Hypertrophic cardiomyopathy in childhood and adolescence – strategies to prevent sudden death
Themed series on ‘ Sudden Cardiac Death – Cardiovascular Therapy’ Meeting June 18–19, 2009 Copenhagen, Denmark
Abstract
Clinically overt hypertrophic cardiomyopathy is the most common cause of sudden unexpected death in childhood and has significantly higher sudden death mortality in the 8- to 16-year age range than in the 17- to 30-year age range. A combination of electrocardiographic risk factors (a limb-lead ECG voltage sum >10 mV) and/or a septal wall thickness >190% of upper limit of normal for age (z-score > 3.72) defines a paediatric high-risk patient with great sensitivity. Syncope, blunted blood pressure response to exercise, non-sustained ventricular tachycardia and a malignant family history are additional risk factors. Of the medical treatments used, only beta-blocker therapy with lipophilic beta-blockers (i.e. propranolol, metoprolol or bisoprolol) have been shown to significantly reduce risk of sudden death, with doses ≥6 mg/kg BW in propranolol equivalents giving around a tenfold reduction in risk. Disopyramide therapy is a very useful adjunct to beta-blockers to improve prognosis in those patients that have dynamic outflow obstruction in spite of large doses of beta-blocker, and its use in patients with hypertrophic cardiomyopathy is not associated with significant pro-arrhythmia mortality. Calcium-channel blockers increase the risk of heart failure-associated death in hypertrophic cardiomyopathy (HCM) patients with severe generalized hypertrophy and should be avoided in such patients. Amiodarone does not protect against sudden death, and long-term use in children usually has to be terminated because of side effects. Therapy with internal cardioverter defibrillator implantation has high paediatric morbidity, 27% incidence of inappropriate shocks, and does not absolutely protect against mortality but is indicated as secondary prevention or in very high-risk patients.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common medical cause of sudden death in older children [1] and in young athletes [2]. 1 illustrates the population-based death rate comparing older children with young adults for some diagnoses commonly associated with sudden cardiac death from Swedish National Death registry data and shows that HCM is the only diagnosis that has a higher death rate in 8- to 16-year-olds than in 17- to 30-year-olds [3]. The disease is not rare, and prevalence studies in several genetically diverse populations have all found a prevalence of 1 in 500 [4–6]. However, many of those individuals were completely unaware of their cardiac disease. The majority of published work on the assessment of risk of sudden death has previously come from adult cardiology centres, but this review is an attempt at focussing specifically on risk assessment in the child and adolescent with HCM and a discussion of possible management strategies. This discussion will deal with a succession of relevant questions.
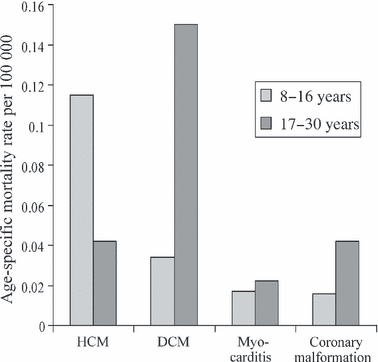
Age-specific mortality rates for the four most commonly diagnosed causes of sudden cardiac death taken from the Swedish National Cause of Death Registry. This distribution recognizes that long QT syndromes are significantly under-diagnosed at post-mortem, so that diagnosis is not included. Abbreviations: HCM, hypertrophic cardiomyopathy; DCM, dilated cardiomyopathy. It is noteworthy that HCM is the only diagnosis where the mortality rate is significantly higher in the 8- to 16-year age range than in the 17- to 30-year age range. Data from [3].
How large is the risk of sudden death in childhood HCM?
In large geographical cohorts, the annual mortality of childhood HCM patients in Sweden and Britain, the majority of whom had had a clinical presentation with symptoms or a murmur, was 6.6% in those patients who did not receive any medical therapy [7]. However, the patients with a clinical presentation in childhood represent the malignant tip of an iceberg of undetected or not yet developed HCM, with a prevalence of only 2.9 per 100 000 age-specific population [8] as distinct from the prevalence detected in echocardiographic population screening of young adults of 200 per 100 000, [4–6]. The population rate of HCM-related deaths based on Swedish National Registry death certificates is 0.112 per 100 000 age-specific population in the 8- to 16-year age range [3].
At what age is the risk greatest?
It has been a general perception that risk of sudden death in HCM is at its highest just after puberty and in young adults, which has led to suggestions that family screening for HCM should be commenced after 12 years [9]. However, subsequent published work has shown unequivocally that the risk of sudden cardiac death in a population with HCM diagnosed in childhood is significantly higher in the 9- to 14-year age range than it is in the 16- to 19-year-olds [3], see age distribution in 2. This surprising finding was confirmed from Swedish National Death Registry figures, which showed that annual mortality for HCM in 8- to 16-year-olds (0.112 per 100 000) was significantly outside the 95% CI for the death rate in 17- to 30-year-olds, which amounted to 0.055 per 100 000 age-specific population [95% CI 0.011–0.099] [3]. Much confusion has arisen in the literature from the uncritical habit of many authors of simply assuming that sudden death risk is uniform over the whole childhood, and thus calculating annual death rates without any consideration of the age composition of the cohort. That this introduces grave errors is clear from the Swedish–British cohort study, which showed that the risk of sudden death is extremely low below 8 years of age, rapidly increases from 8 years, rises to a maximum in 10- to 10.9-year-olds where it reaches an annual mortality of 9.7% and is averaging 7.2% between 9 to 13.9 years. After 16 years, it drops to an average annual mortality of 1.7% (all these figures include patients on medical therapy) [3]. Wren et al. [10] found an annual sudden death mortality of 0.074 per 100 000 in the age range 1–20 years. Considering that they include in their estimates the 0- to 8- year age range where virtually no sudden deaths occur, there is good agreement between their figures and the estimate of a risk of 0.112 per 100 000 in the 8- to 16-year age range [3]. Cohort outcome data from Australian and American Pediatric Cardiomyopathy Registries have recently been published claiming lower mortality than earlier studies; these studies do not separate modes of death, do not include patients presenting with sudden death and have few patients in the high-risk age range; thus, their blanket annual mortality rates are not representative for sudden death risk [11,12].

A frequency distribution plot of the age distribution in numbers of sudden deaths in patients diagnosed with hypertrophic cardiomyopathy below 19 years. As illustrated, a large number of sudden deaths are clustered in the 8- to 16-year age range. Figure modified from [3].
In summary, it can be concluded that the risk of sudden death varies greatly with age in childhood HCM and that in the 8- to 17-year age range, it is significantly higher than the risk both in younger children and in young adults with HCM. This has obvious relevance for timing of risk assessment.
Gender effects
In adults, there is a clear male preponderance in sudden deaths, but this is not the pattern before and during puberty. Between 8–10 years, the annual mortality tends to be higher in girls than in boys, while after 11 years, the sudden death mortality tends to be higher in boys. Overall, the risk of sudden death in girls peaks at 10–11 years, and in boys at 15–16 years. Thus, it is only after 15 years that the male preponderance for sudden death is established [3].
Which children and adolescents should be screened to rule out HCM?
The low prevalence of clinically overt HCM in childhood argues against general population screening for HCM in childhood. However, the situation is different in populations with increased risk.
Pre-participation screening of athletes is compulsory in Italy, including school age as well as adult athletes, and appears largely effective in detecting individuals with HCM [13] with a concomitant reduction in sudden deaths caused by cardiomyopathy [14]. However, few other countries have followed Italy′s lead, with several authors concluding that using ECG screening has too high a false-positive rate to be indicated [15]. A new risk score derived from the 12-lead ECG, where a cut-off of a risk score of 6 points or above gives 100% specificity (i.e. no false positives) and a sensitivity of 85% in detecting individuals with a HCM-related sudden death below the age of 40, might be an argument to re-evaluate the risk–benefit ratio of ECG screening in athletes. The presence of a risk score of 6 or above gave an odds ratio for cardiac arrest of 345 [95% CI 16.9–7064; P < 0.0001] i.e. indicated a very substantial excess risk [16].
Family screening for HCM
The group with the highest risk of having overt HCM is of course children with one parent with HCM and thereby at 50% risk of being a mutation carrier, and a number of working parties have advocated systematic screening of first degree relatives of patients with HCM [17]. It has been suggested that family screening for HCM should be commenced after 12 years of age, [9] but as shown by Östman-Smith et al. [3], those children with familial disease that show disease expression in childhood have a very high annual mortality in the 10- to 13-year age range. In the large Swedish–British cohort study, 8 out of 57 patients with familial HCM (14%) had severe disease expression already in the first year of life, so an initial screening at 6–12 months of age can certainly be justified. A total of 11 out of the 31 sudden deaths occurring in childhood and adolescence were patients with familial HCM, with six sudden deaths occurring at or below 12 years [3]. The risk of sudden death is low but not non-existent below 8 years, and because one clearly wants a window for therapeutic interventions before the child arrives in the high-risk age range, I would advocate that the first screening of a child with a parent with HCM should be carried out no later than 6 years. A child who does not fulfil diagnostic criteria for HCM at 6 years may on subsequent follow-up develop progressive hypertrophy, particularly during the pubertal growth spurt [18]. However, the important thing is that if both ECG and ultrasound are normal, there is no risk of sudden death before significant hypertrophy develops. There are reports of adults with Troponin T mutations suffering cardiac arrest without fulfilling ultrasound criteria of abnormal hypertrophy [19,20], but these patients did not have normal ECGs. In the Swedish–British large cohort study including 150 patients, with 39 sudden arrhythmia deaths, all the patients that died suddenly had cardiac hypertrophy that was of substantial degree for their age [3]. Ultrasound screening alone is not sufficient, as ECG abnormalities may precede clinically pathological hypertrophy by several years in children with HCM-causing mutations [21]. Neither is ECG screening alone sufficient, as the prevalence of a pathological ECG in children with ultrasound-positive familial HCM is only 56% [22], compared with earlier reports of a prevalence of ECG abnormalities in 61–93% of adult HCM patients attending referral centres [23,24]. In childhood, the most helpful ECG features that correlated with overt HCM were the presence of a 12-lead QRS-amplitude sum >24 mV and/or Q-waves >25% of R-wave amplitude in two leads [22].
Non-familial HCM
About one-third of cohorts with childhood HCM consists of patients with Noonan or Leopard syndrome [3,7,11], and these patients have the same risk of sudden death as other childhood HCM patients [3,7] and should be investigated with the same risk-stratification protocols. A further third of childhood HCM consists of patients with HCM caused by sporadic new mutations, and the main way of diagnosing these patients is by some kind of presenting symptom. The most common presenting symptoms are episodes of palpitations, undue breathlessness on exertion and exercise-related syncope. The latter symptom is of particular importance in indicating an increased risk of sudden death.
How do we identify the patient with HCM at increased risk of sudden death?
A considerable amount has been published from adult cardiological centres about risk stratification for sudden death, with non-sustained ventricular tachycardia on Holter monitoring, a maximal wall thickness >3 cm, family history of sudden premature death in relatives, previous cardiac syncope, abnormal blood pressure response on exercise and a left ventricular outflow tract gradient all being identified as positive risk factors [25]. However, these risk factors individually have a low positive predictive accuracy [26–28], and a search for further risk factors has been advocated [29].
Research on risk factors for sudden death in the paediatric age range has been much more limited. The presence of non-sustained ventricular tachycardia on Holter monitoring is certainly a risk factor in the paediatric age range too [30], as is a history of syncope [31,32]. Increased QTc dispersion and the presence of coronary artery myocardial bridges have been claimed to be a paediatric risk factor for sudden death [30], but the cases examined were a tertiary centre referral population, and it is not practicable, and not without risk, to carry out coronary angiography for risk assessment in all paediatric patients with HCM. It seems likely that a malignant family history is a risk factor in the paediatric age range too [33]. There is uncertainty how predictive of risk a poor blood pressure response to exercise is in childhood HCM, but a recent study on isolated HCM in childhood found a blunted exercise blood pressure response to be significantly associated with an increased risk of non-sudden cardiac death [34]. An example of a pathological blood pressure response in a high-risk child is shown in 3. However, it is difficult to perform a proper maximal exercise test in young children, and further risk-stratification strategies are needed for children with HCM.
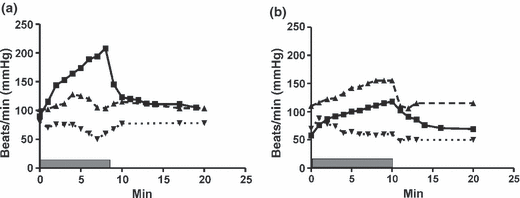
(a, b) Graphs over the response of heart rate (filled squares), systolic blood pressure (upward facing filled triangles,) and diastolic blood pressure (downward-facing triangles) to a standardized bicycle ergometer test starting at a load of 1 W/kg BW and increasing with 10- W increments every minute; duration of exercise is indicated by grey filled bar. (a) A newly diagnosed 11-year-old girl, with mild hypertrophy, but a close family history of sudden death at a young age. There is a rapid rise in heart rate, but a flat blood pressure response and eventually steadily falling blood pressure with increasing load. (b) An 18-year-old boy, receiving beta-blocker and disopyramide therapy because of ventricular arrhythmias. He shows profound beta-receptor blockade with a maximal heart rate of 118 beats/min but has a normal blood pressure response on exercise and manages 2.5 W/kg body weight, within the normal range.
Hypertrophy as risk factor in childhood HCM
Increased degree of cardiac hypertrophy is certainly an incremental risk factor in childhood HCM [7], but a cut-off of >3 cm is meaningless in childhood as illustrated in 4, which shows that the majority of children who died suddenly had a maximal wall thickness <3 cm [35]. A more useful approach is to relate the degree of cardiac hypertrophy to the upper limit of normal for age. This can be done in two ways. For long-axis M-mode measurements the 95th centile diastolic wall thickness for age can be predicted using a published formula [septum: 0.625 + (age in years × 0.0269) cm; posterior left ventricular wall: 0.565 + (age in years × 0.030) cm] [7]. The observed value in the patient is divided by the predicted 95th centile value and multiplied by 100 to give a per cent figure, defined as SEPPER for septal thickness and LVPER for posterior wall thickness. The 95th percentile corresponds to a Z-score of + 1.96. A SEPPER value >190% (i.e. a septal Z-score >3.72) was shown to be an optimal cut-off to indicate high risk of sudden death in a large cohort study with 16 sudden deaths, with a sensitivity of 91% and a negative predictive value of 95% if the value from the latest outpatient assessment was used [35]. Odds ratio was 11.1 [95% CI 1.3–95.2], and P = 0.013, and SEPPER was found to be an independent predictor of sudden death in childhood HCM (P = 0.001) [35]. Wall thickness Z-scores were used in another smaller referral centre study, which had much fewer deaths, only three sudden deaths, and found that a wall thickness Z-score >6 predicted all-cause cardiac death, not specifically sudden death, with a hazard ratio of 3.8 (34). Because of the lower statistical power, it would be unsafe to assume from the later study that a Z-score <6 defines a low-risk patient, in particular as 86% received therapy with beta-blockers and 17/96 (18%) had an implantable cardiac defibrillator (ICD) for sudden death prevention, which may well have contributed considerably to the low rate of sudden deaths [34].
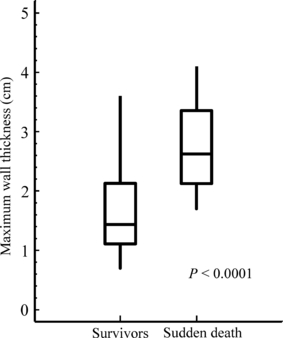
A box-and-whisker plot illustrating the maximal wall thickness (in this cohort always septal thickness) recorded at the last outpatient visit both in surviving patients (survivors) and in those patients that have died suddenly (sudden death). The box encloses the middle two quartiles with the median indicated, and the whiskers the upper and lower quartile values. The patients that died suddenly have significantly greater maximal wall thickness (P < 0.0001), but there is a large overlap. It is noteworthy that the majority of patients that died suddenly had a maximal wall thickness <3 cm and that the 8-year old-patient who died suddenly had a maximal wall thickness of only 1.6 cm. Data from [3].
ECG for risk factor stratification in childhood HCM
Already in 1999, it was found that large electrocardiographic voltages as expressed by a large Sokolow-Lyon index was a risk factor for disease-related death in childhood HCM [7], but as many patients with HCM have dominant S-waves across the precordium, and it cannot be used in children <2 years, the ECG measure of QRS-amplitude voltage sum in all six limb-leads was in addition used in the subsequent larger cohort study, which found that the QRS-voltage sum from all six limb leads already at diagnosis was a significant risk factor for sudden death in childhood HCM (P < 0.0005) and that it was an independent risk factor from that of septal hypertrophy on Cox hazard regression [35]. A cut-off of QRS-limb-lead sum >10 mV gave an odds ratio for sudden death already at the first ECG at diagnosis of 8.4 [95% CI 2.2–33.7], P = 0.001, (see 5). Using the ECG amplitudes from latest outpatient visit, the odds ratio rises to 33.4, with a sensitivity of 94% and a negative predictive value of 97% [35]. QRS-amplitude sum is a significant risk factor for death even in adults with HCM, but with an optimal cut-off for high risk in adults which is lower, ≥7.7 mV [16]. Limb-lead voltage sum has the same normal values in both genders, unlike ECG measures that include chest leads, which makes it a suitable ECG measure for screening [16]. A multifactorial analysis identified that QRS-axis deviation, increased 12 lead QRS-amplitude x duration product, increasing QTc and T-wave inversion, particularly precordial, as well as ST-segment depression were incremental risk factors for sudden death in adults with HCM [16]. On the basis of these findings, a risk score was constructed (see Table I). A risk score ≥6 points signified a high-risk status with a sensitivity of 84% but an area under the curve better than any other ECG measure, and the specificity of 85%, as well as the positive predictive value, was better than that of any other measure [16]. This score has not yet been validated in childhood HCM, but preliminary observations suggest that it has the potential to improve specificity and positive predictive value in risk stratification in childhood HCM too (Östman-Smith, unpublished observation).
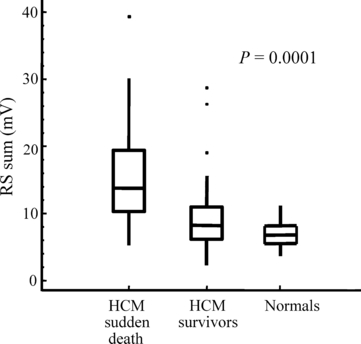
A box-and-whisker plot illustrating the six limb-lead QRS-amplitude sum taken already at the first outpatient visit when the diagnosis was made (abbreviated RS-sum) expressed in mV. Patients that have died suddenly (HCM sudden death) already at diagnosis have strikingly larger ECG amplitudes than long-term survivors with hypertrophic cardiomyopathy (HCM survivors), P = 0.0001, or than normal age-matched children (Normals), P < 0.0001. The box encloses the middle two quartiles with the median indicated, and the whiskers the upper and lower quartile values; outliers are indicated by dots. Data from [35].
| Deviation in QRS-axis present | 1 point | |
| Pathological T-wave inversion limb leads presenta | 1 point | |
| Pathological T-wave inversion precordial leadsb | 2 points | |
| ST-segment depression ≥ 2 mm present | 2 points | |
| S-wave greater than R-wave in V4 | 2 points | |
| Six limb-lead QRS-amplitude sum | ≥7.7 mV | 1 point |
| ≥10.0 mV | 2 points | |
| ≥12.0 mV | 3 points | |
| Twelve-lead amplitude-duration product | ≥2.2 mV.s | 1 point |
| ≥2.5 mV.s | 2 points | |
| ≥3.0 mV.s | 3 points | |
| QTc (Bazett′s formula) | ≥440 ms | 1 point |
| Max score = 14 |
- QTc, corrected QT interval.
- aT-wave inversion in lead III only is not counted as pathological.
- bT-wave inversion in lead V1 is not counted as abnormal, and in children, T-wave inversion is accepted in V2 and V3, but not V4. The two points for precordial T-wave inversion do not get added on top of the 1 point for limb-lead T-wave inversion; thus, total score available for T-wave abnormalities is 2 points.
- A score ≥6 indicates a significantly increased risk of sudden death in adults with hypertrophic cardiomyopathy. Table modified from Eur Heart J 2009, Östman-Smith et al. [16].
Does currently used risk stratification in childhood HCM have a good predictive power?
A cohort study shows that in the absence of any of the ECG- or echocardiographic risk factors at diagnosis, survival is extremely good, with an annual mortality of 0.27%, and the only death in the initial low-risk group was a patient who had rapidly progressive hypertrophy and fulfilled high-risk criteria a full 2 years before her subsequent sudden death [35]. Thus, combination of ECG- and ultrasound information allows very good risk stratification in childhood HCM and can be used to select patients for early pharmacotherapy. That a completely normal ECG that remains normal is a sign of a benign form of HCM is also suggested by studies on 2485 adults with HCM, where there were no deaths among the 135 patients with normal ECGs [36].
How should we treat identified high-risk patients?
Lifestyle modifications
The European Society of Cardiology has issued recommendations about which types of sports should be avoided for patients with HCM [37], and the Italian experience suggests that pre-participation screening of athletes and lifestyle modifications for diagnosed cases of HCM have reduced the sudden deaths attributable to HCM in the Verona region [14]. A majority of children who die suddenly are engaged in some kind of physical activity at the time, and it has been reported that quite a few sudden deaths of children with HCM under follow-up are associated with violations of exercise restrictions [34], so this aspect requires persistent and consistent counselling. It is often more productive to encourage the uptake of a different and more suitable sports activity than to attempt to stop sports activity altogether.
Pharmacotherapy
Unfortunately, there is a complete absence in the literature of long-term prospective randomized drug treatment trials in HCM, which have used mortality as an end-point, both in adult and in paediatric patients. Any conclusions about effects of drug therapy on mortality therefore have to rest on large retrospective cohort studies, where if follow-up is long, the number of patient years can be substantial, and I have quoted those where the controls are equivalent and contemporary.
Beta-adrenoceptor antagonist therapy
Which beta-blocker should be chosen?
In relation to sudden deaths caused by dilated cardiomyopathy and ischaemic heart disease, it has been argued that not all beta-blockers are equally protective, in particular that hydrophilic beta-blockers (for example atenolol) are not as good as lipophilic ones (propranolol, metoprolol, bisoprolol) [38]. Propranolol has in addition to beta-adrenoceptor blocking effects also membrane-stabilizing action and increases the threshold for ventricular fibrillation [38–40], and it is my first line choice in patients exhibiting risk factors for sudden death, unless there is a history of asthma in which case metoprolol or bisoprolol can be used. Furthermore, it has been reported from adult patients with HCM that propranolol therapy reduces inappropriate vasodilation in about half of HCM patients with abnormal blood pressure response to exercise, [41], and some patients with a tendency to severe sinus bradycardia or hypotension on exertion tolerate propranolol better than beta1-selective beta-blockers [see 6].
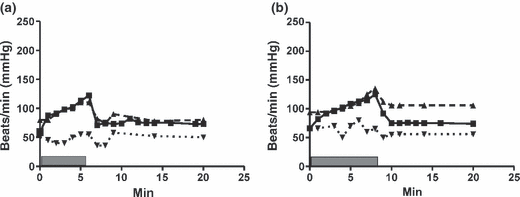
(a, b) Symbols as in 3. (a) shows a 13-year-old boy receiving beta-blocker therapy because of exercise-induced syncope. On initial therapy with metoprolol 6.4 mg/kg BW blood pressure-response is virtually flat, and he becomes hypotensive and dizzy after exercise. (b) After conversion to propranolol 2.9 mg/kg plus metoprolol 3.6 mg/kg, the same boy shows equally profound beta-blockade but has a better blood pressure rise during exercise, manages to perform to a higher load (2.1 W/kg BW) and maintains his blood pressure normally after exercise.
Which beta-blocker dose range is appropriate in children and adolescents?
Modest doses of beta-blockers were introduced for symptomatic treatment early on but did not appear to reduce sudden death mortality in adults [42]. During the 1970s, Frank et al. [43] developed a treatment approach of trying to achieve profound beta-receptor blockade, which in adult HCM patients required on average 6 mg/kg body weight of propranolol. Long-term follow-up of patients maintained in this way showed unusually low mortality rates and a low rate of serious arrhythmias [44], but they had no control groups, and this treatment was not widely taken up within adult HCM therapy. The consensus document on HCM therapy from AAHA/ESC published in 2003 suggests a dose of 2 mg/kg for children (without any references to support benefit) [17]. However, within the paediatric HCM field, it was reported in 1999, on the basis of contemporary retrospective cohorts of patients, that survival was positively correlated with increasing propranolol dose and that a high-dose propranolol regime in a dose of at least 5 mg/kg/day significantly improved survival with a hazard ratio suggesting a five- to ten-fold reduction in the risk of disease-related death [7]. It was subsequently shown that high-dose beta-blocker therapy reduces both sudden deaths and heart failure-related deaths [35]. 7 illustrates the Kaplan–Meier survival curves of patients positive on at least one of the high-risk criteria for sudden death. The high-risk patients treated with high-dose beta-blocker therapy show a significantly improved survival compared with patients on other treatments, P = 0.003, with a 10- year survival of 90.1% and 61.3%, respectively (subgroup analysis of data from [35]).
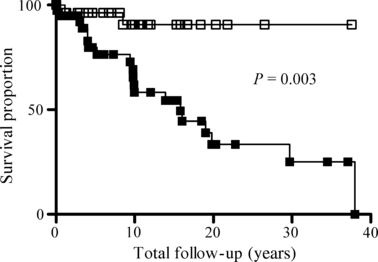
Kaplan–Meier survival curves comparing the survival of patients with hypertrophic cardiomyopathy that are positive for at least one risk factor, with those high-risk patients treated with more than 5 mg/kg BW/day of propranolol or equivalent other beta-blocker dose shown in empty squares (High-risk HDBB, n = 36), compared with those high-risk patients that were treated with other treatment regimes shown in solid black squares (High-risk NOHD). The survival is significantly better for the high-dose beta-blocker group on log-rank test (P = 0.003). Ten-year survival is 90.1% in the high-risk HDBB group and 61.3% in the high-risk NOHD-group. In the HDBB group, half the deaths were sudden and half heart failure-related, so total sudden death mortality was 5% over 10 years of follow-up. Subgroup analysis of data from [35].
It is obviously not very likely that the protective effect of beta-blockers is an on–off effect occurring only above a particular dose level, and if you subdivide paediatric HCM patients who, because they have a higher annual mortality rate than adults, provide more statistical power to detect treatment effects on mortality into subgroups with increasing beta-blocker doses, there is an obvious trend to a dose-related gradual decrease in mortality proportions (P = 0.008 for trend) (see 8) [45]. This mortality trend parallels the effect of therapy on rate of disease progression. In untreated childhood HCM, there is on average a 25% increase in ECG and echocardiographic measures of cardiac hypertrophy over 7.5 years of follow-up, whereas with beta-blocker therapy, there is a dose-dependent effect with partial regression of hypertrophy with the largest doses, and a significant negative correlation between propranolol dose and progression/regression of hypertrophy both for ECG and ultrasound measures [45]. On mortality, it therefore appears that at least from 3 mg/kg upwards, there is some protective effect (8), even if high-dose therapy, the median dose in that group being 9.6 mg/kg, provides better protection. There is probably also an age variation in the dose required. For carvedilol, which, like propranolol, has extensive first pass metabolism by cytochrome P450 in the liver [46], drug kinetics in children have been studied in detail, and it has been shown that weight-adjusted drug clearance was 3.9 times faster in 1-year-olds compared with 19-year-olds and that infants required 4.3 times higher dose, 2- to 11-year-olds 2.9 times higher and 12- to 15-year-olds 1.4 times higher dose to maintain same plasma levels as adult patients [47]. Young children also metabolize propranolol faster than adults and furthermore have a higher sympathetic nervous activity level in general, and in young patients with very severe obstructive HCM, you may have to go as high as 23 mg/kg/BW to achieve satisfactory beta-blockade as revealed by 24-h ECG monitoring (see [7]).
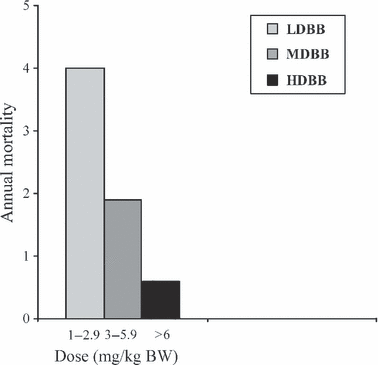
Annual mortality in a subgroup analysis of data dividing beta-blocker treated patients in dose-range bands. There is a dose-related trend of lower mortality proportions with higher doses of beta-blocker (P = 0.008 for trend). The 0.6–2.9 mg/kg consisted of 28 patients, the 3–5.9 mg/kg group of 31 patients and the >6 mg/kg group of 37 patients. Data from [49], 96 patients on beta-blocker therapy, total number of patient years on beta-blocker = 672 years.
How do you titrate the beta-blocker dose in the individual child?
The best tool to ascertain adequate beta-receptor blockade is to use 24 Holter monitoring, or maximal exercise tests; it is insufficient to just look at resting heart rate [7]. On 24-h Holter monitoring, one needs to look at both heart rate variability and maximal heart rates. In an infant with severe HCM, one should strive for maximal heart rate not to exceed 120/min during significant periods, whereas with an adolescent with severe HCM, it may be necessary to achieve a very flat heart rate response with a maximal heart rate below 90 beats/min for full therapeutic effect and % variability in R-R interval to remain below 5–6% (see 9). Children with less severe HCM may have adequate disease control with maximum heart rates not exceeding 110/min [7]. Surprisingly, at least with propranolol, the minimum heart rate does not fall with increasing beta-blocker dose but may instead increase slightly, may be as a result of central anti-vagal effects of propranolol. Increasing sinus bradycardia is usually only seen with very beta-1 selective beta-blockers, particularly atenolol (Östman-Smith, unpublished observation). Using a maximal exercise test to judge beta-receptor blockade, one should aim for a maximal heart rate response of 120–140 beats/min, depending on the age of the child and the disease severity (see 3, 6).
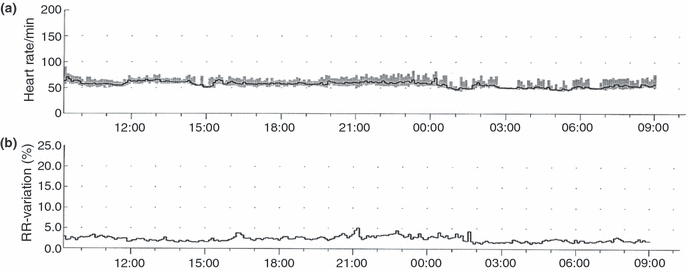
(a) 24-H Holter registration from the 18-year-old whose exercise test is illustrated in 3. Note that this very low heart rate variability is compatible with a normal exercise performance.
Patient acceptability of high-dose beta-blocker therapy
Patient acceptability is best, and side effects are minimized, by a gradual build up of dose, allowing beta-receptors to up-regulate and circulating blood volume to adapt by not increasing the dose more often than about every 2–4 weeks, unless there is an urgent clinical need to move faster. With older children, one may start with 1.5 mg/day of propranolol or equivalent doses of other beta-blockers and then aim to move via 3 mg/kg and 4.5 mg/kg to 6 mg/kg, which should be the minimum target dose in a high-risk patient. In infants, you start with 3 mg/kg/day. If plain propranolol is used, it needs to be given three times daily, and it is advantageous to use slow-release capsules, preferably given twice daily, whenever dosing allows, to achieve smooth serum levels, which reduces side effects. Children followed long term on high-dose beta-blocker therapy have shown normal somatic growth and generally a good tolerance of the therapy, and only about one tenth of patients on high-dose beta-blocker therapy need to change type of beta-blocker because of side effects [7]. If you require unusually large doses to achieve clinical beta-blockade, it is helpful to monitor serum levels if assay is available, both to detect drug non-compliance and to detect individuals with unusually fast (or unusually slow) drug elimination. The serum concentrations of propranolol required in childhood HCM are usually 200–900 μg/L [7], but particularly in young children concentrations up to 1 100 μg/L are sometimes required.
Side effects of beta-blockers
Restless sleep with vivid dreams is the most common side effect complained of with propranolol [7]. Hypoglycaemia after prolonged fasting is a rare problem, five proven or suspected episodes from 430 treatment years with propranolol, but it is the only potentially serious side effect [7]. Parents should be warned to avoid fasting for more than 16 h and informed to watch out for warning symptoms of sweating and shakiness after fasting (for instance caused by intercurrent gastroenteritis) and to administer glucose- or sugar-containing drinks when hypoglycaemia is suspected. A regular meal pattern is important, and access to fruits as extra snack at school is advisable. If hypoglycaemia occurs, the patients ought to be changed to the beta1-selective metoprolol or bisoprolol, because the impairment of glucose mobilization from glycogen is a beta2-receptor effect. Impotence is an often mentioned side effect of non-selective beta-blockers in adult males but is only rarely encountered as a problem in adolescent and young adult males, only in 2 out of 23 individuals on non-selective beta-blockers, and in both cases, it resolved on changing to atenolol (Östman-Smith, unpublished observation). A large review about neuropsychiatric effects of cardiovascular drugs has not found any evidence of propranolol being significantly associated with depression, but on the contrary, it gave significant benefit in reducing performance anxiety [48]. Surprisingly, physical exercise tolerance is not reduced by high-dose beta-blockade in patients with HCM compared with pre-treatment values, in spite of a 30% reduction in maximal heart rate [49,50], see also 3, probably because of the beneficial effects of high-dose beta-blockers on diastolic function [51]. A total of 86% of the children reported by Decker et al. [34] were treated with beta-blockers, and 9% with calcium-channel blockers, but in spite of the medication, 66% had a normal blood pressure response to exercise. This concurs with our own observations that beta-blocker therapy does not reduce blood pressure response to exercise compared with pre-therapy blood pressure reaction [49,50], so a poor blood pressure response on exercise testing cannot be assumed to be caused by beta-blocker therapy (3).
Exchange of beta-blocker drug
If one wishes to change from a non-selective to a selective beta-blocker, one should not exchange the whole dose at once, because the beta2-receptors are up-regulated, but do a gradual change-over with 2 weeks between each step. Propranolol, metoprolol and bisoprolol are metabolized differently, so single-dose potency tables cannot be used for guidance, and effect varies between slow- and fast-metabolizing patients. As a rough guide, propranolol and metoprolol are roughly equipotent in mg doses in children, and 80 mg propranolol can be changed to about 50 mg of atenolol, or 5 mg of bisoprolol, but it is wise to perform a new 24-h Holter recording to compare clinical beta-blockade after completed dose change.
Calcium-channel blocker therapy
Verapamil was introduced more widely in 1980s following reports of beneficial effects on symptoms in HCM [52,53]. Early reports on its use in children and young adults with HCM were also favourable after short follow-up [54]. However, after more widespread use, there were reports of sometimes fatal complications of verapamil therapy, including acute fulminant fatal pulmonary oedema in patients with severe HCM and a raised pulmonary artery wedge pressure, resulting for instance from severe generalized left ventricular hypertrophy [55]. Pelliccia et al. [56] first raised a concern about possible excess late mortality in heart failure, and in paediatric HCM patients, verapamil has been observed to cause an excess mortality in heart failure in those patients that have a marked and generalized left ventricular hypertrophy [57]. There is no evidence that verapamil protects against sudden death in patients with HCM [42], so it would seem a totally unsuitable therapy for paediatric HCM patients who fulfil high-risk criteria.
Disopyramide therapy
Disopyramide has beneficial effects both on diastolic function and on reduction in dynamic outflow obstruction in HCM [58,59], and the effect is additive to that of beta-blockers [60]. Several reports testify to its efficacy in reducing even severe outflow obstruction in childhood [61,62], and an illustrative example of the benefit of completely controlling left ventricular outflow obstruction is shown in 10. It is of course also an anti-arrhythmic drug, which reduces risk of paroxysmal atrial fibrillation, and in HCM, it is useful in reducing/abolishing non-sustained ventricular tachycardias. After the CAST study, which showed significant pro-arrhythmic effects of another Class I anti-arrhythmic drug, flecainide, many cardiologists are very wary about using anti-arrhythmic drugs in this class at all. However, disopyramide is really only pro-arrhythmic if the patient becomes significantly hypokalemic or when there is an interaction with other drugs that prolong the QT interval [63] and is safer than flecainide [64]. That disopyramide in clinical use in HCM patients does not have any pro-arrhythmic effects, but possibly instead a protective effect, is shown by the large multicentre study on the safety of disopyramide for the management of outflow obstruction in HCM, which showed that patients treated with disopyramide had non-significantly lower both total and sudden death mortality than the comparison group [65].
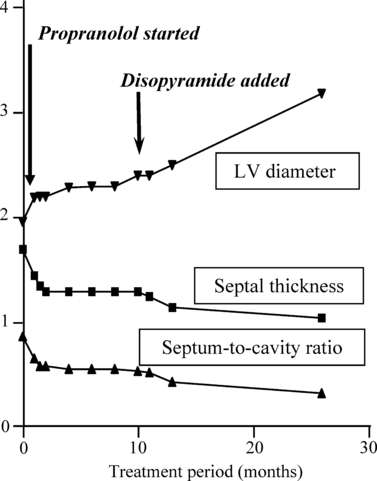
Nine-month infant with severe, non-syndrome-associated, hypertrophic cardiomyopathy, with a left ventricular outflow gradient of 100 mmHg and marked mitral valve reflux at presentation. She was commenced on gradually increasing doses of propranolol, and it required a dose of 20 mg/kg/day to achieve good beta-blockade; 24-h Holter tracings are published in ref [7]. Even on 20 mg/kg BW of propranolol, she continued to have a residual outflow gradient of 24–28 mmHg and mild mitral valve reflux, and thus, disopyramide was added, and within 3 months, the outflow gradient had completely disappeared, and with it the mitral valve reflux and there was a further regression of degree of hypertrophy and improvement in left ventricular volume. She remains well and without outflow gradients on combined propranolol and disopyramide therapy now over 10 years later.
Dose range for disopyramide in children
In terms of pharmacokinetics, disopyramide is cleared more rapidly in children than in adults [66]. Many paediatric drug dose compendiums recommend a dose of 2 mg/kg three times daily for disopyramide, but in my experience, such a low dose has never yet achieved therapeutic plasma levels. I always start with around 8 mg/kg/day, monitor QT prolongation and increase dosage every fourth day until some prolongation of QTc is apparent. If there is no QTc prolongation, you have not reached therapeutic blood levels, but QTc prolongation must never exceed a 25% increase over starting values, and unless there is a clinical need to push doses to maximum, it is safer to aim around a 10–15% prolongation. It is not uncommon to need 10–15 mg/kg/day and occasionally as much as 20 mg/kg/day to achieve therapeutic plasma levels. It is very desirable to monitor plasma levels to guide dose adjustments when using disopyramide. It combines very happily with high-dose propranolol in high-risk patients [7], and my preference would be to always add disopyramide to high-risk patients where the dynamic outflow obstruction is not controlled on high-dose propranolol alone, or where there have been non-sustained ventricular tachycardias on 24-h Holter, or exercise-induced ventricular arrhythmias on adequate beta-blocker therapy. I would use disopyramide before amiodarone for ventricular arrhythmias, as it has a short half-life and quite easily could be replaced with amiodarone after 72-h gap if ineffective, whereas once established on amiodarone therapy, it takes a much longer elimination time before you can safely introduce disopyramide.
Side effects of disopyramide
Anti-cholinergic side effects may occur particularly during the first week of therapy but then usually lessen. A dry mouth can often be relieved by intermittent use of chewing gum, and a tendency to constipation by prophylactic use of lactulose. Difficulty in emptying bladder is exceptionally rare in childhood; if it occurs, one should check serum levels because it may be possible to reduce the dose. The therapeutic range quoted for arrhythmia therapy is 6–16 (exceptionally 20) μmol/L; for adequate effect on dynamic outflow obstructions, plasma levels in the 8–13 μmol/L range are often needed (Östman-Smith, unpublished observation).
Amiodarone therapy
An observational study with historical controls suggested that amiodarone improved survival in patients with hypertrophic cardiomyopathy [67], and in spite of an absence of randomized controlled treatment studies, it has been quite widely used in HCM patients with non-sustained ventricular tachycardia on Holter monitoring.
Side effects of amiodarone
It is a thyroid hormone analogue with unusual pharmacokinetics and a high incidence of side effects, some of which can be serious or even lethal [68]. In children, it is difficult to avoid toxicity even with serum level monitoring [69], and among patients with HCM in childhood, the majority had to discontinue therapy after a while because of side effects [7]. Apart from pulmonary, hepatic, skin and eye toxicity, long-term amiodarone therapy also reduces exercise tolerance and increases pulmonary artery pressure and pulmonary artery wedge pressure in patients with HCM [70].
Thus, although amiodarone may be effective short-term therapy for ventricular arrhythmias and to control paroxysmal atrial fibrillation, it is not really suitable for long-term therapy in paediatric HCM patients. Furthermore, it does not protect against sudden arrhythmia death [7,71,72], and in a recent uncontrolled comparison, the amiodarone-treated patients showed considerably higher annual mortality than patients with HCM treated with other anti-arrhythmic drugs [42].
Dronedarone
Dronedarone is a benzofuran derivative related to amiodarone, with similar electrophysiological properties, but non-iodinated. It has significantly fewer side effects than amiodarone but is also somewhat less effective in preventing relapse of atrial fibrillation [73]. There are neither published pharmacokinetic studies in children nor any studies on its efficacy in preventing ventricular tachycardias, and as paroxysmal atrial fibrillation very rarely is the main arrhythmia in childhood HCM, it would appear that any use in childhood HCM should await pharmacokinetic data in children, in particular as there is an interaction with metoprolol metabolism [74]. Possible exception would be the few children where atrial fibrillation/flutter is the main problem and where amiodarone has caused side effects, but then plasma concentration monitoring should be obtained.
Dofetilide
Like dronedarone, the main indication for dofetilide, a new Class III anti-arrhythmic, is paroxysmal atrial fibrillation, and there are no published pharmacokinetic data in children. Torsades de pointes is a significant dose-related risk, occurring in 0.3–10.5% of adult patients [75], and thus, its use in childhood HCM cannot be recommended before the availability of pharmacokinetic studies in children.
Spironolactone
Hypokalemia greatly increases susceptibility to ventricular fibrillation [76], and in patients where ventricular arrhythmias are present, one should aim at keeping serum potassium between 4.0 and 5.0 mmol/L. Spironolactone in a dose of 0.5–1.0 mg/kg may be useful to achieve this aim. Spironolactone has actually been shown to significantly reduce sudden death in patients with heart failure [77] and possibly also to reduce development of cardiac fibrosis [78]. No specific trials of the effect of spironolactone in patients with HCM have been published as yet; however, spironolactone reduces interstitial fibrosis and improves diastolic function in transgenic mouse models of HCM, so there are theoretical reasons to hope that it may confer additional benefit in patients with HCM [79].
Implantable Cardioverter Defibrillator
Treatment with an ICD offers a way of protecting high-risk patients with HCM from death by acute ventricular fibrillation [80]. However, the procedure has a large impact on quality of life, affects employment opportunities and has both morbidity and mortality [80]. There is no argument that after a resuscitated cardiac arrest, a HCM patient ought to be offered an ICD implantation as secondary prophylaxis [72]. However, the indications for primary prophylaxis with ICD implantation are less clear-cut. Current algorithms for predicting high-risk patients with HCM are not working ideally as shown by a study on 103 adult HCM patients with particularly severe cardiac hypertrophy (LV wall thickness >3 cm), where there were 13 sudden deaths among 87 patients without ICD, whereas there were no device discharges among any of 11 patients that had had ICD implantation for primary prevention [81]. Whereas the annual appropriate discharge rate is 10.6% for patients receiving an ICD for secondary prevention (i.e. after a resuscitated cardiac arrest), the annual discharge rate is only 3.6% in patients having an ICD for primary prevention [80]. Thus, appropriate selection of patients for this therapy remains incompletely resolved, and it is to be hoped that including analysis of electrocardiographic risk factors in risk stratification might improve the selection of appropriate patients for primary prophylaxis with ICD. Implantation of an ICD in childhood HCM deserves particularly careful consideration because for technical reasons, the rate of inappropriate shocks is rather high, around 27%, which is highly distressing for the child, and there was a 17% incidence of complications [82]. Neither does it give complete protection against mortality [82].
Drug therapy for patients with ICD
Treatment with sizeable doses of beta-blockers may reduce inappropriate shocks caused by sinus tachycardia and is to be recommended, not least of course because the prevention of sudden arrhythmia death with an ICD does not cure the myocardial disease, which may also cause heart failure related death [34], unless appropriately treated medically. Effective control of supra-ventricular arrhythmias is also important in reducing risk of inappropriate shocks. Sotalol has been found to significantly reduce the number of delivered shocks in patients with ICD, [83], but its QT-prolonging effect usually precludes doses high enough to give a profound beta-receptor blockade. However, it can of course be combined with another beta-blocker for more effective beta-blockade. An additional consideration for patients on amiodarone therapy is that amiodarone increases defibrillation threshold for monophasic ICD devices [83].
Indications for primary prevention ICD in childhood
There is really no consensus yet about indications in children, but my own view would be that a child who has at least two risk factors remaining while established on therapy with high-dose beta-blockers, and disopyramide in effective doses, should be at least discussed for an ICD implantation, in particular if there is a malignant family history of sudden deaths at a very young age.
Compliance to therapy in childhood
A key factor to obtaining compliance with drug therapy in childhood is a well-motivated patient, and it is necessary to explain the aim and the effect of the drug used not only to the parents, but also in understandable terms to the child him/herself. Both short-term effects and long-term benefits need to be explained, as well as the risks of not taking the drugs, and any additional risks of sudden drug withdrawal. Sudden withdrawal of beta-blocker therapy causes an activation of sympathetic nervous system and may cause an acutely increased arrhythmia risk as the beta-receptors are up-regulated. Common side effects and how they can be minimized need to be mentioned. If mild side effects occur, slow down the dose-increment rate and use much smaller dose increases, and if side effects are significant, either reduce the dose or change to a different better tolerated drug. Advice about always having spare drug supply on travels is important to avoid unintentional interruption of therapy. The use of calendar packs or dose dispensers is helpful aids in identifying when a dose has been forgotten and is useful for parental supervision. Where possible, use slow-release preparations to reduce dosing to no more than twice daily, but even long-acting drugs ought to be split up in two doses to minimize side effects and maximize protection through a smoother plasma concentration profile. Teenagers may go through a rebellious phase where medication refusal can be a part of the picture; this can be identified by surprise 24 Holter monitoring, or unannounced measurement of plasma concentrations, and needs to be managed by patient counselling and perhaps support from a psychologist.
Conclusions
Considerable advances have been made in the identification of paediatric HCM patients at increased risk for catastrophic arrhythmia complications. Active medical pharmacotherapy including substantial doses of beta-blockers for high-risk individuals can reduce the risk substantially to around a tenth of the untreated risk. Children are not miniature adults, and in selecting drug doses, the different pharmacokinetic profile at various ages needs to be allowed for. In individuals with extreme risk, ICDs can significantly improve the prognosis, but with significant morbidity in childhood. As the risk for arrhythmia death is high from 8 years of age, it is particularly important for sudden death prevention that all children with a parent with hypertrophic cardiomyopathy should be screened for the disease no later than 6 years of age.
Abbreviations List
-
- HCM
-
- hypertrophic cardiomyopathy
-
- ECG
-
- electrocardiogram
-
- SEPPER
-
- septal thickness in % of 95th centile value for age
-
- ICD
-
- internal cardioverter defibrillator




