THE TRADING OF ANCIENT GLASS BEADS: NEW ANALYTICAL DATA FROM SOUTH ASIAN AND EAST AFRICAN SODA–ALUMINA GLASS BEADS*
Abstract
The occurrence of similar glass beads at archaeological sites in Africa and Asia bears witness to the trade relationship between the two continents. This paper reports elemental analysis results from a recent in-depth laser ablation – inductively coupled plasma – mass spectrometry (LA–ICP–MS) study of a group of archaeological glass beads with a specific alumina-rich composition from East Africa, India and Sri Lanka. Based on the concentrations of the trace elements, two different subgroups were identified. One subgroup occurs at early periods (fourth century bce to fifth century ace) in South India and Sri Lanka. The second subgroup appears at later dates in Africa and was identified at different Kenyan sites dated from the ninth to the 19th century ace, and at the contemporaneous site of Chaul in western India.
INTRODUCTION
In Sub-Saharan Africa, South and South-East Asia, glass is mostly found as beads. In this vast area, the earliest evidence of glass comes from the northern part of India and dates from the end of the second millennium to the beginning of the first millennium bce (Engle 1976; Francis 1984; Kanungo 2004a). Due to their beauty, affordability, transportability and durability, glass beads rapidly became popular as items of adornment and ceremonialism. Different kinds of beads were made and exchanged, but the small and monochrome type manufactured by the drawn or wound techniques is the most common at archaeological sites. Drawn glass tubes were cut at regular sizes and the edges polished to produce large quantities of beads, with techniques still in use nowadays (Stern 1987; Francis 1991; Kanungo 2004a). The more time-consuming technique to produce wound beads consisted in wrapping a lump of molten glass around a mandrel (Kanungo 2004b). Van Der Sleen (1973) christened drawn and wound glass beads ‘trade wind beads’ because their movement across the Indian Ocean and Persian Gulf relied on monsoon winds for navigation. Francis (1990) named the more ubiquitous of these two types of beads, the drawn beads, Indo-Pacific Monochrome Drawn Beads, or IPMDB, in reference to their aspect and their spatial distribution.
The earliest trade wind beads appear at many South and South-East Asian archaeological sites dated from the middle of the first millennium bce. Based on typological observations and archaeological data, most of these glass beads were thought to originate from South India. The technology would have then been transferred to Sri Lanka and then to South-East Asia during the first half of the first millennium ade, when different glass-bead-making centres would have spread their production across a large area that stretched from Africa to Japan (Francis 1990). This model of technology transfer is now in question, based on the chemical composition of the glass beads. Taking into account recent compositional data from the literature, a more complex picture has appeared (Brill 1999; Dussubieux 2001; Dussubieux and Gratuze 2003; Robertshaw et al. 2003, 2006; Popelka et al. 2005). The identification of chemically distinct glass at multiple contemporaneous and distant locations reveals the emergence at different points across South and South-East Asia of distinct glass technologies and the existence of several independent glass bead-making centres (Lankton and Dussubieux 2006). The most common type of glass used to manufacture glass beads is a mineral soda–alumina glass, also called m-Na–Al or mNA glass. It corresponds to 40% of the glass material studied in the South and South-East Asian regions (Lankton and Dussubieux 2006) and its diffusion area matches the occurrence area of the trade wind beads. The occurrence of this glass dates from the fourth century bce to the 16th century ace (McKinnon and Brill 1987; Dussubieux and Gratuze 2003).
The m-Na–Al glass contains soda as the most abundant constituent after silica. Magnesia in this glass is rather low (lower than 1.5%), suggesting the use of soda taken from a mineral source. The distinctive feature of this type of glass is its high alumina content, which varies from 5% to 15%. Alumina is introduced to the glass with poorly refined sand. The sand composition is similar to that of granite and contains a small amount of potash and lime, and relatively high concentrations of iron, titanium and other trace elements, such as rare earth elements (REE) (Dussubieux 2001; Dussubieux and Gratuze 2003). Our use of ‘high’ and ‘low’ to describe trace elements in this text is relative, given that these elements occur in relatively minor amounts.
The presence of large volumes of this m-Na–Al glass in India led Brill (1987) to conclude that this glass was probably made there. Mineral-soda sources and alumina-rich sands are ubiquitous in India (Bhardwaj 1979, 67; Sen and Chaudhuri 1985, 130). A mixture of carbonate, sodium sulphate and chloride with low proportions of calcium and magnesium called reh is available in the Ganga Valley (Wadia 1975). Several publications report that glass factories using reh mixed with local sands as their raw materials have existed at least since the 19th century ade (Sen and Chaudhuri 1985; Kock and Sode 1995; Sode and Kock 2001; Kanungo 2004a). Also, Brill (1999) demonstrated that the fusion of reh at 1100°C produced a soda-rich vitreous material. However, archaeological evidences of primary glass manufacturing workshops are scarce in India. The site of Kopia in Uttar Pradesh yielded convincing evidence of glass manufacturing in the form of crucibles and a 120 pound piece of glass that may date as early as the fifth century bce (Roy and Varshney 1953; Kanungo and Misra 2004; Kanungo and Shinde 2005; Kanungo 2006). The Sri Lankan site of Giribawa, contemporary with Kopia, yielded furnaces lined with vitrified alumina-rich materials and blocks of raw glass with similar compositions. Alumina sand sources were identified in the close proximity (Bopearachchi 1999, 2002; Gratuze et al. 2000; Dussubieux 2001).
With the exception of the black Nupe glass beads (Carey 1991) and Yoruba beadworks (Freestone 2006; Lankton et al. 2006), archaeologists generally agree that most of the glass beads in Sub-Saharan Africa that predate European arrival were imported from India. Less certain is the mechanism through which Africans procured Indian beads. Davison (1972) used neutron activation analysis and X-ray fluorescence to analyse a large number of glass beads mostly from South Africa. She identified a m-Na–Al composition in a large number of the beads. These beads belonged to the trade wind bead group. She then compared uranium concentrations of this material with those of similar m-Na–Al glass beads excavated at 18 different Indian sites, dating from the second century bce to the 16th century ace. Uranium concentrations for the Indian glass beads were below 50–70 ppm, the detection limits of the XRF system used by Davison. In contrast, the African beads averaged approximately 100 ppm. The results led her to conclude that: the hypothesis of manufacture in India of glass beads found in Africa is neither proven, nor disproven, nor well founded in the first place (Davison 1972, 176). In a later article, Davison and Clark (1974) reported results from some samples from Maski (Deccan culture area, 1518 ace) that showed the presence of both high- and low-uranium glasses at this site. Two decades later, Saitowitz (1996) revisited the question of provenance of African glass beads. Using scanning electron microscopy with energy-dispersive spectrometry to measure the major and minor elements and LA–ICP–MS to determine the trace elements, specifically the rare earth elements, she analysed glass material from Sub-Saharan Africa, Egypt, India and Indonesia. Saitowitz matched rare earth element patterns and cerium and europium anomalies in Sub-Saharan African and non-Sub-Saharan African glass beads. Her efforts were unsuccessful, because she found similar trace element patterns for different glass compositions that were unlikely to have been made in the same area. Despite the inconclusive results, Saitowitz's work remains an important reference for African glass bead compositions (see also Saitowitz et al. 1996). More recently, Robertshaw et al. (2006) compared rare earth anomalies in m-Na–Al glass from Mahilaka, a ninth to 15th century ace site in Madagascar, to different types of rocks. Most of the m-Na–Al archaeological samples exhibited elemental profiles similar to those of granite rocks that are abundant in India and in Sri Lanka.
The previous studies point towards an Indian or Sri Lankan origin for m-Na–Al glass beads found in Sub-Saharan Africa. This paper, reports for the first time, evidence, based on LA–ICP–MS, of a direct connection between a possible Indian m-Na–Al glass bead source area and archaeological sites in Kenya. The production and circulation of m-Na–Al glass will be discussed taking into account glass samples from India, Kenya and Sri Lanka. We identified two subgroups for this type of glass based on differences in trace element concentrations. Combining the results from our chemical analysis with archaeological data, we attempt to define to what extent the different types of glass are a product of chronology, technological style or regionalization. Assuming that the transfer of technologies and goods is conditioned by the existence of economical and political alliances or influences, we believe that the results presented in this paper will provide a foundation for addressing questions related to the economical and political organization in different regions around the Indian Ocean.
SAMPLES AND METHODOLOGY
Samples
A total of 138 trade wind beads from four sites in Kenya and 209 trade wind beads or glass samples from nine Indian and Sri Lankan sites were analysed. The Indian beads were divided into two groups according to their geographical origin: West India (Chaul) and South India (all other Indian sites). Approximately 46%, or 161 samples, are m-Na–Al glass. This paper focuses on this subset of samples. Data for the other beads will be published at a later date.
All of the Kenyan sites (Fig. 1), except Ungwana (Abungu 1990), were excavated by C. Kusimba (1993, 1994, 1999). They range in age from the early Holocene site at Bungule to the colonial-period site at Muasya. The glass beads from Kenya are dated from the ninth to the 19th century ade (Table 1). Bungule and Muasya are located in southeastern Kenya, about 150 km from the East African coast, and were inhabited by agropastoral and hunter–gatherer peoples, whereas Ungwana and Mtwapa were coastal urban settlements inhabited by diverse types of populations, including traders, fishermen and craftspeople. The goal of the research that yielded these samples was to understand the regional and interregional relationships between the coastal and interior societies. The project documented more than 400 sites, of which 15 have been excavated, including five on the coast and 10 in the hinterland.
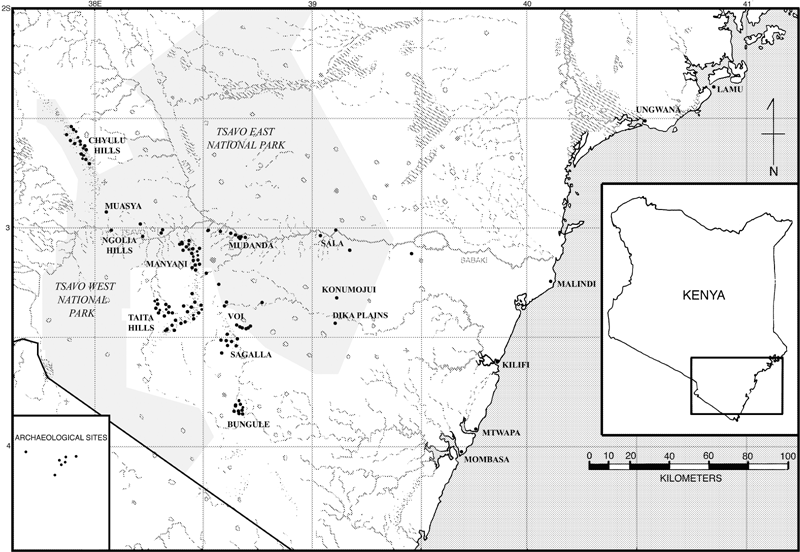
A map of Kenya.
| Country or region | Site | Chronology | Number of m-Na–Al samples/total number of samples |
|---|---|---|---|
| Kenya | Mtwapa | 10th–18th centuries ace | 43/43 |
| Bungule | 17th–19th centuries ace | 5/63 | |
| Muasya | 16th–19th centuries ace | 3/26 | |
| Ungwana | 9th–16th centuries ace | 6/6 | |
| South India | Alagankulam | 3rd century bce–3rd century ace | 10/20 |
| Arikamedu | 2nd century bce–10th century ace | 3/55 | |
| Kalahandi | 1st century ace until possibly 5th century ace | 1/3 | |
| Kodumanal | 3rd century bce–3rd century ace | 8/11 | |
| Karaikadu | 1st century ace until possibly 5th century ace | 6/11 | |
| West India | Chaul | 9th–19th centuries ace | 27/27 |
| Sri Lanka | Giribawa | 3rd century bce–2nd century ace | 26/28 |
| Kelaniya | 3rd century bce–2nd century ace | 9/22 | |
| Ridiyagama | 4th century bce–1st century ace | 14/32 |
The research determined that the coastal towns and hinterland villages were linked by a network of relationships that included kinship and trade partnerships. Towns such as Mtwapa and Ungwana served as important links between the Kenyan hinterlands and the Indian Ocean world system (Kusimba 1999; Kusimba and Kusimba 2001).
The Indian site of Chaul, excavated by V. Gogte, is an ancient port town located at the mouth of the Kundalika river (or Roha creek) about 50 km south of Mumbai (Bombay), on the west coast of India (Fig. 2). The site dates from the Satavahana period (230 bce–230 ace) through the Silahar (c. 1000–1200 ace), and the Bahmani (c. 1400–1600 ace) periods (Gogte 2003). Chaul was one of the leading ports of trade that linked Indian Ocean and Persian societies. Glass beads included in this study were recovered from levels dated from the ninth to the 19th centuries ace, based on the ceramic assemblage.
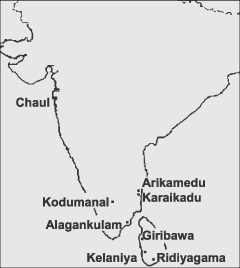
A map of India and Sri Lanka.
The South Indian and Sri Lankan sites (Fig. 2) are dated from the fourth century bce to the fifth century ace. One exception is Arikamedu, which was occupied throughout later periods. As mentioned earlier, Giribawa is the only site that showed evidence of both glass and glass bead productions. This site is located in the west-central part of Sri Lanka, in the Kala Oya valley—a valley that leads to the port site of Pomparippu. Kelaniya is located on the eastern side of the Kelani Ganga, one of the largest rivers in Sri Lanka. Evidence for agricultural and metallurgical (iron) activities was found at the site and habitation areas were identified (Bopearachchi 1999, 2002). Ridiyagama is located 12 km from the mouth of the Walawe Ganga. This site was a trading centre related to Godavaya, which was a deep-water port. Evidence of steel production, probably for export, also was identified at Ridiyagama (Bopearachchi 1998, 2002).
The South Indian archaeological sites included in this study are located in Tamil Nadu state, the exception being Kalahandi, which is located in Orissa state. Arikamedu, on the south-east coast of India, yielded evidence of glass bead production, although so far nothing indicates any primary glass manufacturing (Wheeler et al. 1946; Casal 1949). Karaikadu, 30 km from Arikamedu in the North Arcot district, was probably also a glass bead production centre, as testified by the large number of unfinished beads and glass fragments found at that site (Ramachandran 1980).
Alagankulam and Kodumanal manufactured neither glass nor glass beads. Alagankulam, on the north side of the Vagai River, is a small village, 24 km away from Ramanathapuram, that was probably one of the ports of the south-east coast of India. Roman coins and potteries were excavated at Alagankulam. Many glass beads were found (Majeed et al. 1992). Kodumanal is on the north side of the Noyyal river, 20 km away from Chennimalai, along an ancient road going from Karur to the west coast of India. Megalithic sepulchres, habitation areas, agricultural evidence and craft areas were identified at this site (Rajan 1997). Kalahandi has not been excavated yet and does not appear on Figure 2.
Trading activities between South India and Sri Lanka occurred as early as the third century bce, as demonstrated by the presence of Tamil coins in the southern part of the island of Sri Lanka and the presence of Sri Lankan coins and inscriptions at Arikamedu, Alangakulam and Kodumanal in South India (Bopearachchi 2002).
The majority, or 88%, of the m-Na–Al glass beads included in the study are drawn beads. Only 8% of the beads were manufactured using the wound technique (Table 2). The remaining beads were either moulded or manufactured using a technique that is difficult to identify. Three black fragments of bracelets were included in this study: one was found at Ridiyagama and two at Kelaniya. Most of the glass beads are opaque and coloured in red, green, yellow, orange, black and white. Only some green, turquoise blue and dark blue beads are translucent. The term ‘translucent’ means that even if the light can be transmitted through the glass, the bubbles or inclusions are so dense that one cannot see clearly bodies lying beyond the glass. None of the beads is transparent (3, 4).
| Site | Nature of the artefacts | Technique | Colour | ||
|---|---|---|---|---|---|
| Drawn | Wound | Other | |||
| Mtwapa | 43 beads | 43 | Opaque: yellow, green, red and white | ||
| Translucent: turquoise blue and dark blue | |||||
| Bungule | 5 beads | 4 | Opaque: yellow and black | ||
| 1 | Opaque: yellow | ||||
| Muasya | 3 beads | 3 | Opaque: red and white | ||
| Ungwana | 6 beads | 3 | Opaque: red | ||
| Translucent: turquoise blue | |||||
| 2 | Translucent: green and black | ||||
| 1 | Opaque: yellow | ||||
| Alagankulam | 11 beads | 11 | Opaque: red, green and black | ||
| Translucent: turquoise blue | |||||
| Arikamedu | 2 raw pieces of glass | Translucent: greenish | |||
| 1 bead | 1 | Translucent: greenish | |||
| Kodumanal | 8 beads | 6 | Opaque: red, green and black | ||
| Translucent: turquoise blue | |||||
| 2 | Black | ||||
| Karaikadu | 3 raw pieces of glass | Opaque: red and black | |||
| 3 beads | 2 | Black | |||
| Red core with orange outer surface | |||||
| 1 | Opaque: green | ||||
| Kalahandi | 1 bead | 1 | Translucent: dark blue | ||
| Chaul | 27 beads | 26 | Opaque: red, green, yellow, black and white | ||
| Translucent: turquoise blue and dark blue | |||||
| 1 | Opaque: turquoise blue | ||||
| Giribawa | 6 raw pieces of glass | Translucent: turquoise blue | |||
| Opaque: red and white | |||||
| 2 fragments of tube | 2 | Black | |||
| 16 beads | 16 | Opaque: yellow, black, orange, green and white | |||
| Translucent: turquoise blue | |||||
| Polychrome:* black core with red and white stripes | |||||
| Kelaniya | 2 fragments of bangle | Black | |||
| 7 beads | 7 | Opaque: orange and green | |||
| Translucent: turquoise blue | |||||
| Ridiyagama | 1 fragment of bangle | Black | |||
| 13 beads | 3 | Opaque: green, orange and red | |||
| 5 | Opaque: green and red | ||||
| 5 | Opaque: greyish and green | ||||
| Translucent: turquoise blue | |||||
- * The polychrome bead counts as one bead but three samples, since the different-coloured glasses were sampled separately.

From top left and clockwise, glass beads from Ungwana, Chaul, Mtwapa, Muasya and Bungule (photograph by Laure Dussubieux).

Glass and glass beads from Giribawa (photograph by Laure Dussubieux).
Analytical technique
Most of the samples were analysed by LA–ICP–MS. All samples from Sri Lanka and South India were analysed between 1997 and 2000 at the Centre Ernest Babelon, IRAMAT, CNRS, Orléans, France. Descriptions of the LA–ICP–MS instrumentation, the analytical protocol and the data calibration method are reported in Gratuze (1999). Fast neutron activation analysis (FNAA) was carried out on a few samples at the Centre d’Etudes et de Recherches par Irradiation, CNRS, Orléans, France. Details of this analytical technique and the analytical parameters can be found in Gratuze and Barrandon (1990). The LA–ICP–MS and FNAA results can be directly compared, since it was established that, from a general point of view, the results obtained with the two techniques concur quite well (Gratuze et al. 2001). The compositions for the Sri Lankan and South Indian artefacts are reported in Dussubieux (2001). All the Kenyan and Chaul samples were analysed using a Varian ICP–MS connected to a New Wave UP213 laser, at the Field Museum of Natural History, Chicago, USA, during 2005 and 2006. Analytical protocol and calculation methods were adapted from Gratuze (1999). The isotope 29Si was used as an internal standard and the Standard Reference Materials 610 and 612 from the National Institute for Standards and Technology were used for external standardization, along with the Corning glasses B, C and D (Brill 1999). The consistency of the results over time was checked by analysing, at the Field Museum, samples already analysed at the Centre Ernest Babelon. For elements not involved in the colouring or opacifying of the glass, the relative deviation of the measurements is less than 20%, and for most of the elements ranges from 5% to 10%.
It is important to note that for LA–ICP–MS analysis, no sample preparation is necessary and the analytical technique is virtually non-destructive, given that no visible damage is caused. As many as 55 major, minor and trace elements were measured using this technique, but only 35 elements are reported in Table 3. The detection limits range from 10 ppb to 1 ppm for most of the elements. Accuracy ranges from 5% to 10% depending on the elements and their concentrations.
| Black CIB001 | Black CIB002 | Black CIB003 | T. Blue CIB004 | Green CIB005 | T. blue CIB006 | D. blue CIB007 | D. blue CIB008 | D. blue CIB009 | D. blue CIB010 | Yellow CIB011 | Yellow CIB012 | Yellow CIB013 | Yellow CIB014 | T. blue CIB015 | Green CIB016 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 (%) | 61.1 | 63.6 | 61.6 | 67.2 | 56.9 | 65.3 | 61.8 | 61.4 | 63.1 | 65.8 | 57.9 | 56.9 | 63.1 | 61.6 | 61.7 | 60.7 |
| Na2O (%) | 19.2 | 17.6 | 19.0 | 18.6 | 15.1 | 18.5 | 21.7 | 17.1 | 19.6 | 19.6 | 18.8 | 15.1 | 16.7 | 15.4 | 14.3 | 18.3 |
| MgO (%) | 1.14 | 1.06 | 1.01 | 0.74 | 0.77 | 0.85 | 0.75 | 1.23 | 0.78 | 0.68 | 0.90 | 0.77 | 0.84 | 0.79 | 1.17 | 0.96 |
| Al2O3 (%) | 8.3 | 7.4 | 7.4 | 5.1 | 7.5 | 6.5 | 6.5 | 7.9 | 6.6 | 5.6 | 7.5 | 7.5 | 4.7 | 6.7 | 8.2 | 7.3 |
| P2O3 (%) | 0.13 | 0.29 | 0.17 | 0.10 | 0.13 | 0.12 | 0.10 | 0.18 | 0.12 | 0.07 | 0.22 | 0.13 | 0.10 | 0.18 | 0.12 | 0.10 |
| K2O (%) | 2.1 | 2.6 | 2.4 | 1.8 | 2.8 | 1.9 | 2.0 | 2.5 | 2.1 | 1.2 | 2.1 | 2.8 | 1.6 | 2.0 | 2.9 | 1.5 |
| CaO (%) | 5.6 | 4.8 | 5.8 | 4.3 | 4.4 | 4.5 | 4.0 | 5.6 | 4.0 | 3.5 | 5.6 | 4.4 | 3.4 | 5.5 | 6.1 | 4.6 |
| MnO (%) | 0.07 | 0.09 | 0.07 | 0.04 | 0.05 | 0.06 | 0.08 | 0.16 | 0.15 | 0.05 | 0.08 | 0.05 | 0.04 | 0.06 | 0.07 | 0.05 |
| Fe2O3 (%) | 2.1 | 2.4 | 2.2 | 1.6 | 1.6 | 1.8 | 2.2 | 2.5 | 2.0 | 1.8 | 1.9 | 1.6 | 1.6 | 2.5 | 4.9 | 3.3 |
| CuO (%) | 0.19 | 0.13 | 0.05 | 0.41 | 0.05 | 0.52 | 0.06 | 0.03 | 0.02 | 0.04 | 0.02 | 0.05 | 0.02 | 0.05 | 0.30 | 0.59 |
| PbO2 (%) | 0.01 | 0.04 | 0.18 | 0.01 | 8.31 | 0.02 | 0.44 | 0.01 | 0.01 | 0.07 | 2.83 | 8.31 | 5.43 | 3.39 | 0.05 | 2.17 |
| Be (ppm) | 1.8 | 1.2 | 1.5 | 0.9 | 1.8 | 1.0 | 1.5 | 2.2 | 2.2 | 2.1 | 2.1 | 1.8 | 1.4 | 2.5 | 2.1 | 2.8 |
| B (ppm) | 131 | 128 | 123 | 120 | 96 | 122 | 179 | 109 | 91 | 136 | 130 | 95.6 | 107 | 96 | 104 | 150 |
| Ti (ppm) | 2049 | 1930 | 1643 | 1404 | 1870 | 1447 | 1824 | 1988 | 1793 | 1626 | 2137 | 1870 | 1389 | 2047 | 2075 | 1625 |
| V (ppm) | 70 | 126 | 103 | 111 | 55 | 91 | 88 | 79 | 84 | 82 | 114 | 54.9 | 83 | 87 | 121 | 102 |
| Cr (ppm) | 42 | 29 | 32 | 8.4 | 16 | 13 | 32 | 26 | 39 | 32 | 17 | 15.6 | 11 | 18 | 24 | 28 |
| Ni (ppm) | 16 | 20 | 12 | 22.1 | 13 | 33 | 489 | 93 | 59 | 87 | 21 | 13.2 | 14 | 25 | 29 | 28 |
| Co (ppm) | 8.2 | 18 | 37 | 89 | 6 | 176 | 1180 | 947 | 548 | 954 | 11 | 5.8 | 6.1 | 9.1 | 12 | 82 |
| Zn (ppm) | 91 | 68 | 149 | 37 | 239 | 88 | 163 | 61 | 48 | 60 | 582 | 239 | 1021 | 460 | 88 | 204 |
| As (ppm) | 8.9 | 11 | 17 | 61 | 2.7 | 138 | 2081 | 1088 | 618 | 965 | 4.8 | 2.7 | 4.2 | 4.2 | 4.7 | 40.0 |
| Rb (ppm) | 60 | 83 | 51 | 47 | 78 | 53 | 69 | 75 | 66 | 42 | 62 | 78 | 43 | 62 | 59 | 40 |
| Sr (ppm) | 275 | 273 | 288 | 176 | 193 | 199 | 141 | 276 | 188 | 147 | 245 | 193 | 147 | 148 | 235 | 221 |
| Zr (ppm) | 146 | 118 | 128 | 79 | 176 | 105 | 125 | 104 | 100 | 99 | 188 | 176 | 85 | 107 | 93 | 116 |
| Nb (ppm) | 8.0 | 6.2 | 7.0 | 5.4 | 9.7 | 7.2 | 8.7 | 7.3 | 6.5 | 6.4 | 7.7 | 9.7 | 5.9 | 9.6 | 7.4 | 6.8 |
| Ag (ppm) | 1.0 | 1.6 | 0.6 | 0.6 | 2.7 | 1.1 | 2.6 | 0.1 | 0.2 | 0.8 | 1.3 | 2.7 | 3.5 | 1.5 | 0.7 | 15.2 |
| Sn (ppm) | 16 | 56 | 432 | 31 | 6290 | 48 | 554 | 6.2 | 17 | 46 | 2771 | 6290 | 3969 | 2692 | 82 | 1321 |
| Sb (ppm) | 1.6 | 0.7 | 1.1 | 6.2 | 6.8 | 9.1 | 2.2 | 0.2 | 0.2 | 0.6 | 23.8 | 6.8 | 1.9 | 1.7 | 7.5 | 11.2 |
| Cs (ppm) | 0.7 | 1.2 | 0.5 | 0.5 | 0.4 | 0.5 | 0.8 | 0.6 | 0.6 | 0.3 | 0.5 | 0.4 | 0.2 | 0.6 | 0.3 | 0.2 |
| Ba (ppm) | 381 | 349 | 331 | 230 | 408 | 286 | 273 | 398 | 360 | 259 | 540 | 408 | 215 | 308 | 291 | 304 |
| La (ppm) | 29 | 20 | 28 | 22 | 27 | 26 | 28 | 30 | 25 | 21 | 35 | 27 | 18 | 41 | 23 | 23 |
| Ce (ppm) | 61 | 47 | 59 | 43 | 54 | 58 | 71 | 59 | 57 | 52 | 69 | 54 | 40 | 92 | 50 | 49 |
| Pr (ppm) | 6.6 | 5.1 | 6.2 | 4.9 | 6.6 | 6.0 | 6.5 | 6.9 | 5.6 | 5.0 | 8.0 | 6.6 | 4.4 | 10.1 | 5.4 | 5.3 |
| Y (ppm) | 13 | 11 | 12 | 8.0 | 11 | 10 | 13 | 11 | 11 | 9.7 | 15 | 14 | 8 | 14 | 10 | 9.4 |
| Bi (ppm) | 1.9 | 0.4 | 1.0 | 0.4 | 0.7 | 0.5 | 1708 | 0.2 | 0.2 | 1.4 | 0.2 | 0.7 | 0.2 | 2.8 | 0.5 | 0.6 |
| U (ppm) | 58 | 150 | 96 | 80 | 51 | 89 | 204 | 47 | 116 | 178 | 97 | 51 | 60 | 136 | 63 | 118 |
| T. blue CIB017 | Red CIB018 | Red CIB019 | Red CIB020 | Red CIB021 | Red CIB022 | White CIB023 | Green CIB024 | Yellow CIB025 | White CIB026 | D. blue CIB027 | Black BUK018 | Black BUK051 | Yellow BUK053 | Yellow BUK055 | Black BUK057 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 (%) | 57.2 | 61.0 | 61.5 | 59.7 | 61.7 | 63.0 | 64.0 | 62.7 | 58.5 | 63.8 | 62.4 | 65.8 | 61.3 | 58.9 | 60.3 | 59.6 |
| Na2O (%) | 18.3 | 18.0 | 17.0 | 17.9 | 18.5 | 17.9 | 18.4 | 17.3 | 14.2 | 18.7 | 19.9 | 14.8 | 17.6 | 17.0 | 14.4 | 17.3 |
| MgO (%) | 1.35 | 0.81 | 0.92 | 0.94 | 0.86 | 0.87 | 0.87 | 0.83 | 1.60 | 0.84 | 0.87 | 0.46 | 0.75 | 0.36 | 0.43 | 0.65 |
| Al2O3 (%) | 9.9 | 7.9 | 8.2 | 8.3 | 7.6 | 7.8 | 7.3 | 5.0 | 9.1 | 6.5 | 7.0 | 9.4 | 10.5 | 9.0 | 8.7 | 8.7 |
| P2O3 (%) | 0.18 | 0.14 | 0.21 | 0.19 | 0.17 | 0.19 | 0.30 | 0.17 | 0.42 | 0.14 | 0.13 | 0.19 | 0.32 | 0.10 | 0.10 | 0.18 |
| K2O (%) | 2.2 | 2.5 | 2.2 | 2.4 | 1.9 | 2.0 | 2.5 | 1.7 | 2.6 | 2.5 | 1.6 | 4.7 | 4.0 | 3.2 | 3.1 | 3.2 |
| CaO (%) | 5.9 | 4.2 | 4.7 | 4.8 | 4.6 | 3.4 | 3.2 | 3.8 | 4.4 | 3.9 | 4.3 | 2.7 | 2.7 | 1.6 | 3.4 | 3.5 |
| MnO (%) | 0.07 | 0.07 | 0.09 | 0.08 | 0.07 | 0.06 | 0.06 | 0.05 | 0.08 | 0.06 | 0.07 | 0.05 | 0.04 | 0.03 | 0.10 | 0.18 |
| Fe2O3 (%) | 4.0 | 4.1 | 3.8 | 4.7 | 3.6 | 2.9 | 1.8 | 1.3 | 2.8 | 2.0 | 1.9 | 1.4 | 1.5 | 1.3 | 1.2 | 1.3 |
| CuO (%) | 0.53 | 0.76 | 0.51 | 0.58 | 0.38 | 0.42 | 0.07 | 0.84 | 0.11 | 0.10 | 0.05 | 0.005 | 0.01 | 0.02 | 0.01 | 0.01 |
| PbO2 (%) | 0.08 | 0.26 | 0.64 | 0.15 | 0.37 | 0.07 | 0.10 | 3.90 | 4.55 | 0.06 | 0.07 | 0.01 | 0.00 | 6.69 | 4.77 | 0.02 |
| Be (ppm) | 1.7 | 1.8 | 1.5 | 1.8 | 1.5 | 1.3 | 1.5 | 1.0 | 1.7 | 1.3 | 1.4 | 1.1 | 1.2 | 1.0 | 1.0 | 1.2 |
| B (ppm) | 140 | 99 | 133 | 122 | 123 | 176 | 103 | 136 | 160 | 120 | 152 | 108 | 102 | 47 | 83 | 124 |
| Ti (ppm) | 2599 | 1884 | 1968 | 2567 | 2132 | 2047 | 1478 | 1285 | 2973 | 1699 | 1633 | 3612 | 1833 | 1423 | 3057 | 2060 |
| V (ppm) | 120 | 128 | 129 | 172 | 136 | 169 | 99 | 68 | 92 | 85 | 105 | 94 | 95 | 57 | 125 | 116 |
| Cr (ppm) | 16 | 44 | 45 | 49 | 37 | 26 | 15 | 9 | 18 | 22 | 21 | 17 | 26 | 12 | 18 | 24 |
| Ni (ppm) | 29 | 25 | 28 | 27 | 23 | 22 | 20 | 29 | 41 | 23 | 69 | 10 | 14 | 12 | 14 | 12 |
| Co (ppm) | 15 | 59 | 71 | 59 | 42 | 15 | 12 | 20 | 13 | 9.6 | 918 | 6.1 | 6.6 | 4.2 | 11 | 6.5 |
| Zn (ppm) | 57 | 72 | 120 | 71 | 91 | 42 | 60 | 60 | 907 | 45 | 34 | 194 | 102 | 38 | 24 | 780 |
| As (ppm) | 4.2 | 50 | 71 | 47 | 33 | 14 | 9.0 | 18 | 12 | 8.9 | 210 | 59 | 27 | 19 | 12 | 5.9 |
| Rb (ppm) | 55 | 64 | 61 | 61 | 49 | 50 | 69 | 47 | 82 | 56 | 47 | 104 | 122 | 111 | 113 | 98 |
| Sr (ppm) | 299 | 204 | 220 | 237 | 207 | 225 | 263 | 216 | 323 | 215 | 249 | 244 | 159 | 132 | 255 | 278 |
| Zr (ppm) | 159 | 185 | 210 | 245 | 225 | 362 | 153 | 112 | 153 | 122 | 128 | 555 | 174 | 248 | 335 | 179 |
| Nb (ppm) | 10.0 | 8.1 | 8.4 | 9.8 | 8.4 | 9.0 | 6.4 | 6.5 | 14.4 | 8.6 | 7.4 | 15.9 | 6.9 | 7.1 | 11.7 | 10.0 |
| Ag (ppm) | 0.7 | 2.0 | 2.2 | 1.5 | 1.1 | 0.8 | 2.7 | 2.6 | 4.2 | 0.7 | 0.4 | 0.2 | 0.2 | 11.4 | 3.9 | 0.7 |
| Sn (ppm) | 51 | 182 | 412 | 112 | 203 | 59 | 53 | 7722 | 1273 | 26 | 26 | 11 | 1.9 | 3659 | 1509 | 6.8 |
| Sb (ppm) | 8.4 | 8.0 | 8.2 | 9.4 | 5.3 | 7.6 | 0.8 | 6.4 | 9.2 | 0.8 | 0.5 | 20 | 1.5 | 14 | 4.1 | 0.2 |
| Cs (ppm) | 0.4 | 0.4 | 0.5 | 0.5 | 0.3 | 0.6 | 0.6 | 0.4 | 1.3 | 0.6 | 0.6 | 0.9 | 1.6 | 0.8 | 1.2 | 1.2 |
| Ba (ppm) | 411 | 312 | 357 | 351 | 325 | 276 | 496 | 243 | 459 | 290 | 338 | 548 | 371 | 361 | 563 | 360 |
| La (ppm) | 35 | 35 | 32 | 39 | 30 | 35 | 22 | 23 | 54 | 31 | 29 | 29 | 32 | 12 | 44 | 24 |
| Ce (ppm) | 72 | 70 | 67 | 81 | 67 | 72 | 56 | 49 | 115 | 64 | 62 | 72 | 72 | 30 | 96 | 59 |
| Pr (ppm) | 8.5 | 8.2 | 7.3 | 8.7 | 7.1 | 7.1 | 5.0 | 5.0 | 11.9 | 6.6 | 6.2 | 7.6 | 7.8 | 3.0 | 10.1 | 6.1 |
| Y (ppm) | 14 | 13 | 13 | 13 | 12 | 14 | 13 | 11 | 22 | 13 | 12 | 8.8 | 25 | 18 | 38 | 32 |
| Bi (ppm) | 0.3 | 0.4 | 0.6 | 0.7 | 0.3 | 0.3 | 0.4 | 0.9 | 11 | 0.9 | 0.6 | 0.1 | 0.1 | 0.3 | 13.7 | 0.2 |
| U (ppm) | 71 | 98 | 79 | 119 | 116 | 158 | 113 | 101 | 95 | 122 | 162 | 41 | 63 | 17 | 96 | 75 |
| Red MRS005 | Red MRS007 | White MRS012 | T. blue MTK001 | T. blue MTK002 | Red MTK004 | T. blue MTK005 | White MTK006 | Green MTK007 | D. blue MTK008 | T. blue MTK009 | Green MTK010 | Green MTK011 | Red MTK012 | Green MTK013 | Green MTK014 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 (%) | 66.0 | 64.6 | 63.9 | 59.8 | 68.9 | 61.7 | 69.6 | 60.6 | 57.0 | 63.0 | 63.6 | 58.3 | 58.0 | 60.5 | 57.0 | 63.5 |
| Na2O (%) | 15.0 | 17.0 | 23.8 | 18.3 | 18.5 | 17.6 | 13.7 | 19.3 | 15.6 | 17.0 | 16.4 | 13.8 | 16.2 | 19.2 | 20.6 | 16.6 |
| MgO (%) | 1.08 | 1.11 | 0.36 | 1.50 | 0.64 | 1.57 | 1.31 | 0.84 | 1.41 | 0.98 | 1.13 | 1.35 | 1.34 | 1.22 | 1.03 | 1.35 |
| Al2O3 (%) | 8.8 | 8.7 | 7.2 | 9.4 | 5.6 | 6.0 | 4.9 | 9.5 | 9.9 | 8.2 | 7.5 | 9.3 | 9.6 | 7.7 | 9.6 | 9.1 |
| P2O3 (%) | 0.26 | 0.18 | 0.03 | 0.36 | 0.18 | 0.26 | 0.18 | 0.15 | 0.32 | 0.18 | 0.15 | 0.30 | 0.35 | 0.43 | 0.19 | 0.47 |
| K2O (%) | 2.3 | 3.2 | 2.4 | 2.4 | 2.0 | 2.4 | 1.4 | 2.6 | 2.6 | 4.2 | 3.1 | 3.8 | 2.5 | 2.1 | 3.4 | 2.8 |
| CaO (%) | 1.8 | 1.6 | 1.1 | 4.1 | 1.7 | 3.0 | 3.8 | 3.8 | 4.9 | 3.1 | 4.0 | 4.6 | 4.6 | 3.3 | 2.9 | 3.3 |
| MnO (%) | 0.05 | 0.06 | 0.04 | 0.07 | 0.05 | 0.06 | 0.20 | 0.07 | 0.08 | 0.08 | 0.05 | 0.07 | 0.08 | 0.10 | 0.07 | 0.08 |
| Fe2O3 (%) | 3.3 | 2.1 | 1.1 | 2.1 | 1.8 | 4.3 | 1.9 | 1.5 | 2.7 | 1.7 | 1.7 | 2.5 | 2.6 | 2.8 | 3.1 | 2.2 |
| CuO (%) | 1.40 | 1.38 | 0.005 | 0.54 | 0.61 | 0.95 | 1.50 | 0.01 | 0.71 | 0.06 | 0.52 | 0.73 | 0.78 | 0.51 | 0.04 | 0.50 |
| PbO2 (%) | 0.04 | 0.07 | 0.01 | 0.05 | 0.01 | 0.04 | 0.03 | 0.01 | 3.18 | 0.13 | 0.04 | 3.40 | 2.57 | 0.42 | 0.22 | 0.01 |
| Be (ppm) | 2.0 | 1.8 | 1.2 | 1.3 | 0.8 | 0.6 | 0.7 | 1.1 | 1.1 | 0.9 | 1.0 | 1.2 | 1.1 | 1.2 | 1.3 | 1.5 |
| B (ppm) | 164.9 | 153 | 84 | 124 | 113 | 117 | 262 | 152 | 109 | 77 | 127 | 114 | 119 | 154 | 110 | 84 |
| Ti (ppm) | 2435.9 | 2460 | 1593 | 2811 | 1924 | 2983 | 2270 | 2376 | 2513 | 1455 | 1677 | 2402 | 2519 | 2238 | 3052 | 2790 |
| V (ppm) | 82 | 75 | 54 | 77 | 88 | 116 | 67 | 79 | 93 | 60 | 61 | 86 | 91 | 113 | 107 | 70 |
| Cr (ppm) | 41 | 39 | 21 | 16 | 28 | 31 | 52 | 14 | 18 | 17 | 17 | 16 | 17 | 25 | 37 | 19 |
| Ni (ppm) | 30 | 24 | 12 | 31 | 97 | 30 | 37 | 14 | 44 | 46 | 30 | 46 | 46 | 36 | 31 | 40 |
| Co (ppm) | 21 | 17 | 7 | 9 | 191 | 33 | 32 | 6 | 16 | 494 | 8 | 19 | 17 | 41 | 15 | 73 |
| Zn (ppm) | 72 | 217 | 16 | 80 | 55 | 45 | 120 | 57 | 218 | 73 | 53 | 210 | 232 | 167 | 48 | 82 |
| As (ppm) | 56 | 46 | 2 | 11 | 284 | 36 | 42 | 5 | 24 | 133 | 14 | 28 | 26 | 48 | 4 | 25 |
| Rb (ppm) | 97 | 94 | 54 | 72 | 82 | 36 | 27 | 79 | 65 | 78 | 52 | 65 | 67 | 80 | 112 | 79 |
| Sr (ppm) | 128 | 108 | 89 | 340 | 115 | 112 | 310 | 222 | 293 | 230 | 245 | 284 | 289 | 478 | 210 | 328 |
| Zr (ppm) | 151 | 195 | 103 | 186 | 101 | 247 | 174 | 261 | 187 | 251 | 147 | 173 | 182 | 175 | 151 | 178 |
| Nb (ppm) | 11.6 | 12 | 9.7 | 13 | 8.5 | 5.8 | 6.9 | 14 | 11 | 8 | 6 | 10 | 10 | 10 | 13 | 12 |
| Ag (ppm) | 5.8 | 7.1 | 0.2 | 0.5 | 1.9 | 1.9 | 5.3 | 1.9 | 2.1 | 0.2 | 0.8 | 1.4 | 3.9 | 1.3 | 0.4 | 0.3 |
| Sn (ppm) | 274 | 959 | 3.8 | 124 | 21 | 122 | 148 | 9.9 | 4486 | 116 | 162 | 2145 | 2160 | 530 | 275 | 18 |
| Sb (ppm) | 21 | 4.0 | 55 | 4.5 | 11 | 32 | 18 | 0.3 | 18 | 1.1 | 2.4 | 13 | 16 | 13 | 1.1 | 3.2 |
| Cs (ppm) | 5.0 | 4.9 | 0.4 | 0.7 | 0.7 | 0.4 | 0.6 | 0.5 | 0.8 | 0.4 | 0.5 | 0.7 | 0.8 | 0.9 | 1.4 | 1.0 |
| Ba (ppm) | 323 | 330 | 267 | 629 | 226 | 258 | 276 | 514 | 395 | 558 | 356 | 358 | 396 | 312 | 401 | 729 |
| La (ppm) | 35 | 35 | 26 | 41 | 23 | 23 | 19 | 37 | 36 | 26 | 23 | 32 | 35 | 29 | 37 | 51 |
| Ce (ppm) | 75 | 69 | 57 | 86 | 72 | 44 | 43 | 74 | 70 | 57 | 48 | 63 | 70 | 79 | 87 | 104 |
| Pr (ppm) | 8.9 | 8.7 | 6.2 | 9.1 | 5.2 | 4.8 | 4.9 | 8.1 | 7.7 | 5.4 | 4.8 | 6.9 | 7.6 | 7.3 | 8.4 | 10.4 |
| Y (ppm) | 26 | 26 | 25 | 34 | 18 | 21 | 26 | 26 | 21 | 24 | 17 | 24 | 31 | 36 | 33 | 28 |
| Bi (ppm) | 36 | 20 | 0.4 | 0.4 | 272 | 15 | 3.2 | 0.2 | 0.6 | 0.8 | 0.6 | 4.3 | 0.6 | 41 | 0.1 | 0.8 |
| U (ppm) | 346 | 244 | 72 | 74 | 217 | 41 | 26 | 55 | 102 | 150 | 73 | 91 | 101 | 221 | 202 | 76 |
| Green MTK015 | Green MTK016 | Red MTK017 | Red MTK018 | Green MTK019 | Yellow MTK020 | Green MTK021 | Red MTK022 | Red MTK024 | Green MTK025 | Red MTK026 | Red MTK027 | T. blue MTK028 | T. blue MTK029 | D. blue MTK030 | D. blue MTK031 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 (%) | 58.1 | 57.1 | 59.7 | 59.8 | 58.5 | 57.2 | 60.4 | 63.2 | 54.0 | 54.2 | 60.8 | 61.7 | 64.5 | 64.5 | 63.8 | 59.4 |
| Na2O (%) | 15.1 | 14.8 | 18.3 | 20.8 | 20.0 | 15.1 | 21.2 | 19.0 | 22.1 | 22.5 | 20.2 | 16.4 | 18.1 | 16.5 | 19.7 | 22.4 |
| MgO (%) | 1.43 | 1.39 | 1.01 | 0.91 | 1.57 | 1.35 | 1.09 | 1.66 | 1.55 | 0.93 | 1.71 | 2.30 | 1.81 | 0.97 | 0.75 | 1.00 |
| Al2O3 (%) | 9.7 | 9.5 | 7.7 | 8.0 | 7.1 | 8.6 | 7.4 | 5.5 | 10.2 | 9.0 | 4.7 | 4.8 | 5.8 | 8.7 | 5.8 | 7.8 |
| P2O3 (%) | 0.31 | 0.29 | 0.16 | 0.23 | 0.24 | 0.31 | 0.12 | 0.22 | 0.16 | 0.11 | 0.14 | 0.47 | 0.37 | 0.21 | 0.17 | 0.11 |
| K2O (%) | 2.5 | 3.8 | 2.3 | 2.1 | 1.7 | 3.8 | 1.1 | 1.4 | 2.3 | 2.3 | 1.3 | 1.4 | 1.1 | 3.1 | 1.6 | 1.7 |
| CaO (%) | 4.9 | 4.7 | 4.7 | 2.7 | 3.1 | 4.1 | 4.1 | 3.6 | 3.4 | 2.2 | 3.5 | 4.4 | 4.0 | 2.9 | 3.4 | 2.8 |
| MnO (%) | 0.09 | 0.08 | 0.06 | 0.07 | 0.07 | 0.11 | 0.05 | 0.48 | 0.09 | 0.09 | 0.62 | 0.94 | 0.10 | 0.07 | 0.06 | 0.07 |
| Fe2O3 (%) | 2.1 | 2.6 | 4.2 | 3.7 | 4.0 | 2.1 | 2.2 | 4.2 | 2.8 | 3.3 | 4.0 | 5.0 | 2.9 | 2.1 | 1.7 | 2.3 |
| CuO (%) | 0.80 | 0.76 | 0.42 | 0.20 | 0.68 | 0.02 | 0.57 | 0.73 | 0.24 | 0.39 | 0.49 | 0.28 | 1.34 | 0.61 | 0.84 | 0.02 |
| PbO2 (%) | 2.67 | 3.26 | 0.15 | 0.13 | 1.51 | 4.72 | 1.46 | 0.10 | 0.10 | 1.93 | 0.02 | 0.14 | 0.01 | 0.09 | 0.04 | 0.02 |
| Be (ppm) | 1.3 | 1.3 | 1.6 | 0.9 | 1.8 | 1.1 | 1.5 | 0.8 | 1.1 | 1.3 | 0.5 | 0.7 | 1.0 | 0.9 | 0.8 | 1.3 |
| B (ppm) | 112 | 111 | 112 | 78 | 153 | 117 | 152 | 141 | 121 | 107 | 194 | 206 | 357 | 88 | 134 | 129 |
| Ti (ppm) | 2488 | 2413 | 1875 | 3923 | 2526 | 2430 | 2006 | 3133 | 3031 | 3448 | 2896 | 2870 | 2924 | 2477 | 1838 | 2832 |
| V (ppm) | 95 | 87 | 144 | 151 | 94 | 80 | 104 | 114 | 115 | 128 | 111 | 116 | 86 | 99 | 116 | 114 |
| Cr (ppm) | 15 | 17 | 38 | 55 | 27 | 19 | 13 | 25 | 34 | 13 | 16 | 30 | 45 | 23 | 21 | 31 |
| Ni (ppm) | 46 | 44 | 29 | 25 | 41 | 23 | 54 | 28 | 109 | 39 | 25 | 33 | 23 | 35 | 126 | 696 |
| Co (ppm) | 17 | 16 | 44 | 13 | 141 | 9 | 37 | 29 | 66 | 30 | 33 | 29 | 12 | 16 | 270 | 1727 |
| Zn (ppm) | 214 | 202 | 141 | 131 | 320 | 376 | 151 | 142 | 99 | 175 | 55 | 83 | 52 | 366 | 72 | 57 |
| As (ppm) | 25 | 28 | 45 | 15 | 134 | 6 | 14 | 28 | 11 | 23 | 25 | 15 | 21 | 20 | 356 | 1427 |
| Rb (ppm) | 67 | 62 | 49 | 62 | 35 | 61 | 34 | 30 | 58 | 73 | 28 | 34 | 15 | 105 | 53 | 51 |
| Sr (ppm) | 302 | 267 | 254 | 211 | 146 | 277 | 203 | 150 | 260 | 162 | 125 | 251 | 223 | 208 | 174 | 164 |
| Zr (ppm) | 190 | 177 | 254 | 460 | 111 | 152 | 127 | 91 | 150 | 145 | 74 | 74 | 182 | 138 | 95 | 143 |
| Nb (ppm) | 11 | 10 | 7.7 | 14 | 6.3 | 11 | 9.6 | 5.6 | 10 | 13.6 | 4.2 | 5.4 | 8.0 | 9.8 | 7.4 | 11.4 |
| Ag (ppm) | 1.4 | 2.4 | 2.1 | 2.3 | 4.3 | 1.1 | 23.0 | 2.5 | 2.6 | 8.0 | 0.5 | 2.2 | 7.4 | 4.4 | 2.3 | 1.6 |
| Sn (ppm) | 2457 | 2087 | 240 | 280 | 1251 | 3941 | 2158 | 148 | 301 | 3359 | 36 | 193 | 64 | 731 | 65 | 18 |
| Sb (ppm) | 18 | 15 | 13 | 4.9 | 22 | 7.0 | 20 | 19 | 5.7 | 11 | 12 | 5.3 | 27 | 9.3 | 21 | 1.5 |
| Cs (ppm) | 0.8 | 0.8 | 1.1 | 0.9 | 1.3 | 0.7 | 0.6 | 0.4 | 1.0 | 1.1 | 0.2 | 0.4 | 0.3 | 1.6 | 1.2 | 0.8 |
| Ba (ppm) | 406 | 369 | 295 | 434 | 428 | 565 | 320 | 306 | 355 | 336 | 273 | 247 | 222 | 313 | 289 | 382 |
| La (ppm) | 37 | 34 | 27 | 42 | 27 | 33 | 31 | 17 | 28 | 38 | 13 | 15 | 19 | 26 | 25 | 36 |
| Ce (ppm) | 72 | 65 | 61 | 82 | 48 | 70 | 61 | 36 | 60 | 87 | 26 | 32 | 38 | 55 | 48 | 73 |
| Pr (ppm) | 7.9 | 7.2 | 6.3 | 9.2 | 6.0 | 7.1 | 6.6 | 3.9 | 6.6 | 8.6 | 3.0 | 3.8 | 4.4 | 6.1 | 5.4 | 7.8 |
| Y (ppm) | 16 | 25 | 17 | 38 | 15 | 28 | 18 | 16 | 18 | 23 | 12 | 14 | 19 | 18 | 14 | 21 |
| Bi (ppm) | 0.8 | 0.5 | 1.4 | 1.8 | 12 | 0.3 | 2.6 | 7.1 | 1.2 | 24.2 | 13 | 5 | 1.0 | 1.4 | 431 | 2563 |
| U (ppm) | 102 | 93 | 127 | 167 | 26 | 80 | 155 | 32 | 79 | 203 | 21 | 25 | 18 | 115 | 144 | 258 |
| Red MTK033 | Red MTK034 | Green MTK037 | Green MTK038 | Red MTK039 | Red MTK040 | Red MTK041 | Red MTK043 | Red MTK044 | Red MTK045 | Red MTK046 | Red MTK048 | Red MTK049 | D. blue MTK050 | D. blue UNK001 | Red UNK002 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 (%) | 61.1 | 60.7 | 55.6 | 60.2 | 60.5 | 60.4 | 62.2 | 60.8 | 64.8 | 63.6 | 64.0 | 62.3 | 57.8 | 73.8 | 71.2 | 56.4 |
| Na2O (%) | 16.7 | 16.9 | 21.0 | 18.8 | 15.5 | 17.4 | 18.9 | 20.4 | 18.2 | 17.3 | 20.9 | 19.8 | 20.7 | 14.0 | 14.4 | 23.0 |
| MgO (%) | 1.59 | 0.97 | 1.37 | 1.14 | 1.19 | 2.11 | 0.74 | 0.80 | 1.56 | 2.06 | 0.68 | 1.63 | 0.99 | 0.68 | 0.61 | 0.81 |
| Al2O3 (%) | 5.9 | 8.1 | 7.0 | 5.3 | 11.0 | 6.8 | 8.7 | 7.6 | 5.6 | 5.1 | 5.7 | 5.5 | 8.9 | 4.3 | 5.2 | 9.1 |
| P2O3 (%) | 0.18 | 0.23 | 0.21 | 0.18 | 0.32 | 0.26 | 0.24 | 0.19 | 0.24 | 0.25 | 0.16 | 0.20 | 0.26 | 0.17 | 0.10 | 0.12 |
| K2O (%) | 3.8 | 3.2 | 2.4 | 1.3 | 2.4 | 2.0 | 2.0 | 2.0 | 1.2 | 1.7 | 1.6 | 1.6 | 2.8 | 1.5 | 2.3 | 2.2 |
| CaO (%) | 3.2 | 3.1 | 2.7 | 3.2 | 3.7 | 4.2 | 2.4 | 2.4 | 2.8 | 3.2 | 2.8 | 3.0 | 3.2 | 2.9 | 3.5 | 4.5 |
| MnO (%) | 0.30 | 0.05 | 0.05 | 0.21 | 0.06 | 0.51 | 0.07 | 0.08 | 0.24 | 0.07 | 0.04 | 0.24 | 0.07 | 0.03 | 0.05 | 0.10 |
| Fe2O3 (%) | 4.1 | 3.6 | 3.5 | 1.9 | 4.0 | 4.9 | 3.3 | 3.0 | 3.8 | 4.6 | 1.7 | 3.9 | 2.7 | 1.2 | 1.6 | 3.3 |
| CuO (%) | 0.46 | 0.74 | 0.91 | 2.08 | 0.63 | 0.38 | 0.62 | 0.52 | 0.42 | 0.53 | 0.74 | 0.39 | 0.78 | 0.35 | 0.80 | 0.22 |
| PbO2 (%) | 0.02 | 0.16 | 2.22 | 3.50 | 0.03 | 0.07 | 0.08 | 0.40 | 0.01 | 0.02 | 0.19 | 0.02 | 0.14 | 0.004 | 0.12 | 0.11 |
| Be (ppm) | 0.8 | 1.2 | 0.9 | 1.0 | 1.5 | 1.8 | 1.2 | 1.3 | 0.6 | 0.4 | 0.7 | 0.6 | 1.2 | 0.6 | 0.9 | 1.1 |
| B (ppm) | 134 | 104 | 135 | 425 | 74 | 152 | 120 | 158 | 135 | 169 | 150 | 131 | 101 | 105 | 108 | 116 |
| Ti (ppm) | 2877 | 2478 | 2361 | 2232 | 3206 | 3796 | 2493 | 2272 | 2782 | 3671 | 1708 | 2712 | 3077 | 1469 | 1355 | 2840 |
| V (ppm) | 99 | 116 | 78 | 95 | 120 | 140 | 136 | 114 | 110 | 136 | 130 | 116 | 110 | 74 | 83 | 111 |
| Cr (ppm) | 25 | 46 | 15 | 41 | 43 | 25 | 32 | 23 | 25 | 26 | 21 | 24 | 44 | 22 | 18 | 29 |
| Ni (ppm) | 28 | 32 | 32 | 45 | 31 | 34 | 32 | 34 | 25 | 32 | 70 | 24 | 27 | 23 | 56 | 22 |
| Co (ppm) | 29 | 40 | 54 | 46 | 33 | 60 | 24 | 41 | 33 | 40 | 168 | 33 | 14 | 113 | 139 | 33 |
| Zn (ppm) | 44 | 65 | 119 | 620 | 51 | 397 | 128 | 132 | 63 | 98 | 58 | 59 | 194 | 30 | 48 | 108 |
| As (ppm) | 33 | 49 | 50 | 62 | 47 | 57 | 38 | 48 | 33 | 44 | 314 | 30 | 19 | 124 | 228 | 22 |
| Rb (ppm) | 43 | 55 | 31 | 22 | 76 | 51 | 78 | 74 | 35 | 25 | 54 | 40 | 90 | 45 | 42 | 54 |
| Sr (ppm) | 159 | 175 | 139 | 314 | 253 | 183 | 147 | 147 | 127 | 97 | 141 | 127 | 231 | 133 | 135 | 194 |
| Zr (ppm) | 97 | 136 | 150 | 160 | 160 | 130 | 136 | 179 | 99 | 58 | 103 | 111 | 163 | 95 | 121 | 193 |
| Nb (ppm) | 6.1 | 10 | 5.0 | 7.8 | 11 | 9.1 | 11 | 10 | 4.8 | 4.2 | 6.7 | 5.6 | 14 | 8.3 | 5.7 | 9.3 |
| Ag (ppm) | 1.1 | 1.8 | 3.8 | 47.4 | 3.2 | 1.9 | 2.9 | 2.2 | 0.9 | 1.2 | 2.2 | 1.1 | 49 | 1.0 | 1.4 | 4.7 |
| Sn (ppm) | 20 | 145 | 609 | 976 | 59 | 149 | 145 | 512 | 30 | 36 | 228 | 30 | 655 | 18 | 115 | 172 |
| Sb (ppm) | 16 | 18 | 21 | 23 | 17 | 23 | 13 | 13 | 10 | 19 | 11 | 11 | 7.4 | 6.5 | 9.3 | 5.4 |
| Cs (ppm) | 0.4 | 1.0 | 0.3 | 0.5 | 1.2 | 8.0 | 0.9 | 0.7 | 0.4 | 0.2 | 0.6 | 0.5 | 1.1 | 0.7 | 0.4 | 0.7 |
| Ba (ppm) | 300 | 401 | 423 | 279 | 669 | 343 | 342 | 293 | 262 | 120 | 262 | 274 | 379 | 183 | 222 | 314 |
| La (ppm) | 18 | 33 | 25 | 19 | 42 | 30 | 33 | 30 | 17 | 9 | 22 | 19 | 39 | 23 | 19 | 28 |
| Ce (ppm) | 37 | 69 | 46 | 40 | 81 | 48 | 83 | 73 | 35 | 22 | 48 | 36 | 87 | 49 | 43 | 57 |
| Pr (ppm) | 4.0 | 6.9 | 5.0 | 4.6 | 8.9 | 6.1 | 7.5 | 7.0 | 3.6 | 2.5 | 4.8 | 3.9 | 9.0 | 4.8 | 4.8 | 7.0 |
| Y (ppm) | 19 | 28 | 16 | 14 | 34 | 20 | 31 | 36 | 19 | 19 | 21 | 14 | 36 | 15 | 10 | 18 |
| Bi (ppm) | 13 | 4.9 | 4.6 | 1.0 | 3.8 | 16 | 19 | 40 | 8.5 | 12 | 252 | 8.9 | 3.2 | 0.4 | 0.02 | 1.4 |
| U (ppm) | 23 | 323 | 11 | 27 | 153 | 165 | 161 | 213 | 31 | 29 | 198 | 31 | 242 | 53 | 144 | 139 |
| D. green UNK003 | T. blue UNK004 | Yellow UNK005 | Black UNK006 | D. green UNK003 | T. blue UNK004 | Yellow UNK005 | Black UNK006 | ||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 (%) | 64.4 | 67.8 | 52.8 | 69.7 | Be (ppm) | 1.3 | 1.0 | 1.0 | 1.0 |
| Na2O (%) | 14.3 | 16.4 | 11.3 | 14.0 | B (ppm) | 44 | 90 | 105 | 60 |
| MgO (%) | 0.41 | 0.66 | 0.27 | 0.32 | Ti (ppm) | 3975 | 1989 | 1130 | 2038 |
| Al2O3 (%) | 9.1 | 6.7 | 5.4 | 8.3 | V (ppm) | 69 | 75 | 45 | 60 |
| P2O3 (%) | 0.11 | 0.07 | 0.06 | 0.18 | Cr (ppm) | 15 | 15 | 10 | 28 |
| K2O (%) | 3.7 | 2.7 | 2.5 | 3.5 | Ni (ppm) | 13 | 19 | 9.6 | 11 |
| CaO (%) | 4.2 | 2.9 | 1.3 | 2.2 | Co (ppm) | 6 | 7.9 | 3.1 | 6.8 |
| MnO (%) | 0.10 | 0.09 | 0.04 | 0.06 | Zn (ppm) | 189 | 113 | 615 | 30 |
| Fe2O3 (%) | 3.0 | 1.9 | 0.9 | 1.6 | As (ppm) | 28 | 5.8 | 2.4 | 2.7 |
| CuO (%) | 0.48 | 0.63 | 0.02 | 0.004 | Rb (ppm) | 87 | 62 | 89 | 88 |
| PbO2 (%) | 0.10 | 0.08 | 23.66 | 0.004 | Sr (ppm) | 313 | 137 | 79 | 146 |
| Zr (ppm) | 576 | 134 | 113 | 234 | |||||
| Nb (ppm) | 16 | 9.2 | 4.3 | 8.1 | |||||
| Ag (ppm) | 3.9 | 2.5 | 16 | 0.9 | |||||
| Sn (ppm) | 151 | 197 | 9944 | 2.7 | |||||
| Sb (ppm) | 18 | 11 | 3.4 | 0.2 | |||||
| Cs (ppm) | 0.6 | 0.7 | 1.7 | 0.7 | |||||
| Ba (ppm) | 569 | 256 | 199 | 551 | |||||
| La (ppm) | 28 | 24 | 12 | 20 | |||||
| Ce (ppm) | 62 | 58 | 29 | 55 | |||||
| Pr (ppm) | 7.8 | 6.0 | 3.2 | 5.6 | |||||
| Y (ppm) | 23 | 16 | 10 | 21 | |||||
| Bi (ppm) | 0.8 | 3.1 | 73 | 0.1 | |||||
| U (ppm) | 7 | 202 | 51 | 130 |
To discuss the major and minor elements related to the sand and flux, we consider the reduced compositions of the samples limited to the concentrations for SiO2, Na2O, MgO, Al2O3, CaO, K2O and Fe2O3, after recalculation so that their sum is 100%, as suggested by Brill (Brill 1999, vol. 2, p. 9).
RESULTS
This paper is primarily focused on the presentation of the analytical results of the samples from Chaul and Kenya, while data from Sri Lankan and South Indian artefacts are considered for comparison purposes. Therefore, we will report the results by site for the former and for the whole South Indian and Sri Lankan area for the latter. Another reason for presenting a global average of the Sri Lankan and South Indian compositions is that no subgroup could be identified for this geographical area, even if the composition of the samples varied over a large range (Dussubieux 2001).
Elements related to the flux and the sand
The average concentrations for the reduced compositions of the glass samples by site or region are recapitulated in Table 4. For all the samples, average soda concentrations range from 16.3% to 18.8%. Average magnesia concentrations are always below 1.5%. Average alumina concentrations are high in all groups, and vary from 7.4% for Chaul to 10.4% for the glass samples from India and Sri Lanka. The glass beads from Bungule have the highest potash contents (3.8% on average), whereas glass beads from Chaul have the lowest ones, with only 2.2% of potash on average. Average lime concentrations remain fairly low (< 5%) and range from 1.6% to 3.1%. The Muasya samples have the lowest average lime concentration (1.6%). The compositions that we identified correspond to the definition of m-Na–Al glass given in the introduction, regardless of from where the samples originate.
| Bungule | Muasya | Mtwapa | Ungwana | Chaul | South India and Sri Lanka | |
|---|---|---|---|---|---|---|
| SiO2 | 64.5 ± 1.7 | 63.6 ± 1.6 | 63.2 ± 3.4 | 67.2 ± 5.7 | 63.7 ± 2.3 | 63.5 ± 4.9 |
| Na2O | 17.1 ± 1.7 | 18.8 ± 4.7 | 18.8 ± 2.4 | 16.3 ± 3.5 | 18.5 ± 1.6 | 17.3 ± 3.5 |
| MgO | 0.6 ± 0.2 | 0.9 ± 0.4 | 1.3 ± 0.4 | 0.5 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.9 |
| Al2O3 | 9.7 ± 0.6 | 8.3 ± 1.0 | 7.8 ± 1.9 | 7.7 ± 1.5 | 7.4 ± 1.2 | 10.4 ± 2.2 |
| K2O | 3.8 ± 0.6 | 2.7 ± 0.5 | 2.4 ± 0.9 | 3.0 ± 0.7 | 2.2 ± 0.5 | 2.9 ± 1.1 |
| CaO | 2.9 ± 0.8 | 1.5 ± 0.4 | 3.6 ± 0.8 | 3.2 ± 1.1 | 4.7 ± 0.8 | 3.1 ± 1.6 |
| Fe2O3 | 1.4 ± 0.1 | 2.2 ± 1.1 | 3.0 ± 1.0 | 2.1 ± 0.8 | 2.6 ± 1.0 | 1.9 ± 1.3 |
The ranges of concentrations for the major and minor elements within each geographical group overlap between groups, with the exception of the samples from Muasya. This small group, with only three glass beads, has low lime concentrations compared to the samples from the other locations.
As the information provided by the major and minor elements is quite limited, trace elements were examined. In previous studies (Davison 1972; Davison and Clark 1974; Robertshaw et al. 2003, 2006; Popelka et al. 2005), relatively high uranium concentrations were measured in m-Na–Al glass found in Sub-Saharan Africa. Figure 5 shows the repartition of the uranium concentrations for each Kenyan site, Chaul and for South India and Sri Lanka.
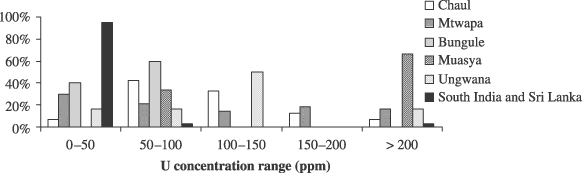
The proportion of samples for each site or region, based on their uranium concentrations.
Among the 78 glass samples from South India or Sri Lanka that were analysed, 74 samples have uranium concentrations below 50 ppm, two have uranium concentrations in the 50–100 ppm range, and the two remaining beads have more than 200 ppm of uranium. At Chaul, the distribution of the uranium concentrations is very different. Only 6% of the samples have less than 50 ppm of uranium, while 76% of the samples have uranium concentrations ranging from 50 to 150 ppm. At Mtwapa, 30% of the beads have uranium concentrations in the lowest values. The average uranium concentration, in the interval 0–50 ppm is higher for the beads from Mtwapa (26 ppm) compared to the Sri Lankan and South Indian material (only 14 ppm). The rest of the material from Mtwapa is distributed evenly across the spectrum of concentrations. The Bungule material has uranium concentrations that are always lower than 100 ppm, while 66% of the samples from Muasya have uranium concentrations higher than 200 ppm. For these two sites and Ungwana, too few samples are available to define general trends.
The consideration of additional trace elements confirms the differences between the samples from Chaul and those from South India and Sri Lanka, and also underscores the similarity between the Chaul and Kenyan samples. The barium and uranium concentrations are reported in Figure 6. The glass samples from Sri Lanka and South India have low uranium concentrations and rather high barium concentrations compared to the concentrations for the same elements encountered in the Kenyan and Chaul samples.
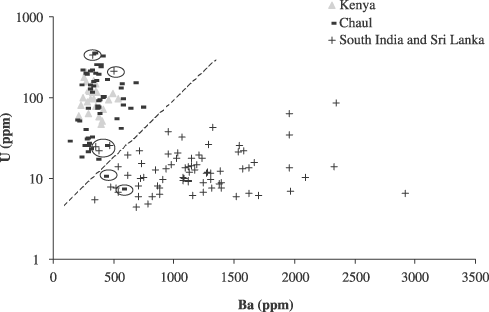
Barium and uranium concentrations. The dotted line delineates the hypothetical limit between the two groups of m-Na–Al glass. Circled samples are discussed in the text.
The zirconium and strontium concentrations confirm the observation made for uranium and barium. The concentrations for these two elements are similar for the samples from Mtwapa and Chaul. They are higher in the group formed by the samples from Sri Lanka and South India (Table 5). Compositions for the samples from Bungule, Ungwana and Muasya seem to follow a trend close to that of Mtawapa and Chaul, even if the Bungule samples are generally slightly lower in uranium and higher in zirconium, the Muasya samples are slightly higher in uranium and lower in strontium and the Ungwana samples are slightly higher in zirconium. Here again, the small number of samples for these three sites limits the discussion.
| Bungule | Muasya | Mtwapa | Ungwana | Chaul | South India and Sri Lanka | |
|---|---|---|---|---|---|---|
| U | 58 ± 30 | 221 ± 138 | 109 ± 80 | 112 ± 70 | 105 ± 41 | 22 ± 44 |
| Ba | 440 ± 105 | 307 ± 35 | 357 ± 124 | 351 ± 165 | 340 ± 79 | 1050 ± 502 |
| Sr | 213 ± 64 | 108 ± 19 | 213 ± 79 | 167 ± 80 | 222 ± 49 | 416 ± 166 |
| Zr | 298 ± 157 | 150 ± 46 | 157 ± 68 | 228 ± 176 | 148 ± 61 | 585 ± 296 |
Figure 6 also shows that five samples from South India and Sri Lanka fit into the group formed by the samples from Chaul and Kenya (RID23, IKIBC1, IART1, IART8 and IARBN), whereas two samples from Kenya (MTK037 and UNK003) seem to fit into the South Indian and Sri Lankan sample group (see the circled samples on Fig. 6). Sample RID23, from Ridiyagama, Sri Lanka, is a red wound bead. Visually, this bead is very close to bead BUK053, from Bungule, which is an opaque yellow wound bead. Sample IKIBC1, from Kalahandi, India, is a cobalt blue bead. These two beads have high uranium concentrations (243 and 381 ppm, respectively) and relatively low barium concentrations (564 and 367 ppm). The zirconium (250 and 471 ppm) and strontium (203 and 135 ppm) concentrations are compatible with a classification of these beads into the group of samples from Chaul and Kenya.
Three glass samples IART1, IART8 and IARBN from Arikamedu, the only ones with a m-Na–Al composition among 55 samples from this site that we analysed, have low barium concentrations and uranium concentrations around 20 ppm. The zirconium and strontium concentrations of these three glass samples vary within a large range (from 150 to 576 ppm for strontium, and from 68 to 385 ppm for zirconium), making it difficult to attribute these three beads to either one of the groups that we identified. The colour of these three samples is identical but very unusual for m-Na–Al glass: they have a translucent light greenish hue. No other m-Na–Al glass samples from Kenya, India or Sri Lanka have such a colour. These glass samples will be considered as a group of their own.
On the other hand, two Kenyan glass beads, MTK037 from Mtwapa and UNK003 from Ungwana, have relatively low uranium concentrations. If MTK037, with a strontium concentration of 139 ppm and a zirconium concentration of 150 ppm close to those encountered in the other Kenyan glass samples, is, perhaps, the lowest extreme for the uranium concentrations encountered in the Kenyan and Chaul glass beads, UNK003, with a strontium concentration of 313 ppm and a zirconium concentration of 577 ppm, definitely fits better into the group defined by the South India and Sri Lanka glass samples. Sample UNK003 is a translucent dark green bi-pyramidal bead, manufactured using the wound technique. A very similar-looking bead, although with a different composition, was recovered at Ridiyagama (RID20; Dussubieux 2001, 102).
Few data from the literature feature measurements for trace elements. The study of Robertshaw et al. (2006) reports compositional analysis of glass beads from a Malagasy site called Mahilaka, dated from the ninth to the 15th century ade. LA–ICP–MS was used by Robertshaw, who revealed that 15 samples have a m-Na–Al composition as defined earlier. Fourteen beads have trace element patterns similar to the one defined for our samples from Kenya and Chaul, whereas only one bead has a trace element pattern similar to the samples from South India and Sri Lanka (Table 6).
| High-uranium/low-barium | Low-uranium/high-barium | |
|---|---|---|
| U | 98 ± 79 | 43 |
| Sr | 193 ± 52 | 522 |
| Zr | 322 ± 138 | 588 |
| Ba | 499 ± 98 | 1791 |
Colouring elements
In this section, we will assess whether the nature of the colouring ingredients is identical in all chemical groups or if some differences exist. We will focus our attention on two specific colours: red produced by the addition of copper and dark blue resulting from the presence of cobalt in the glass.
The colour red is rather common in our sampling: 19 red glass beads from Mtwapa were analysed, two from Muasya, one from Ungwana, five from Chaul and 12 from Sri Lanka and South India.
Red glass beads from Mtwapa, Chaul and Ungwana consistently contain concentrations of copper less than 1%. Copper is higher in the two red glass beads found at Muasya. Both beads contain 1.4% of copper. Most of the samples from Sri Lanka and South India have copper concentrations ranging from 1% to 2%. Not only are the copper concentrations in these beads higher than those in the Chaul, Mtwapa and Ungwana red beads, but also the iron concentrations are generally lower in the Sri Lanka and South India material (Fig. 7). Iron concentrations in the red glass from Mtwapa, Ungwana and Chaul are twice the levels encountered in the glass beads of other colours from the same sites. For the samples from South India and Sri Lanka, the quantities of iron are the same whatever the colour (Table 7). Ahmed and Ashour (1977) showed that iron has a reducing effect on copper cations. In the presence of iron, metallic copper results in an easily obtained red colorant for glass. We therefore assume that the ancient glassmakers who produced the red glass found in Kenya (except for Muasya) and in Chaul used the reducing property of iron, whether consciously or unconsciously.
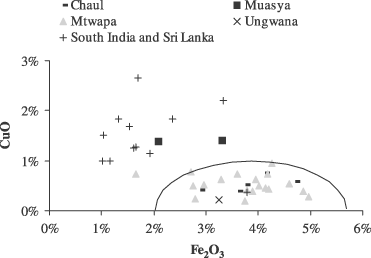
Iron and copper concentrations for red glass samples. The line delineates the hypothetical limits of the different groups of red glass.
| South India and Sri Lanka | Kenya | Chaul | |
|---|---|---|---|
| Red glass samples | 1.8 ± 0.9 | 3.7 ± 0.8 | 4.1 ± 0.6 |
| Other colours | 1.5 ± 0.9 | 2.0 ± 0.8 | 2.1 ± 0.5 |
Sample RID23 has the same behaviour in terms of copper and iron concentrations as the samples from Mtwapa, Chaul and Ungwana, confirming its membership in that group.
Cobalt blue glasses are extremely rare in the South India and Sri Lanka sample group. Dark blue glass samples coloured with cobalt are not specifically rare at Indian and Sri Lankan archaeological sites; they just belong systematically to other glass types (Dussubieux and Gratuze 2003). Actually, only one sample, IKIBC1 from Kalahandi, contains cobalt in a significant amount. We showed earlier that this sample does not belong to the same m-Na–Al glass group that includes the samples from Sri Lanka and South India but, rather, to the group formed by Chaul and most of the Kenyan glass beads. Five glass samples in the Kenya group contain cobalt for colouring purposes (four beads from Mtwapa and one bead from Ungwana). Five glass beads from Chaul were coloured using cobalt.
The cobalt blue glass samples contain, along with cobalt, more arsenic and sometimes more nickel and bismuth than glass samples of other colours. There is no correlation between the trace element patterns and the sites (Table 8). These trace elements may help to identify the type of cobalt mineral that was used by the glassmakers. In some glass beads from Madagascar, Robertshaw et al. (2006) identified the use of two different cobalt minerals, erythrite and cobaltite, using the arsenic-to-cobalt ratios in the glass of 2:3 and 1:1, respectively. However, cobalt-based pigments may be obtained by roasting cobalt-containing minerals, resulting in the volatilization of arsenic, and therefore modifying the arsenic-to-cobalt ratio. Robertshaw et al. (2006) report the existence of cobaltite in Rajasthan, India, from the copper mines of Khetri. Wadia (1975) mentions that a sulphide of both cobalt and nickel found in the copper mines of Khetri is used by Indian jewellers for the making of blue enamel. Yaocheng et al. (1994) published an analysis of Indian cobaltite (CoAsS) without precise information about its provenance. The arsenic-to-cobalt ratio for the Indian cobalt sample is 1.6, which is quite close to the ratios measured in some of the samples from Mtwapa, Ungwana and Chaul. However, bismuth was not tested and no nickel in any significant quantity was reported for the Indian cobaltite analysed by Yaocheng et al. (1994). Based on the information available at this point, an Indian origin for the cobalt used to colour the m-Na–Al dark blue glass can neither be ruled out nor confirmed. We know also that the association Co–As–Bi–Ni has been identified in European glazed ceramics dated from the 15th to the 18th centuries ace (Gratuze et al. 1996).
| As:Co | Bi:Co | Ni:Co | |
|---|---|---|---|
| CIB027 | 0.2 | 0.0 | 0.1 |
| IKIBC1 | 0.3 | 0.0 | 0.0 |
| MTK008 | 0.3 | 0.0 | 0.1 |
| MTK031 | 0.8 | 1.5 | 0.4 |
| CIB010 | 1.0 | 0.0 | 0.1 |
| MTK050 | 1.1 | 0.0 | 0.2 |
| CIB009 | 1.1 | 0.0 | 0.1 |
| CIB008 | 1.1 | 0.0 | 0.1 |
| MTK030 | 1.3 | 1.6 | 0.5 |
| UNK001 | 1.6 | 0.0 | 0.4 |
| CIB007 | 1.8 | 1.4 | 0.4 |
For brevity, we will provide short sketches of the other colouring techniques. Translucent turquoise glass beads usually contain copper. Opaque yellow glass samples contain usually lead and tin; they are opacified by a compound combining these two elements: lead stannate (Turner and Rooksby 1959). Opaque green glass contains copper that gives a green colour to the glass in the presence of lead; lead stannate causes the opacity of the glass. A greenish colour can also be produced by the presence of iron, accidentally included in the glass with the sand. Black and white glass samples do not contain any element in specifically high concentrations that could explain their colour. Black glass can be produced by the combination of iron and sulphur ions (Fe3+–Sn2−) (Schreurs and Brill 1984).
DISCUSSION
The major and minor element concentrations of the trade wind beads from Kenya, Sri Lanka and India with a m-Na–Al glass composition do not allow for the reliable identification of subgroups. Only the beads from Muasya can be separated from the rest of the samples, due to their low lime concentrations and the relatively high copper concentrations in the opaque red glass beads. Muasya is one of the more recent of the Kenyan sites (16th–19th centuries ace). The presence of this specific composition at Muasya may be an indication of some technological evolutions in time or of the use of some different sources of glass beads at later periods. Only three samples are from Musaya, which is insufficient to draw reliable conclusions.
Trace elements, and more especially the uranium, barium, zirconium and strontium concentrations, allow for the separation of samples into two main groups: the low-uranium/high-barium group (lU-hBa), which also has high strontium and high zirconium concentrations, and the high-uranium/low-barium group (hU-lBa), with low strontium and low zirconium concentrations. It is important to note that the concentrations vary in a wide range (e.g., the concentrations vary from 4 to 87 ppm for uranium in the lU-hBa group). By combining several trace elements, a more reliable attribution to a group for any given sample is made possible. A larger sample of beads would be necessary to verify whether additional subgroups exist, or if this variability in the composition of the glass is simply a reflection of the variability in the composition of the raw materials. No specific correlation between compositions and glass bead types was observed: wound and drawn beads are part of both compositional groups.
Red glass beads from these two groups were coloured using two different techniques. In the hU-lBa group, high iron concentrations were detected in the opaque red glass beads, while in the lU-hBa group, iron concentrations are as high in the red glass beads as they are in the others. An iron-rich ingredient was selected to facilitate the red colour in the hU-lBa glass.
No cobalt blue glass beads have been identified so far in the lU-hBa glass group. This is not due to an absence of demand for this colour, since cobalt blue glass beads are recovered very often mixed with beads of the lU-hBa glass type (e.g., Giribawa). These dark blue glass beads have systematically different compositions. The reason for the absence of cobalt blue lU-hBa glass remains unknown. Several hypotheses exist: the lU-hBa glassmakers may have been unaware of the use of cobalt; they may have been denied access to cobalt sources; or perhaps economic reasons prevented them from using cobalt. Further research should be able to clarify this point.
The lU-hBa group includes all of the samples from the South India and Sri Lanka sites, with a few exceptions. The dating of this material ranges from the fourth century bce to the fifth century ade. The lU-hBa glass is also quite common in South-East Asia at sites dated from the third century bce to the 10th century ade and represents about 60% of the analysed glass from this region (Dussubieux 2001; Dussubieux and Gratuze 2003). No primary lU-hBa glass workshop in South-East Asia has been identified in the earliest periods, which suggests that this glass was only manufactured in Sri Lanka and possibly in South India, and traded as the raw material or as beads to South-East Asia.
The hU-lBa group gathers samples from Chaul, Bungule, Ungwana and Mtwapa. It seems that this second type of glass is more recent and that it circulated between the east coast of Africa and Madagascar and the west coast of India, possibly with Chaul as a trading port to Africa, over a period ranging from the ninth to the 19th centuries ade. It is quite likely that the high-uranium glass samples identified by Davison (1972) and Davison and Clark (1974) are part the hU-lBa glass group, even if this would need to be verified by determining barium, strontium and zirconium concentrations in the samples. If we take Davison's results into account, the region of occurrence of the hU-lBa glass could be extended in Africa to the two Kenyan sites of Fort Jesus and Gedi, and would encompass Mozambique, Zimbabwe, Zambia, South Africa (eastern part), the site of Vohémar in Madagascar and the site of Maski in India. The chronology of these sites is consistent with the chronology of our Kenyan sites, since it spans from the 11th to the 19th centuries ace. Rare specimens of hU-lBa glass have been identified in South-East Asia: six beads from Hitam cave, Sarawak (ninth century ace) (Dussubieux 2001), three beads from Timor (surface findings) (Gratuze and O’Connor pers. comm.) and a few glass samples discovered in a Chinese junk shipwreck located 22 nautical miles off the Sultanate of Brunei (from the 15th century to the beginning of the 16th century ace; Gratuze in press). Few late (later than the 10th century ace) glass bead samples from South-East Asia have been investigated so far and this may explain the low number of South-East Asian hU-lBa glass specimens in our records.
At this stage, it is difficult to define a precise chronology for the hU-lBa and the lU-hBa glass samples in Africa, India, Sri Lanka and South-East Asia for several reasons. Some time periods were poorly investigated in some areas—or not investigated. Some of the glass samples—and, more especially, the samples from South and South-East Asia—belong to very broad time periods and some of the beads are surface findings. In addition, glass beads are durable artefacts that can be transmitted from one generation to the next; therefore, the archaeological level yielding the glass beads can post-date the manufacturing period of the beads by at least one century or more. Glass recycling should also be mentioned. In this case, the artefact is more recent than the glass itself. The proportion of the m-Na–Al (hU-lBa) glass beads at Muasya and Bungule is very small—11.5% and 8%, respectively—and one may wonder whether the presence of this type of glass bead at these two late sites reflects the persistence of some trade with India at later periods or if it is just an older type of bead that survived over a long period of time.
If we can hypothesize safely that the hU-lBa glass was manufactured in India, it is impossible at this point to localize the provenance of this glass more precisely. Future excavations and glass analyses will perhaps reveal where exactly this glass was produced. On the other hand, our results show that Chaul was a port from which some hU-lBa glass beads may have been transported to Africa. Another question for further investigation is whether Chaul was the single glass bead trade centre or one among many other competing traders. Glass was recovered from early period levels at Chaul, with some evidence of glass bead production. It will be possible to establish a chronology and to find out how early the hU-lBa glass beads appeared at this site and whether some lU-hBa glass preceded the hU-lBa glass.
CONCLUSIONS
This study demonstrates the usefulness of trace element concentrations in the study of glass. These concentrations provided key information for the discrimination of different groups of m-Na–Al glass in South Asia and in Sub-Saharan Africa, and allowed for the determination of two distinct trading areas and periods for these glass types. We defined a low-uranium/high-barium glass that was present at an early period in Sri Lanka and South India, and a high-uranium/low-barium glass that was present on the west coast of India and in Sub-Saharan Africa after the ninth century ade. A number of questions still remain unanswered. Further research will investigate the evolutionary relationships between the two types of glass and other types of glass present in the area.
This paper results from a collaborative effort by scholars studying both coasts of the Indian Ocean to address the concept of pre-modern global interactions. We will continue to analyse large volumes of samples from our own excavations and from other sites in the region in order to address the questions raised by this study. We are convinced that LA–ICP–MS analysis of some glass beads from contemporary sites from different regions will provide the best means for understanding how ancient interaction spheres shaped the technological, cultural and ecological landscapes of modern society.
ACKNOWLEDGEMENTS
Several archaeologists have provided us with material for analysis. In particular, the authors would like to thank O. Bopearachchi (CNRS, Paris, France) and K. Rajan (Tamil University, Thanjavur, India). The samples from Karaikadu, Kalahandi and Arikamedu were provided by the late P. Francis Jr. The LA–ICP–MS laboratory at the Field Museum of Natural History was built with funds from the National Science Foundation (BCS-0320903), an anonymous donation and the Anthropology Alliance.




