19 in the treatment group and 16 in the control group. Power analysis was not performed as part of the design of the study, but post hoc analysis revealed that 47 dogs would be needed in each group to give the study a power of 0.80 at a significance level of 0.05 based on measurement of outcome. Only 1 dog that was eligible during the trial period was not included due to lack of owner consent. All dogs that entered the study completed the trial. There were 3 shelter dogs included in the study, all of which were in the control group. Most dogs (30/35) were of the mixed-breed variety, with the purebreds consisting of 2 American Pit Bull Terriers and 1 each of Dachshund, Beagle, and Labrador Retriever. The median age of dogs in the control group was 14 weeks (8, 36 w), while that of the treatment group was 12 weeks (8, 44 w) (P=0.50). Twenty-one of 35 (60%) dogs were female, with 10 of 21 (48%) randomized to the control group and 11 of 21 (52%) randomized to the treatment group. Fourteen of 35 (40%) dogs were male, with 6 of 14 (43%) randomized to the control group and 8 of 14 (57%) to the treatment group (P=1.0). All dogs were sexually intact. Vaccination status was known for 10 of 16 (62%) dogs in the control group and 13 of 19 (68%) dogs in the treatment group. Of these, 5 of 10 (50%) dogs in the control group had received at least 1 vaccination against CPV, whereas 6 of 19 (32%) in the treatment group had been vaccinated at least once against CPV (P=1.0). The duration of clinical signs before presentation was known for 15 of 16 (94%) control dogs and 16 of 19 (84%) treatment dogs. Mean days sick before presentation for the control group was 1.4 days (0.9 d), while that for the treatment group was 1.8 days (1.0 d) (P=0.31). No statistically significant differences were found between groups in the baseline characteristics of age, sex, vaccination status, or duration of clinical signs before presentation.
There was no significant difference between groups in the degree of estimated dehydration at entry. The control dogs were estimated to have a mean fluid deficit of 6.3% (1.5%), while the treatment dogs were estimated at 6.9% (1.7%) (P=0.35). In addition, there was no statistical difference among the weights at entry or the weights at discharge between the 2 groups. Dogs in the control group had a median entry weight of 6.7 kg (1.8, 28.2 kg), and those of the treatment group 4 kg (1.6, 25 kg) (P=0.21). At discharge, the median weight of control dogs was 6.5 kg (1.8, 27.3 kg) and that of the treatment group was 4.4 kg (1.6, 28.6 kg) (P=0.42). However, a significant difference was found in the weight change from entry until discharge. Dogs in the control group experienced a median change of −0.21 kg (−2.8, 0.5 kg), while those in the treatment group had a median change of 0.07 kg (−1, 3.6 kg) (P=0.01). This correlates also to a significant difference in the percentage of change in body weight ([discharge weight−entry weight/entry weight] × 100) (Figure 1). Dogs in the control group had a mean change of −4.5% (6.9%), and those in the treatment group a mean change of 2.6% (7.1%) (P=0.006). When this analysis was repeated for survivors only, the results were still found to be significant. The control group contained all 3 nonsurvivors, and the new calculation without these dogs resulted in a mean percent weight change of −5.2% (7.5%), compared with the mean percent weight change of 2.6% (7.1%) for the treatment dogs (P=0.006).
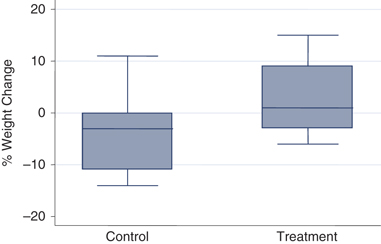
Boxplots of percent weight change during hospital stay for control and treatment dogs. A significant difference (P=0.006) was found in the change in weight from entry until discharge between the control and treatment groups. The control group lost a mean of 4.5% (6.9%) of their body weight, and the treatment dogs gained a small amount with the mean change of 2.6% (7.1%) of their body weight. Percent weight change was calculated as (discharge weight−entry weight)/entry weight × 100.
The percentage of days in the hospital that SIRS criteria were met was calculated for each dog. There was no significant difference between groups, with control dogs meeting SIRS criteria a mean of 52% of days, versus 54% for treatment dogs (P=0.91).
The WBC values that were evaluated (initial counts for WBC, NEUT, and LYMPH; the nadir values and day of nadir; and clinically relevant decreases in counts for WBC, NEUT, and LYMPH) were compared between groups. No significant differences were found for any of these values between groups. When entry (Day 1) WBC counts were compared with counts on Days 2, 3, 4, and 5, significant differences were found for the control group but not the treatment group. The control dogs, with a median WBC count on entry of 7.83 (0.59, 18.3) × 109/L, showed a significant decline on Day 2 (6.1 [0.39, 14.6] × 109/L) (P=0.04), Day 3 (6.3 [0.5, 10.9] × 109/L) (P=0.04), and most notably on Day 4 (2.49 [0.41, 13.6] × 109/L) (P=0.009). No difference from entry to Day 5 (3.33 [0.4, 11.0] × 109/L) was found (P=0.08). The treatment dogs, on the other hand, had no significant changes in their median WBC counts from entry (4.95 [0.5, 24.1] × 109/L) to Day 2 (2.75 [0.46, 14.5] × 109/L) (P=0.08), Day 3 (3.46 [0.46, 11.9] × 109/L) (P=0.10), Day 4 (4.7 [0.71, 16,28] × 109/L) (P=0.55), or Day 5 (4.79 [0.78, 24.4] × 109/L) (P=0.97) (Figure 2).
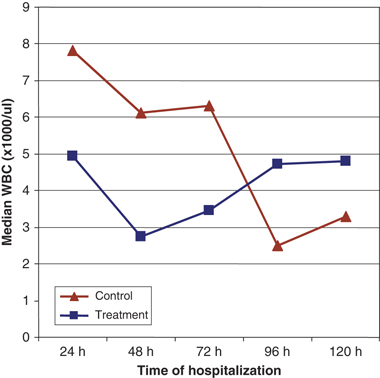
Change in median white blood cell (WBC) count. Control dogs showed a significant decrease in WBC count from entry (0 h) to hours 48, 72, and 96. *Significant decrease from presentation.
It was noted that an approximately equal proportion of dogs in each group experienced a severe decline in WBC, with nadirs occurring at <0.1 × 109/L in 7 of 16 (44%) control dogs and 8 of 19 (42%) treatment dogs. Of these, 2 of the control dogs did not survive (1 died, 1 euthanized) while there were no deaths in the treatment group. Because of the small number of dogs involved, statistical analysis was not performed.
When the clinical scores were compared day by day for each category as well as the cumulative total for that day, no significant differences were found between groups with the exception of Day 6 for the appetite score (P=0.02), when the treatment group scores were significantly lower (more normal). The trends in WBC and clinical scores for both groups are presented in Table 2. While there were no significant differences found in scores between groups, it can be seen that there was a slight trend for lower scores over time in the treatment group. Vomiting, when it did occur, did not appear to be associated with administration of oral medications.
| Treatment | Control | |||||
|---|---|---|---|---|---|---|
| Initial | Day 4 | Day 5 | Initial | Day 4 | Day 5 | |
| WBC | 4.95 | 4.71 | 4.79 | 7.83 | 2.49* | 3.28 |
| Attitude | 2 | 1 | 1 | 2 | 2 | 2 |
| Appetite | 5 | 2 | 2 | 6 | 4 | 3 |
| Vomit | 2 | 1 | 1 | 2 | 3 | 2 |
| Fecal | 3 | 2 | 2 | 2 | 3 | 3 |
| Cumulative | 12 | 7 | 6 | 13 | 11 | 9 |
- Median values for white blood cells (WBC) (× 109/L), and average daily total clinical scores at presentation and on Days 4 and 5 of hospitalization. There was a significant decrease in WBC count from entry to Day 4 in the control group, but no significant changes over time in WBC count in the treatment group.
- * Significant difference from initial count; P=0.009.
No difference was found in the duration of hospitalization between the 2 groups. Dogs in the control group had a mean stay of 5.9 days (2.6 d), and those of the treatment group 6.0 days (2.3 d) (P=1.0). Colloid therapy was not required often, as the median number of days on colloids for the control group was 0 days (0, 3 d) and also 0 days (0, 5 d) for the treatment group (P=0.5). None of the 16 dogs in the control group received a blood transfusion, while 2 of 19 (10%) dogs in the treatment group did (P=0.5). The addition of chlorpromazine was necessary in 10 of 19 (53%) dogs in the treatment group, and 9 of 16 (56%) dogs in the control group. Dogs in the treatment group that did receive chlorpromazine did so an average of 21% of their days in the hospital, while the dogs requiring it in the control group were given chlorpromazine an average of 30% of their days in the hospital. The overall survival rate was 91% (32/35). Three dogs died, all from the control group, giving this group a survival rate of 81% (13/16) compared with a survival rate of 100% in the treatment group. The difference between the 2 groups, however, was not significant (P=0.09). Only 1 of the dogs that died was from the animal shelter, the others were client owned. One animal was euthanized after severe progression of clinical signs despite treatment, and it was deemed that the dog was suffering and would not recover, while the other 2 had natural deaths.
Postmortem examinations were performed on all nonsurviving dogs. Findings were consistent with a diagnosis of CPV enteritis. All dogs had diffuse, severe, necrotizing enteritis. Bone marrow was examined in 2 of 3 dogs, both showing sections of moderate hypocellularity. While 2 dogs were noted to have diffuse congestion and edema in their lungs, the third dog was found to have a mild interstitial pneumonia.
Discussion
The use of oseltamivir in addition to standard therapy for naturally occurring CPV enteritis did not result in a significant decrease in hospitalization time, treatments needed, clinical scores, morbidity, or mortality. Dogs in the treatment group, however, gained significantly more weight than those in the control group, which on average, lost weight. Initial dehydration was not different between groups and therefore was unlikely to influence observed weight gain. In addition, it was noted that the control group experienced a significant decrease in WBC count from entry (Day 1) to Days 2, 3, and 4 of hospitalization, while those dogs in the treatment group had no significant decline in WBC count for any of the initial 5 days of hospitalization.
CPV does not rely on NA for replication. However, anecdotal reports of the use of oseltamivir in dogs with CPV enteritis have claimed decreased morbidity and shortened recovery time in the treated dogs. It is speculated that the drug may inhibit bacterial translocation that subsequently leads to endotoxemia, sepsis, SIRS, and death. Bacterial adherence and colonization of respiratory epithelial cells is potentiated in the presence of viral NA, and inhibited with NA blocking agents.19–21 It is believed that the bacteria that commonly invade the lower respiratory tract express their own NA, thus enabling them to penetrate the protective mucin layer and infect the epithelial cells.19 Although unproven, a similar mechanism may exist in the gastrointestinal tract. Oseltamivir may exert a beneficial effect by inhibiting NA on enteric bacteria, preventing their translocation across the gastrointestinal mucosal barrier. In CPV enteritis, the mucosal barrier is already impaired, allowing easier passage of bacteria. If bacterial NA plays a role similar to that in the lungs, the NA would cleave sialic acid residues on the gut epithelium, exposing receptor sites for bacterial adherence and further encouraging translocation. In addition, CPV suppresses the dog's immune system, both humoral and cell-mediated factors, allowing for systemic spread of bacteria and the resultant deleterious effects. Further studies are needed to accurately define the actual mechanism behind the observed anecdotal benefits of the use of oseltamivir to treat CPV enteritis.
The importance and implications of weight gain associated with oseltamivir treatment are unknown at this time. Other studies have shown that a significant change in weight in study subjects is associated with an improved outcome from infectious disease. In 1 investigation,20 mice treated prophylactically with oseltamivir lost an average of only 5% of their body weight, and had a survival of 100% after an influenza and bacterial challenge. In contrast, the control mice lost an average of 25% of their body weight, and no mice survived the same viral/bacterial challenge. Weight loss was correlated with increased severity of disease and decreased survival rate in the mice in the influenza study. In our study, an increased survival rate and increased weight gain were seen in dogs receiving oseltamivir. While the actual mechanisms behind the weight gain in the dogs in the treatment group are unknown, one possible explanation is that disease severity was decreased in dogs receiving oseltamivir. While a direct link between preservation of weight and outcome was not found, these findings were both present with oseltamivir administration and not in the control subjects.
A veterinary study investigating the effect of EEN on dogs with parvoviral enteritis10 found that dogs in the EEN group had a significant increase in weight from entry on all days of the study, while the conventional group had no significant change in weight. In addition, dogs in the EEN group showed a more rapid clinical improvement, based on normalization of clinical scores, than did the conventional group. These results also suggest that the change in weight might be a marker of disease severity. Dogs with less severe disease, as interpreted based on clinical scores, are more apt to maintain their body weight. The mechanism for this effect, while yet to be truly elucidated, is thought to be associated with improved enterocyte health secondary to the trophic effects of enternal nutrition, resulting in less bacterial translocation. Oseltamivir is postulated to also help decrease bacterial translocation across the gut endothelial cells by blocking the action of NA. Decreased bacterial adherence and subsequent bacterial translocation would result in less cytokine release from the gut-associated lymphatic tissue. By blocking bacterial translocation through NA inhibition, oseltamivir could decrease disease severity both locally in the gastrointestinal tract, and systemically. Reduced severity of disease would likely contribute to weight gain in treated animals. Although the treatment group in the present study showed an increase in weight during hospitalization, suggesting a less severe form of disease, this improvement in disease morbidity was not demonstrated with our clinical scoring system. This discrepancy could be due to an inherent limitation of sensitivity with the scoring system utilized, or the possibility that there really was no effect. Although weight gain in treated animals appears to be a beneficial effect associated with oseltamivir administration, the interpretation of the weight change and assignment of associations with this finding must be done with caution because the actual mechanism is unknown and interpretations are speculative.
A decline in WBCs is a considered a hallmark sign of CPV infection. This decline is postulated to be due to multiple effects, including lymphoid necrosis, a direct toxic effect of CPV on neutrophil precursors in the bone marrow, as well as overwhelming consumption secondary to acute inflammation of the gut. The decline in WBC count has been associated with clinical signs in CPV enteritis, both in timeline11 and outcome.9 Neutrophil nadir has been shown to coincide with the period when clinical signs are at their worst, and leukopenic dogs have been demonstrated to have a decreased survival. This decreased survival is speculated to be due to the impaired ability of the immune system to fight off a systemic infection and its associated inflammatory effects secondary to bacterial translocation. A higher WBC count could mean a stronger immune system and an increased ability to protect against the negative effects of sepsis development. The control dogs in this study showed a significant decline in WBC count from entry to Days 2, 3, and most notably to Day 4. The dogs receiving oseltamivir had no significant changes in their WBC count from entry to any of the initial 5 days of hospitalization. Although clinical scores did not show a difference in disease severity, and survival rates, while different, were not significantly so, it can be argued that this lack of WBC decline could be a protective effect of oseltamivir. Given a more sensitive clinical scoring system and a larger number of dogs, this protective effect could potentially be associated with a decreased disease morbidity and mortality. Further studies are needed to more fully define this relationship.
Although the treatment dogs had a slightly lower median WBC count on entry than the control dogs, this difference was not found to be significant. Being that there was no difference in entry values for WBC count, nor in the reported duration of clinical signs before entry, both groups should have been at approximately equivalent stages of their disease process and the timeline of disease progression should not have differed significantly between the groups. Thus, it appears that the lack of WBC count decline in the treatment group as opposed to the control group is a beneficial effect of oseltamivir administration.
The main adverse effects of oseltamivir reported in humans are gastrointestinal effects apparently due to direct local irritation of the gastric mucosa.15 In the experience of the authors, dogs will also often react to the taste of the oseltamivir suspension and nausea and vomiting can be encountered. Dilution with water just before administration appears to minimize these effects. This practice was utilized in this study in an effort to not only avoid uncovering group assignment and thereby instituting a bias, but also in an effort to keep the clinical scores an accurate representation of the disease process in the animal and not obscure these scores with drug reaction. However, it is still possible that oseltamivir caused increased vomiting and nausea as a side effect of the drug, thereby concealing any benefit evident by analysis of the clinical scores. There was a lack of any significant difference in clinical scores between groups, specifically vomiting and appetite scores, as well as the subjective observation that administration of the oral medications (oseltamivir or placebo) was not associated with increased nausea or vomiting directly afterwards. Both of these observations support the interpretation of results indicating that adverse effects of oseltamivir were minimal, as were beneficial effects on decreasing disease morbidity.
The intensity of treatment required and the expected cost of treatment were inferred based on additional therapy, such as colloid infusion or blood transfusions, as well as prolonged hospitalization times. Colloids were not frequently used, in contrast to a previous report.25 Indications for the institution of colloid therapy were not elucidated in the previous study. Protocol in this study required decrease in total protein to 35 g/L. Much more modest decreases, often around 40–45 g/L, are frequently used in the clinic setting as indication for treatment with colloids. A higher cutoff value as a trigger for colloid infusion likely would have increased the incidence of its use in this study. Blood transfusions were also rarely indicated. The 2 cases that did require a transfusion received fresh whole blood, rather than packed red blood cells. Packed red blood cell availability was very limited at the time of the first required transfusion. In order to minimize differences in treatment between dogs, when the second transfusion became necessary, although packed red blood cells were available, fresh whole blood from the previous donor was again administered. The total volume of crystalloids as well as colloids administered between the 2 groups may have been helpful to evaluate. Dogs experiencing greater fluid loss from vomiting or diarrhea as well as having less voluntary intake per os would have a greater IV fluid need. This would correlate with an increased severity of clinical signs and manifestation of the disease process. Unfortunately, the collection of this data was not possible from certain dogs due to recording errors or technical difficulties. Therefore this variable was not analyzed.
The duration of clinical signs before presentation was not different between the groups. It has been reported that for human influenza viral infections, oseltamivir is most effective if started within the first 12 hours of clinical signs, with efficacy decreasing up to 48 hours.26 For every 6-hour delay in starting oseltamivir, the duration of illness is predicted to increase by approximately 8%.26 Whether this also holds true for its use in CPV enteritis is unknown. Because replication of CPV does not depend on NA, administration of oseltamivir in the early stages of infection is unlikely to diminish viral replication and dissemination as with the influenza virus, and thus a time-efficacy response is not expected. Rather, with the proposed mechanism against bacterial translocation, oseltamivir may have a greater impact when administered during the period of leukopenia and severe clinical signs. Further investigation is needed to expand on these speculations.
A clinical scoring system was utilized in order to evaluate the subjective criteria of attitude and appetite, as well as to quantify the severity of vomiting and characterize the feces to allow for comparison across dogs. One investigator (M.R.S.) had the responsibility of assigning scores to all dogs to minimize interobserver variability. This investigator was also blinded to group assignment in order to minimize bias.
Values for each category and cumulative scores were compared between the 2 groups for each day, and although mild trends could be seen for lower scores in the treatment group, there were no significant differences. This could be attributed to the small sample size in this study, as well as the variability in the timeline of illness among dogs. Because dogs presented in all stages of their disease (ie, clinical symptoms for <12 h to up to 4 d), a comparison of scores per day may not illustrate true differences and a larger group would be needed to further examine this effect. In addition, the clinical scoring system utilized is a very simple system, and as such, was relatively insensitive in its ability to differentiate between various stages of aberrancy in each of the clinical attributes. This scoring system failed to identify subtle yet clinically significant differences. A scoring system with a greater degree of stratification between assigned values may allow for a greater sensitivity and a more accurate representation of the clinical status of the patient.
Limitations of this study do involve the concern of administration of an oral medication to a vomiting patient, and its potential for variable systemic absorption in the face of a diseased gastrointestinal tract. The specific site of oseltamivir absorption from the gastrointestinal tract has not yet been fully elucidated. It has been shown to be absorbed equally from the stomach, proximal, and distal small intestinal segments in an experimental study.27 Colonic absorption does appear to occur, but to a lesser extent and with a greater delay than other sites. Early safety studies of oseltamivir show that it has a bioavailability of 73% in healthy dogs, with detectable levels of the drug in plasma within approximately 30 minutes after oral administration.27 Oseltamivir does require transformation to its active metabolite by esterases located within the liver, and to a certain degree, within the intestinal system.28,29 The importance of the intestinal system esterases is unknown. Because of this need for transformation, it is believed that oseltamivir effects are not due to a purely local action, but do require systemic distribution. The effect of a diseased gastrointestinal system such as that seen in CPV enteritis on the absorption, systemic distribution, and transformation is unknown at this time and future pharmacokinetic studies, in this situation especially, are needed.
The reasoning behind any beneficial effect of oseltamivir in the treatment of CPV enteritis suggests that it helps decrease bacterial translocation and therefore the ensuing endotoxemia, SIRS, and organ dysfunction that can develop. In this study, although certain physical and clinic pathologic parameters were monitored, there was no direct test for the presence of bacterial translocation or sepsis. SIRS criteria were evaluated, but this is a crude assessment, especially considering the confounding factors. As the dogs were starting to feel better, they would oftentimes become very excitable when being handled. This frequently resulted in an elevation of their heart rate to over 140/min, or their respiratory rate to >40/min. The presence of these 2 variables would classify these healthy, excitable puppies as fulfilling the criteria SIRS. In addition, the effect of the virus itself on the WBC count confounds the definition slightly. A WBC of <6.0 × 109/L can simply reflect destruction of progenitor cells in the bone marrow and is not necessarily associated with systemic inflammation. Other methods to evaluate for the presence of bacterial translocation, endotoxemia, or SIRS may be more fruitful. Culture of mesenteric lymph nodes is considered the gold standard in human medicine and animal models for evaluation of bacterial translocation.30 The feasibility of this procedure in this patient population (client-owned, live animals) and setting is questionable. Other methods, such as blood cultures or measurement of serum endotoxin levels or other inflammatory mediators could be used to differentiate animals in which bacterial translocation is present from those in which it is not. Further investigation would be needed before any true conclusions can be made.
Oseltamivir administration to animals is not an approved use of the drug. However, this would not be the first instance of off-label use of a medication in veterinary medicine. Yet concern exists over the acceptability of using this drug in animals. This concern revolves around the potential that use of the drug may enhance resistance to this medication, specifically by the avian influenza virus. Oseltamivir is considered the first line agent in the prevention of a viral epidemic if the avian influenza virus appears in the United States. There are a very limited number of drugs currently available to treat or prevent avian influenza in humans; oseltamivir is one of these. If resistance to oseltamivir develops, the ability to treat and prevent a viral epidemic could be severely hampered. Recently, the World Health Organization has found that the number of strains of the avian influenza virus exhibiting resistance to oseltamivir has increased.31 However, the significance, etiology, and impact of this increased resistance are unknown at this time. While oseltamivir was used in a companion pet population in this study, the appropriateness of its continued use in this setting in light of this increased resistance has yet to be determined.
Conclusions
CPV enteritis can be a devasting disease process. The financial constraints often encountered with treatment can be very frustrating given the treatable nature of this disease. Despite the anecdotal reports touting the success of oseltamivir to decrease the disease morbidity and mortality of CPV enteritis, concrete scientific evidence of this was not found in this study. However, a significant difference in the change in body weight during hospitalization stay was established, as was the apparent safety of the drug in this patient population. It was also shown that the control dogs had a significant decline in their WBC counts from Day 1 to Days 2 through 4 of hospitalization, while treatment dogs seemed to be protected from this effect, and had no significant change during this time period. Whether this represents a true benefit of oseltamivir administration or rather a difference in timelines of disease between groups is open to debate, although the latter appears to be unlikely in this study. It is believed that, given the paucity of adverse effects and the findings presented in this study, further investigation is warranted for its effects in CPV enteritis.
Acknowledgement
The authors would like to acknowledge Dr. Kenneth Drobatz for his assistance with the statistical analysis.
Footnotes
aSNAP Parvo antigen test, IDEXX Laboratories Inc, Westbrook, ME.
bLactated Ringer's solution, Abbott Laboratories, North Chicago, IL.
cNormosol-R, Abbott Laboratories.
dPotassium chloride, Phoenix Pharmaceutical Inc, St Joseph, MO.
eDextrose 50% solution, Veterinary Laboratories Inc, Lenexa, KS.
fAmpicillin, Abraxis Pharmaceutical Products, Schaumburg, IL.
gEnrofloxacin injectable solution, 2.27%, Bayer HealthCare LLC, Animal Health Division, Shawnee Mission, KS.
hMetoclopramide injection USP, SICOR Pharmaceuticals Inc, Irvine, CA.
iChlorpromazine HCl injection USP, Baxter, Deerfield, IL.
jPyrantel pamoate 50 mg/mL, Pfizer Animal Health, New York, NY.
k6% Hetastarch, Hospira, Lake Forest, IL.
lScience Diet Canine I/D, Hill's Pet Nutrition Inc, Topeka, KS.
mOseltamivir phosphate, Roche Laboratories Inc, Nutley, NJ.
nStataCorp. 2003, Stata Statistical Software: Release 8, College Station, TX.




