Alzheimer’s β-amyloid peptide blocks vascular endothelial growth factor mediated signaling via direct interaction with VEGFR-2
Abstract
J. Neurochem. (2010) 112, 66–76.
Beta-amyloid peptides (Aβ) are the major constituents of senile plaques and cerebrovascular deposits in the brains of Alzheimer’s disease patients. We have shown previously that soluble forms of Aβ are anti-angiogenic both in vitro and in vivo. However, the mechanism of the anti-angiogenic activity of Aβ peptides is unclear. In this study, we examined the effects of Aβ1–42 on vascular endothelial growth factor receptor 2 (VEGFR-2) signaling, which plays a key role in angiogenesis. Aβ inhibited VEGF-induced migration of endothelial cells, as well as VEGF-induced permeability of an in vitro model of the blood brain barrier. Consistently, exogenous VEGF dose-dependently antagonized the anti-angiogenic activity of Aβ in a capillary network assay. Aβ1–42 also blocked VEGF-induced tyrosine phosphorylation of VEGFR-2 in two types of primary endothelial cells, suggesting an antagonistic action of Aβ toward VEGFR-2 signaling in cells. Moreover, Aβ was able to directly interact with the extracellular domain of VEGFR-2 and to compete with the binding of VEGF to its receptor in a cell-free assay. Co-immunoprecipitation experiments confirmed that Aβ can bind VEGFR-2 both in vitro and in vivo. Altogether, our data suggest that Aβ acts as an antagonist of VEGFR-2 and provide a mechanism explaining the anti-angiogenic activity of Aβ peptides.
Abbreviations used:
-
- Aβ
-
- beta-amyloid peptide
-
- AD
-
- Alzheimer’s disease
-
- APP
-
- amyloid precursor protein
-
- BBB
-
- blood brain barrier
-
- bFGF
-
- basic fibroblast growth factor
-
- BSA
-
- bovine serum albumin
-
- DMSO
-
- dimethylsulfoxide
-
- EC
-
- endothelial cell
-
- FD4
-
- fluorescein isothiocyanate-dextran
-
- flt
-
- fms-like tyrosine kinase
-
- HBMEC
-
- human brain microvascular endothelial cells
-
- HFIP
-
- 1,1,1,3,3,3-hexafluoro-2-propanol
-
- HUVEC
-
- human umbilical vein endothelial cells
-
- PBS
-
- phosphate buffered saline
-
- VEGFR-2
-
- vascular endothelial growth factor receptor-2
One of the pathological hallmarks of the Alzheimer’s disease (AD) brain is senile or neuritic plaques which are composed primarily of 39–43 amino acid beta-amyloid peptides (Aβ) (Gorevic et al. 1986; Selkoe et al. 1986) derived from the amyloid precursor protein (APP) after sequential action of β- and γ-secretases. The most abundant Aβ peptides found in AD brain are the 40 and 42 amino acid forms. In the AD brain, Aβ peptides can form fibrillar deposits around blood vessels, leading to cerebral amyloid angiopathy (Pardridge et al. 1987; Jellinger and Attems 2005). The progressive accumulation of Aβ in the brain is believed to produce the clinical phenotype of AD and it was hypothesized that extracellullar amyloid fibrils, which compose senile plaques, were cytotoxic. However, more recent work has lead to the identification of small soluble oligomers of Aβ as being the main pathological culprit in AD since soluble Aβ species appear to disrupt long-term potentiation, impair memory, and decrease dendritic spine density (Cerpa et al. 2008; Selkoe 2008; Harmeier et al. 2009). In addition, damage to the vasculature resulting from Aβ deposition and from increased soluble levels of Aβ can result in a reduction of cerebral blood flow (Johnson et al. 2005). In support of this observation, we and others have shown that vascular functional impairments, reduced brain capillary density, and reduced cerebral blood flow are features of the AD brain (Farkas and Luiten 2001; Beckmann et al. 2003; Paris et al. 2004a,b; Wu et al. 2005). In addition, endothelial cells and arterial explants harvested from the brains of transgenic mice harboring the ‘Swedish’ mutation of APP, K670M/N671L, display an impaired ability to form capillary-like structures on reconstituted basement membrane, suggesting alterations in the angiogenic response in these mice (Paris et al. 2004a). We have also previously shown that angiogenesis is inhibited by soluble Aβ peptides in multiple different in vitro and in vivo assays (Paris et al. 2004c), whereas fibrillar forms of the peptide are not anti-angiogenic (Paris et al. 2005). Anti-angiogenic peptides have amyloidogenic properties, suggesting that β-sheet structures may mediate their inhibitory effects (Gebbink et al. 2004). In addition, Aβ peptides can inhibit the growth and vacularization of human gliobastoma and human lung adenocarcinoma cells xenografted into athymic mice (Paris et al. 2004c) and oppose vascular endothelial growth factor (VEGF)-A induced angiogenesis in vivo (Patel et al. 2008).
Vascular endothelial growth factor is a key factor required for the initiation of angiogenesis. Initially discovered as ‘vascular permeability factor’Senger et al. (1983), VEGF was later characterized for its effects on vascular growth and function by Leung et al. (1989). In humans, at least five different VEGF isoforms exist, each varying in amino acid length: VEGF-121, VEGF-145, VEGF-165, VEGF-189, and VEGF-206. Although cells produce all five isoforms, it is important to note that significant functional differences exist between the different VEGF isoforms. VEGF-121, VEGF-145, and VEGF-165 are secreted, whereas VEGF-189 and VEGF-206 remain bound to the cell surface and their biological activity has not been well characterized (Neufeld et al. 1999). The three secreted VEGF isoforms (VEGF-121, VEGF-145, and VEGF-165) are all able to induce angiogenic responses in endothelial cells. However, VEGF-121 lacks a heparin-binding domain and is therefore not able to bind cell surface proteoglycans, and VEGF-145 is believed to be a slow release form of VEGF as it binds to the extracellular matrix with high affinity. We chose VEGF-165 (which is a pro-angiogenic isoform of VEGF-A) for all experiments in this study, as it is one of the most abundant VEGF isoforms involved in angiogenesis. In this study, VEGF is used to indicate the VEGF-165 isoform of VEGF-A.
Vascular endothelial growth factor-165 has been well characterized as a potent angiogenesis-inducing factor in vivo (Connolly et al. 1989; Cao et al. 1998). The importance of VEGF in angiogenesis was discovered from studies using animals defective for particular VEGF genes (Carmeliet et al. 1996; Ferrara et al. 1996). The survival of endothelial cells in newly formed vessels is critically dependent upon VEGF (Alon et al. 1995), and VEGF is also known to be up-regulated in a variety of pathological conditions, such as hypoxia/ischemia and in tumor growth (Liu et al. 1995; Ankoma-Sey et al. 2000; Kim et al. 2006). Tumors secrete, in addition to other growth factors, large amounts of VEGF to promote angiogenesis and vascularization (Ferrara 2002; Jain 2002). For this reason, inhibitors of the VEGF pathway represent attractive targets for anti-tumoral therapies (Zhu and Witte 1999; Shinkaruk et al. 2003).
Vascular endothelial growth factor is also an important signaling molecule within neurons of the CNS. Both VEGF and one of its main pro-angiogenic receptors, VEGF receptor 2 (VEGFR-2), are expressed in the developing CNS. In addition, brain injury and ischemia can cause the up-regulation of VEGF and VEGF receptors on astrocytes (Krum and Khaibullina 2003; Krum et al. 2008) indicating that astrocytes play a role in reparative mechanisms within the brain.
There are currently three known receptor tyrosine kinase VEGF receptors [VEGFR-1 (fms-like tyrosine kinase or flt-1), VEGFR-2 (fetal liver kinase-1/kinase insert domain-containing receptor) and VEGFR-3 (flt-4)]. VEGFR-2 is the primary transducer of pro-angiogenic signals. VEGFR-1 has the highest affinity for VEGF but negatively regulates VEGFR-2-induced mitogenic signaling. Upon ligand binding, VEGF receptors dimerize and undergo autophosphorylation, initiating multiple intracellular signal transduction cascades.
Although we have shown previously that Aβ peptides and a short peptide derived from the Aβ sequence (HHQKLVFF) are able to inhibit angiogenesis, the molecular mechanism behind this effect remains unclear. In this study, we investigated the ability of soluble Aβ1–42 to interfere with VEGF signaling. Using in vitro and in vivo assays, we show that Aβ1–42 can block the activity of VEGFR-2 and that this effect is caused by a direct binding of Aβ to VEGFR-2.
Materials and methods
Cell culture and reagents
Human umbilical vein endothelial cells (HUVEC) (Cell Applications, San Diego, CA, USA) and human brain microvascular endothelial cells (HBMEC) (Sciencell, Carlsbad, CA, USA) were used for in vitro experiments. HUVEC were cultured in endothelial cell growth medium (Cell Applications). HBMEC were cultured in endothelial cell (EC) medium (with 5% serum, 1% penicillin/streptomycin, and 1% endothelial cell growth supplement). At all times, cells were maintained in a sterile cell culture incubator at 37°C and 5% CO2. Fibronectin solution was purchased from Sigma Chemical Co. (St Louis, MO, USA). VEGF-165 was purchased from Calbiochem (San Diego, CA, USA) and was used for all assays involving addition of VEGF. VEGFR-2 was purchased from Sigma-Aldrich (St Louis, MO, USA). Anti-VEGFR-2 antibody was purchased from Cell Signaling Technology (Beverly, MA, USa). Antibodies to VEGF, phospho-VEGFR-2(Tyr996), Akt-1, and phospho-Akt-1 (Ser473) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). 6E10 antibody was purchased from Covance (Denver, PA, USA). Actin antibody was purchased from Millipore (Billerica, MA, USA). Biotinylated peptides HHQKLVFF and GGQGLVFF were purchased from Invitrogen (Carlsbad, CA, USA).
Preparation of Aβ peptides
Aβ1–42 (purity greater than 95%) was purchased from Biosource (Carlsbad, CA, USA). 1 mg of lyophilized peptide was dissolved in 1 mL of 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) to minimize formation of β-sheet structures and promote α-helical secondary structure. The peptide was allowed to air dry in a chemical fume hood for 1 h, followed by further drying in a speed-vac (Thermo-Savant, Holbrook, NY, USA) for 30 min. The resulting clear film was re-suspended in 100% dimethylsulfoxide (DMSO) to a concentration of 1 mM, followed by aliquoting and storage at −80°C.
Solutions used for the cell and cell-free treatments were prepared by directly diluting 1 mM stock solutions to the appropriate final concentrations either in cell culture medium (for the cell-based assays) or in phosphate buffered saline (PBS; for the cell-free binding assays). In the different assays that we are using, Aβ peptides have been treated with HFIP prior to being solubilized in DMSO to minimize the formation of β-sheet structures. Such preparations of HFIP-treated Aβ peptide appear to form essentially soluble Aβ dimers in the culture medium surrounding endothelial cells (Paris et al. 2005). Once in solution (cell culture medium or PBS), these species are likely to change over time, and it is likely that higher order oligomers will form eventually. However, the cell treatments are over a short period of time (15 min) and the cell-free binding assays are likely to involve a relatively rapid binding of Aβ to VEGFR-2. Therefore, high-order oligomers are unlikely to form during this time frame.
Tube formation by HBMEC
Human brain microvascular endothelial cells (7.5 × 104 cells/mL) in 500 μL of medium were seeded in 24-well plates, on top of a layer of Matrigel basement membrane matrix (Invitrogen) in F12K medium (ATCC, Manassas, VA, USA) containing 4% serum (Invitrogen), 0.1 mg/mL Heparin, and 0.03 mg/mL endothelial cell growth supplement (Sigma-Aldrich). Cells were incubated with treatment or control conditions for 24 h. Control wells received the same volume of vehicle (DMSO) used to dilute the peptides. The Matrigel matrix contains, among other growth factors, VEGF therefore allowing the endothelial cells to form a capillary network. Capillary network formation experiments were performed in quadruplicate, and at least two randomly chosen fields were photographed for each well using a 4× objective. Capillary length was measured using Image Pro Plus software (Media Cybernetic, Inc., Bethesda, MD, USA). For the capillary length quantification in Fig. 1a, at least six pictures per treatment group were taken at 4× magnification. Each picture was subsequently analyzed for total capillary length by manually delineating the capillary tubes formed in the Matrigel. For each picture, the software collated the total capillary length in mm. The results (average total capillary length per picture) for each treatment were then expressed as a percentage of the control treatment. The black bar in the control field represents 250 μm length.
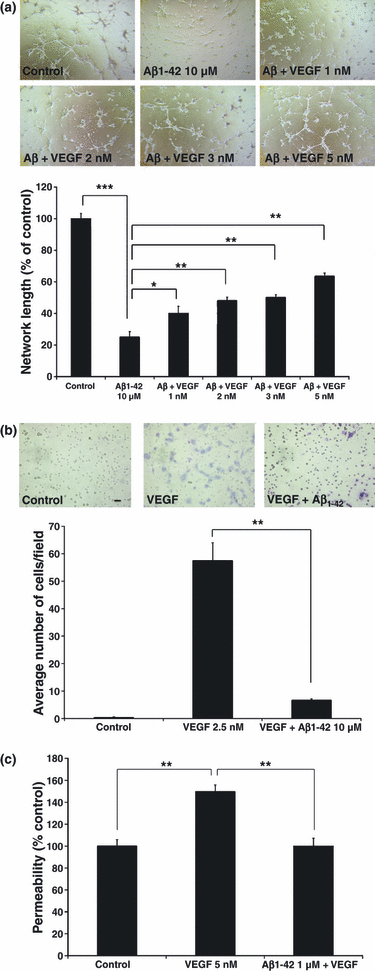
Effect of Aβ1–42 on physiologic functions of VEGF-165. (a) Capillary network formation by human brain microvascular endothelial cells (HBMEC). Capillaries were photographed at 4× magnification following 24 h incubation with 10 μM Aβ1–42 alone, or with varying doses of VEGF-165. Total capillary length was quantified for each treatment group (n = 6), and expressed as a percentage of control treatment. Post hoc analysis revealed significant differences between control and Aβ1–42 (***p < 0.001) and between Aβ1–42 and Aβ1–42 + VEGF-165 at all doses (*p < 0.05 for VEGF-165 1 nM, and **p < 0.01 for VEGF-165 2, 3, 5 nM). (b) Cell migration of human umbilical vein endothelial cells (HUVEC) in response to 2.5 nM of VEGF-165. HUVEC were incubated with or without Aβ1–42 for 24 h (n = 6 for each treatment group). anova followed by post hoc analysis revealed a significant effect for Aβ1–42 (**p < 0.01). Representative images at 20× magnification are shown for each treatment group. (c) VEGF-165 induced permeability of HBMEC monolayers. HBMEC were incubated with or without treatments for 30 min (n ≥ 6 for each treatment group). anova followed by post hoc analysis revealed a significant effect for Aβ1–42 (**p < 0.01).
Cell migration
Human umbilical vein endothelial cells (1 × 105 cells per well) were plated onto cell culture inserts containing 8 μm pores (BD Bioscience, San Jose, CA, USA) and allowed to adhere for 4 h in medium containing 1% serum. Inserts were then placed into 24-well companion plates (BD Bioscience) containing medium with either 1% serum (for negative control) or 2.5 nM VEGF (for treatment conditions). Medium in the inserts was then treated with 10 μM freshly solubilized Aβ1–42 peptide (or DMSO control). Following 24 h incubation, non-migrated cells on the upper side of the inserts were removed by scraping with cotton tips. Inserts were then washed in PBS, migrated cells on the underside of the membrane fixed with methanol, and stained with Giemsa solution (Sigma-Aldrich). Membranes were then cut out from the inserts and mounted onto glass cover slips. Cell migration was quantified by counting the total number of cells in each membrane at 4× magnification. The black bar in the control field represents 250 μm length. All treatment groups were performed in triplicate.
Apical permeability assay
Human brain microvascular endothelial cells (1 × 105 cells/cm2) were seeded onto fibronectin-coated (4 μg/cm2) 24-well translucent 0.4 μm membrane inserts (0.3 cm2/well) (Fisher Scientific, St. Louis, MO, USA) in regular EC medium. Media replacement was carried out every other day until the monolayers reached confluence (3–4 days). EC medium containing 10 μM 4kD fluorescein isothiocyanate-dextran (FD4) and 0.1% bovine serum albumin (BSA) was placed in the basolateral (donor) compartment. The treatment groups also contained 5 nM VEGF or 1 μM Aβ1–42 + 5 nM VEGF. The apical (receiver) side of the membrane was exposed to endothelial cell medium (ECM) media alone. The donor compartment was sampled at time 0 to establish the initial concentration of FD4 in each group. Following exposure of the insert to the well containing FD4, samples were collected from the apical compartment at 30 min to assess the movement of FD4 across the HBMEC monolayer (basolateral-to-apical). The samples were analyzed (λex = 485 nm and λem = 516 nm) for FD4 using a BioTek Synergy HT multi-detection microplate reader (Winooski, VT, USA). The apparent permeability (Papp) of FD4 was determined using the equation Papp = 1/AC0*(dQ/dt), where A represents the surface area of the membrane, C0 is the initial concentration of FD4 in the basolateral compartment, and dQ/dt is the amount of FD4 appearing in the apical compartment in the given time period. The Papp of FD4 in the treatment groups was compared with control and expressed as a percentage.
Western blotting of HUVEC and HBMEC cell cultures
Seventy to 80% confluent HUVEC in six-well plates were serum starved for 4 h in medium with 0% serum. Following starvation, HUVEC was treated with 10 μM Aβ1–42 for 5 min followed by 3.5 nM VEGF-165 for 10 min. Control wells received DMSO, or VEGF alone (for positive control). Following treatment, cells were washed thrice with ice cold PBS containing 1 mM sodium orthovanadate and lysed in 150 μL ice cold lysis buffer (Mammalian Protein Extraction Reagent; Thermo-Fisher Scientific, Rockford, IL, USA) containing 1X protease inhibitor cocktail (Calbiochem), and 1 mM each of phenylmethylsulfonyl fluoride and sodium orthovanadate. Lysates were collected and subjected to one freeze-thaw cycle in liquid nitrogen. Lysates were then clarified by centrifugation at 15 000 g for 5 min and the resulting supernatants analyzed by western blot. Briefly, 35 μL of supernatant were separated on a 4–20% Tris-HCl gel, and transferred overnight onto a polyvinylidene fluoride membrane. Membranes were probed with appropriate antibodies to phospho proteins, followed by stripping and re-probing with antibodies to total proteins. Bands were subjected to densitometric analysis using Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA). Phospho proteins were normalized against total protein levels, and results expressed as a percentage of control.
For experiments using HBMEC, 70–80% confluent HBMEC were treated with or without a dose range of Aβ1–42 for 15 min. Cells were then subject to the same protocol as for HUVEC. Results are expressed as a percentage of control treatment.
Enzyme immunosorbent assay
Five μg/mL VEGFR-2-Fc (Human recombinant extracellular domain of VEGFR-2; Calbiochem) (or 3% BSA for control wells) was plated onto high absorbency 96-well plates (Fisher Scientific, Pittsburgh, PA, USA) and incubated overnight at 4°C. The following day, the plates were washed and blocked in PBS containing 3% BSA and 0.1% Tween-20 for 2 h. Plates were then washed thrice and incubated with treatments. For Aβ/VEGF competition experiments, different concentrations of Aβ1–42 were added to the plate, followed by 263 nM of VEGF (for Aβ binding experiments, no VEGF was added). Following 2 h incubation, plates were washed thrice with wash buffer (1X Tris buffer saline). Bound VEGF or bound Aβ was quantified using anti-VEGF antibody or 6E10, respectively, followed by appropriate horse radish peroxidase-linked secondary antibodies. For short Aβ peptide (HHQKLVFF and GGQGLVFF) binding experiments, biotinylated peptides (Invitrogen) were used at 4 μM. Bound peptides were detected using anti-biotin horse radish peroxidase-linked antibody (Cell Signaling Technology). SuperSignal West Femto Maximum Sensitivity chemiluminescent substrate (Thermo-Fisher Scientific) was added and signal read in a plate reader (BioTek).
Immunoprecipitation of cellular lysates
For immunoprecipitation of cell cultures, confluent HUVECs were seeded in six-well plates and serum starved in 0% serum for 30 min. Cells were then treated with control (DMSO) or 10 μM Aβ peptide for 10 min. Following treatment, cells were washed thrice with ice cold PBS, and lysed in 150 μL ice cold lysis buffer. Lysates were collected and subjected to one freeze-thaw cycle in liquid nitrogen. Lysates were then clarified by centrifugation at 15 000 g for 5 min and the resulting supernatants were immunoprecipitated with either 0.16 μg/mL anti-VEGFR-2 antibody or 100 μg/mL 6E10 antibody. Following overnight incubation at 4°C with shaking, Protein Sepharose G beads were added, and the mixture was incubated for an additional 2 h at 4°C with shaking. Beads were then centrifuged at 800 g for 5 min, washed thrice in 1 mL of PBS, and finally boiled for 20 min in 45 μL laemmli sample buffer containing β-mercaptoethanol. Beads were then centrifuged at 800 g for 5 min and the supernatant analyzed by western blot.
Preparation of mouse brain homogenates
Mice were killed, brains were collected and snap-frozen in liquid nitrogen. 500 μL lysis buffer was added to one half of the brain. The resulting mixture was sonicated using a sonic dismembrator (Fisher Scientific) and centrifuged at 15 000 g for 10 min at 4°C. Supernatants were used for subsequent analysis. Protein content in each sample was determined using the bicinchonic acid protein reagent kit (Pierce-Endogen, Rockford, IL, USA), as per the manufacturer’s protocol. For immunoprecipitation of brain lysates, 2 mg of total brain lysate was used for each mouse sample, and VEGFR-2 was immunoprecipitated using 0.16 μg/mL of anti-VEGFR-2 antibody, as outlined above in the protocol for cell culture.
Molecular model of HHQKLVFF/VEGFR-2 binding
An in silico docking experiment was conducted to identify the binding region of an Aβ derived peptide, HHQKLVFF, on the VEGFR-2 extracellular domain. The 3D structure for VEGFR-2 was built using a homology model with the MPACK software (Mathura et al. 2005) since the crystal structure of VEGFR-2 has not been determined. An initial sequence alignment between the extracellular domain of VEGFR-2 (Uniprot entry: P35968, residues 122–223) and VEGFR-1 was created using CLUSTALW (Larkin et al. 2007). We used the secondary structure information from VEGFR-1 to modify this alignment such that insertions and deletions occur in the loop region. Using the structural information available for the extracellular domain of VEGFR-1 [Protein Data Bank (PDB): 1FLT, complex of extracellular domain of VEGFR-1 complexed with VEGF], we extracted geometric constraints (distance and dihedral angles) by the MPACK (Exdis) program. The threshold for upper and lower distanced constrains were kept at 0.25Å. A 3D VEGFR-2 model was built using the DIAMOD software (University of Texas Medical Branch, Galveston, TX, USA) and was subsequently energy minimized using the FANTOM program in MPACK. The 3D structure of the Aβ13–20 peptide (HHQKLVFF) was extracted from our previous model of Aβ (Mathura et al. 2005). We used the AutoDock program (release 4.0) (Morris et al. 1998) to dock the HHQKLVFF peptide to the model of the extracellular domain of VEGFR-2. Using Autogrid, we generated surface grids over the entire 3D structure of the receptor (box size, grid points). We selected Lamarckian Genetic Algorithm to perform a stochastic search for the best docking pose onto the entire surface of the extracellular domain of VEGFR-2. We set the HHQKLVFF peptide backbone and aromatic bonds as non-rotatable. The initial population size was set to 250 and the genetic cross-over rate was set at 0.8. The termination criteria were set using the number of evaluations to 2 600 000 and the number of generations to be 27 000. We ranked the 250 models using their binding energy predicted by the docking experiment and selected the lowest energy cluster for further analysis. The best peptide–receptor complex model had a favorable negative binding energy (−0.36 kcal/mol).
Statistical analyses
For statistical analyses, anova and t-tests were performed where appropriate using SPSS for Windows release 10.1 (SPSS Inc., Chicago, IL, USA).
Results
Reversal of the anti-antiangiogenic activity of Aβ1–42 by VEGF-165
Since VEGF-165 is a potent pro-angiogenic molecule and we have shown that Aβ can antagonize VEGF-induced angiogenesis in vivo (Patel et al. 2008), we investigated whether adding an excess of VEGF could oppose the anti-angiogenic effects of Aβ1–42. The Matrigel matrix contains VEGF, thereby allowing the endothelial cells to form a capillary network in the control (no exogenous VEGF addition) condition. Here, we now show that the addition of exogenous VEGF-165 is able to dose-dependently reverse the anti-angiogenic effects of Aβ1–42 in the Matrigel network assay (Fig. 1a) (p < 0.05 for VEGF 1 nM, p < 0.01 for VEGF 2, 3, and 5 nM). These results show that stimulation of VEGF signaling can prevent the anti-angiogenic activity of Aβin vitro, raising the possibility that Aβ can interfere with VEGF signaling.
Inhibition of VEGF-165 induced cell migration and apical membrane permeability
Since we showed that application of exogenous VEGF-165 can partially reverse the anti-angiogenic effect of Aβ1–42, we investigated whether Aβ could affect other known biological activities associated with VEGF-165, such as endothelial cell migration and vascular permeability. First, application of 2.5 nM VEGF-165 was able to induce cellular migration of HUVEC through a porous membrane (Fig. 1b). Application of Aβ1–42 in the inserts was able to block endothelial cell migration induced by VEGF-165 (p < 0.01). Next, we used an in vitro model of the blood brain barrier (BBB) as another assay to monitor Aβ’s effect on VEGF activity. The average basal apparent permeability of FITC-labeled dextran (FD4) in our BBB model employing HBMEC was 5.2 × 10−6 cm/s. This falls within the range of the basal apparent permeability values reported for FD4 (0.3–7.3 × 10−6 cm/s) in a previously published in vitro BBB model (Gaillard and de Boer 2000), indicating comparable junctional resistance. In this study, we used a 4 kDa fluorescent dextran marker (FD4) to monitor the effect of VEGF on vascular permeability. Exposure of HBMEC monolayers to VEGF-165 resulted in a significant increase (40%) in the basolateral-to-apical permeability to FD4 (Fig. 1c) and this effect was completely abolished by Aβ1–42 (p < 0.01).
Inhibition of phosphorylation of VEGFR-2 and Akt-1 by Aβ1–42
To determine whether Aβ1–42 affects VEGF signaling directly at the level of VEGFR-2, we investigated the effect of Aβ on the phosphorylation of VEGFR-2 triggered by VEGF-165 in HUVEC. Aβ appears to antagonize the phosphorylation of Tyr996 of VEGFR-2 induced by VEGF-165 in HUVEC (Fig. 2a). VEGF signaling is also known to lead to a stimulation of Akt phosphorylation (Gerber et al. 1998). Aβ1–42 also appears to antagonize Akt phosphorylation induced by VEGF-165 in HUVEC, further showing that Aβ1–42 suppresses VEGF signaling (Fig. 2a). In addition, Aβ1–42 was able to dose-dependently inhibit basal phosphorylation of VEGFR-2 and Akt-1 in HBMEC (Fig. 2b) which produce VEGF (Fischer et al. 1995).
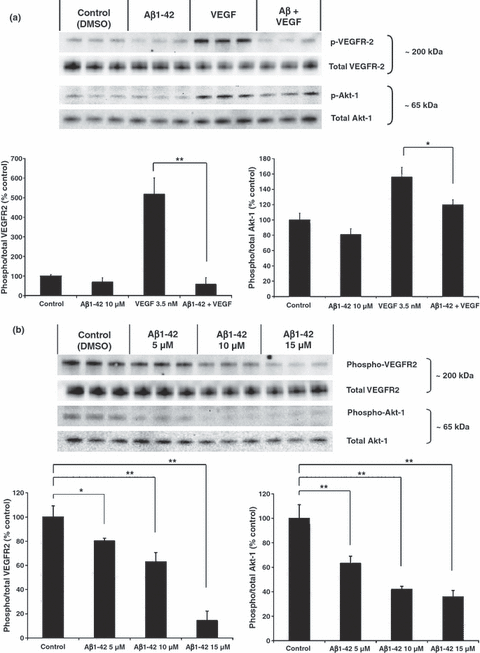
Effect of Aβ1–42 on VEGFR-2 signaling. (a) Serum starved human umbilical vein endothelial cells were pre-treated with Aβ1–42 for 5 min followed by VEGF-165 for 10 min. Control wells received dimethylsulfoxide (DMSO), or VEGF alone (n = 3 for each treatment group). anova followed by post hoc analysis revealed a significant effect for VEGF versus Aβ1–42 + VEGF (**p < 0.01 for phospho-VEGFR-2, and *p < 0.05 for phospho-Akt-1). (b) Human brain microvascular endothelial cells were treated with varying doses of Aβ1–42 for 15 min. Control wells received DMSO. anova followed by post hoc analysis revealed a significant dose dependent effect of Aβ on VEGFR-2 phosphorylation (*p < 0.05 for 5 μM, and **p < 0.01 for 10 μM and 15 μM), and a significant effect of Aβ on Akt-1 phosphorylation at all doses (**p < 0.01).
Aβ inhibits VEGF/VEGFR-2 binding and directly interacts with VEGFR-2
We next investigated whether Aβ can interact directly with VEGFR-2, employing a cell-free enzyme immunosorbent assay with human recombinant VEGFR-2 containing the amino acid residues 1–764 of the extracellular domain of human VEGFR-2. In this assay, Aβ1–42 appears to block the binding of VEGF-165 to the recombinant VEGFR-2 in a dose-dependent manner, with an IC50 of approximately 224 nM (Fig. 3a, p < 0.01 for all doses). Interestingly, Aβ1–42 also appears to directly bind to the extracellular domain of VEGFR-2 in a dose-dependent manner (Fig. 3b, p < 0.05 for Aβ 560 nM, p < 0.01 for Aβ 1120 nM) suggesting that Aβ1–42 directly competes with the binding of VEGF-165 to its receptor.
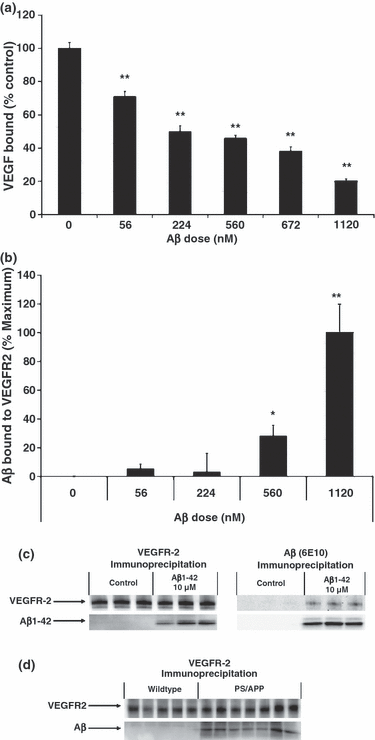
Binding of Aβ to VEGFR-2 in vitro and in vivo. (a) Varying concentrations of Aβ1–42 were added to the immobilized extracellular domain of VEGFR-2, followed by 263 nM of VEGF-165 (n = 8 for each treatment group). Control wells (0 nM Aβ) received dimethylsulfoxide (DMSO) alone. VEGF bound to VEGFR-2 was measured and expressed as a percentage of control. anova followed by post hoc analysis revealed a significant effect of Aβ for all doses (**p < 0.01). (b) Varying concentrations of Aβ1–42 were added to the immobilized extracellular domain of VEGFR-2 (n = 8 for each treatment group). Control wells (0 ng Aβ) received DMSO alone. Results are expressed as a percentage of maximal Aβ binding. anova followed by post hoc analysis revealed a dose-dependent binding of Aβ to VEGFR-2 (*p > 0.05 for Aβ 56 nM and 224 nM, **p < 0.05 for Aβ 560 nM, p < 0.01 for Aβ 1120 nM). (c) Human umbilical vein endothelial cells were treated with 10 μM Aβ1–42 for 10 min (n = 3 for each treatment group). Control wells received DMSO alone. Cells were washed and immunoprecipitated with either anti-VEGFR-2 or 6E10 antibody. Probing of the membrane revealed that Aβ1–42 can co-immunoprecipitate with VEGFR-2, and vice versa. (d) Whole brain lysates from 99-week-old wild-type and transgenic (PS1/APPsw) mice were immunoprecipitated with anti-VEGFR-2 antibody (n = 5 for wild-type mice, n = 7 for transgenic mice). Probing of the membrane revealed that Aβ can co-immunoprecipitate with VEGFR-2 in vivo.
Co-immunoprecipitation of VEGFR-2 and Aβin vitro and in vivo
Since we have shown that Aβ can bind to a human recombinant VEGFR-2 peptide in a cell-free assay, we investigated whether Aβ would display the same ability in whole cells. We treated HUVECs with Aβ1-42 for 10 min and washed the cells thrice in PBS to remove any Aβ bound non-specifically to the cell surface. Substantial amounts of Aβ were co-immunoprecipitated by an antibody to VEGFR-2 (Fig. 3c). VEGFR-2 was also co-immunoprecipitated by an antibody to Aβ (6E10), highlighting the strength of the Aβ/VEGFR-2 interaction (Fig. 3c). Furthermore, to demonstrate whether this interaction between VEGFR-2 and Aβ also occurs in vivo, we show that VEGFR-2 and Aβ can co-immunoprecipitate in brain lysates from 99-week-old Tg Presenilin-1 (PS1)(M146L)/APPsw(K670M/N671L) double transgenic mice (Fig. 3d). These results suggest that VEGFR-2 and Aβ can interact both in vitro and in vivo in a transgenic mouse model of AD.
Molecular docking of Aβ peptide to VEGFR-2
The 3D structure of the soluble form of Aβ1–42 has not been elucidated which precludes its use in developing a molecular docking model. In addition, this peptide is too large to predict its 3D structure with a computational approach. We have shown previously that a short peptide 13–20 (HHQKLVFF) derived from the Aβ sequence retains the anti-angiogenic activity of the full-length peptide (Patel et al. 2008). We show here that, like the full-length peptide, this short peptide binds to the extracellular domain of VEGFR-2 and opposes VEGF-induced endothelial cell migration (Fig. 4). Interestingly, mutations within this short peptide can abolish its anti-angiogenic activity (Patel et al. 2008) and its ability to bind to VEGFR-2 and oppose VEGF-induced endothelial cell migration (Fig. 4). Overall, this short peptide fragment of Aβ retains the biological activities of the full-length peptide and is therefore suitable for developing a docking model of Aβ to the VEGFR-2 receptor. We built a 3D model of the short HHQKLVFF peptide and docked it to a 3D model of the VEGFR-2 based on its homology with VEGFR-1 (covering residues 122–223 of the extracellular domain of the receptor). The docking model reveals that the VEGFR-2 receptor surface area of interaction with the short Aβ peptide and the VEGF dimer significantly overlap (Fig. 5). This supports our observation that the Aβ peptide may directly compete with the binding of VEGF to VEGFR-2. In particular, the docking model reveals that residue K16 of the Aβ peptide can form a salt bridge with residue E167 of VEGFR-2. In addition, amino acids L17–F20 of the Aβ peptide show hydrophobic interactions with the surface formed by residues Y165, P166, G196, M197, and Y221 of VEGFR-2. These hydrophobic residues of the receptor also interact with the VEGF dimer.
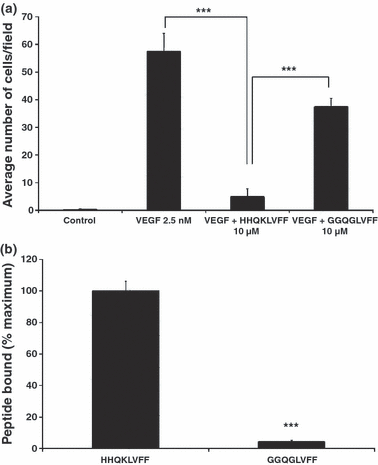
Effect of short Aβ peptide fragments on cell migration of human umbilical vein endothelial cells (HUVEC) and binding of short peptides to VEGFR-2 in vitro. (a) Cell migration of HUVEC in response to 2.5 nM of VEGF-165. HUVEC were incubated with or without peptides for 24 h (n = 6 for each treatment group). anova revealed a significant difference between VEGF and VEGF + HHQKLVFF (***p < 0.001) and between VEGF + HHQKLVFF and VEGF + GGQGLVFF (***p < 0.001). (b) Binding of short peptides to VEGFR-2. 4.3 μM of biotinylated peptides were added to the immobilized extracellular domain of VEGFR-2 (n = 8 for each treatment group). Results are expressed as percentage of wild-type peptide (HHQKLVFF) binding. anova revealed a significant difference between binding of HHQKLVFF and GGQGLVFF to VEGFR-2 (***p < 0.001).
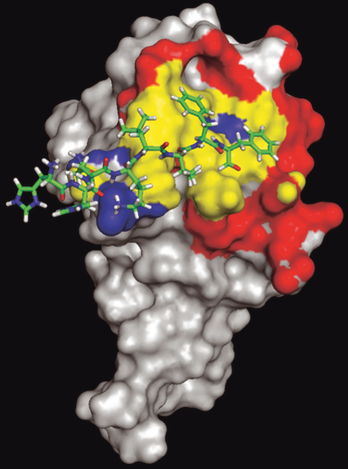
Model of the Aβ fragment HHQKLVFF bound to the extracellular domain of VEGFR-2. The Aβ fragment is shown in wire representation and VEGFR-2 in a surface representation. The interacting surface of VEGFR-2 unique to the VEGF dimer is shown in red (identified as atoms less than 5Å between VEGF and VEGFR-2) and Aβ interaction shown in blue (identified as atoms less than 5Å between the Aβ peptide and VEGFR-2). A common overlapping binding region between the Aβ fragment and VEGF dimmer is shown in yellow (interacting surface).
Discussion
We have previously shown that soluble Aβ peptides are anti-angiogenic both in vitro and in vivo (Paris et al. 2004c). We and others have also shown that Aβ peptides are able to antagonize basic fibroblast growth factor (bFGF) and VEGF-induced angiogenesis in vivo, in a rat corneal model of angiogenesis (Paris et al. 2004c; Donnini et al. 2006; Patel et al. 2008). In addition, it has been suggested that the anti-angiogenic activity of Aβ peptides is related to effects on cell migration (Magrane et al. 2006; Patel et al. 2008). However, the molecular mechanism responsible for the anti-angiogenic activity of Aβ peptides remains unclear. The aim of this study was to elucidate a molecular mechanism explaining the anti-angiogenic activity of Aβ1–42.
Angiogenesis, the formation of new blood vessels from pre-existing ones, is critically dependent upon adequate growth factor signaling. It has been well documented that the vasculature is compromised in the AD brain (Weller et al. 2002, 2004; Paris et al. 2004a; Meyer et al. 2008), despite an increase in VEGF levels (Kalaria et al. 1998; Tarkowski et al. 2002). The increase in VEGF expression in the cerebral vasculature and CSF of AD patients compared with controls (Kalaria et al. 1998; Tarkowski et al. 2002; Thirumangalakudi et al. 2006) suggests that compensatory mechanisms may be activated to oppose the reduced perfusion. However, despite increased VEGF levels, reduced blood flow, capillary degeneration, and reduced capillary density are prominent features in AD brain, suggesting an impairment of growth factor signaling.
Vascular endothelial growth factor is important for maintaining vascular integrity, and is also a key factor in vascular remodeling following stroke or head injury (Slevin et al. 2000; Shore et al. 2004). VEGF is also an important neurotrophic molecule (Rosenstein et al. 2003). The primary receptor responsible for the pro-angiogenic and mitogenic effects of VEGF is VEGFR-2 (Waltenberger et al. 1994). We therefore focused our attention on the possible impact of Aβ on VEGFR-2 signaling, using three different functional cell-based assays. First, we tested whether VEGF-165 can dose-dependently antagonize the anti-angiogenic activity of Aβ in a capillary network assay. The application of exogenous VEGF-165 was able to dose-dependently reverse the anti-angiogenic effect of Aβ. Second, we assessed the effect of Aβ on VEGF-165-induced endothelial cell migration. We show that Aβ can block VEGF-induced cellular migration. Third, we used an in vitro model of the BBB to further demonstrate the ability of Aβ to antagonize VEGF activity. We show that Aβ can abolish VEGF-induced apical membrane permeability, providing further evidence for Aβ’s ability to block the biological activities of VEGF. In Fig. 1(a), we demonstrate that the addition of excess VEGF can partially rescue the anti-angiogenic effect of Aβ1–42 in vitro. In this assay, there are likely to be a number of other growth factors each of which Aβ may be opposing. However, the addition of VEGF is sufficient to partially reverse this. Similarly, in Fig. 2(b), basal phosphorylation of VEGFR-2 (and Akt-1) is reduced by Aβ treatment, but one cannot rule out the possibility that Aβ may be acting also on other growth factor receptors. In support of this, other growth factors such as Insulin-like growth factors-1 and -2 and bFGF can oppose some toxic and anti-angiogenic effects of Aβ peptides (Donnini et al. 2006; Jarvis et al. 2007). The fact that addition of VEGF can only partially reverse the anti-angiogenic effects of Aβ peptides further suggests that other angiogenic growth factor signaling pathways may be impaired by Aβ. In addition to VEGF, other growth factors such as bFGF and Platelet Derived Growth Factor (PDGF) can influence angiogenesis and it is known that cross-talk exists between various growth factor signaling pathways, implying that reducing basal phosphorylation of VEGFR-2 can affect basal phosphorylation levels of other growth factor receptors. Altogether, these results provide evidence that Aβ1–42 can effectively antagonize various biological activities of VEGF in endothelial cells. Next, we investigated whether Aβ can block VEGFR-2 signaling. Interestingly, we found that Aβ can dose-dependently antagonize the phosphorylation of both VEGFR-2 and Akt-1 in primary human endothelial cells.
Having established that Aβ is able to inhibit VEGFR-2 activation, we investigated whether Aβ could affect the binding of VEGF to its receptor VEGFR-2. We show that Aβ can dose dependently prevent the binding of VEGF-165 to VEGFR-2 in a cell-free assay. Additionally, we show that Aβ is able to directly bind VEGFR-2 in a dose dependent manner suggesting that Aβ acts as an antagonist of the VEGF receptor. We further validated the interaction of Aβ with VEGFR-2 in a cell-based assay and also in vivo in the brain of transgenic mice over-expressing Aβ. Pull-down experiments effectively confirmed that VEGFR-2 and Aβ interact both in vitro and in vivo. For our docking study, we used the peptide region HHQKLVFF derived from the Aβ sequence since we have shown previously that this short fragment containing the HHQKLVFF region is critical for blocking angiogenesis (Patel et al. 2008). Since the 3D structure of full-length Aβ is unknown, we used a model for the short Aβ peptide fragment, HHQKVLFF, for our molecular docking study. From our model (Mathura et al. 2005) of soluble Aβ10–42, which is an anti-parallel beta sheet, we extracted the region Aβ13–20 representing the peptide HHQKLVFF (as a beta strand). Soluble oligomeric forms of amyloid beta may complex with the extracellular surface of VEGFR-2 through the residues HHQKLVFF that are part of the beta sheet of Aβ as shown in Fig. 5. Our docking experiments further suggest that the HHQKLVFF peptide (or Aβ) competes with the VEGF binding region in VEGFR-2. Our in silico prediction indicates that the hydrophobic core LVFF is important to mediate this interaction and supports our experimental observation that Aβ peptide acts as an antagonist by sterically preventing VEGF binding to VEGFR-2. Further mutation experiments may reveal the validity of this docking model.
Previous studies have suggested that Aβ may interfere with the bFGF signaling pathway, since over-expression of FGF-2 can oppose endothelial cell dysfunction induced by Aβ peptides (Donnini et al. 2006). However, cross-talk has been shown between the bFGF and VEGF signaling pathways (Presta et al. 2005), and it is possible that the protective effects of bFGF over-expression are, at least in part, as a result of an increased activity of the VEGF pathway (Donnini et al. 2006).
In summary, we provide evidence for the first time that Aβ1–42 is able to interact directly with VEGFR-2 and is able to prevent VEGF binding to its receptor, providing a mechanism to explain the anti-angiogenic activity of Aβ. The molecular mechanism underlying the anti-angiogenic activity of Aβ peptides may help us to better understand the contribution of Aβ to the AD process and in particular the contribution of the cerebrovasculature to the pathogenesis of AD. In addition, our data suggest that pro-angiogenic therapies or those that interfere with Aβ-VEGFR-2 binding may be beneficial in AD.
Acknowledgements
This work was supported by NIH grant # R01A619250 and the James and Esther King Biomedical Research Program. We would like to thank Diane and Robert Roskamp for their generosity in helping to make this work possible.
Conflict of interest
The authors declare that they have no conflict of interest.




