Retinoid X receptor-α mediates (R )-flurbiprofen’s effect on the levels of Alzheimer’s β-amyloid
Abstract
Alzheimer’s disease (AD) is characterized by the formation of extracellular senile plaques in the brain, whose major component is a small peptide called β-amyloid (Aβ). Long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) has been found beneficial for AD and several reports suggest that NSAIDs reduce the generation of Aβ, especially the more amyloidogenic form Aβ42. However, the exact mechanism underlying NSAIDs’ effect on AD risk remains largely inconclusive and all clinical trials using NSAIDs for AD treatment show negative results so far. Recent studies have shown that some NSAIDs can bind to certain nuclear receptors, suggesting that nuclear receptors may be involved in NSAID’s effect on AD risk. Here we find that (R)-flurbiprofen, the R-enantiomer of the racemate NSAID flurbiprofen, can significantly reduce Aβ secretion, but at the same time, increases the level of intracellular Aβ. In addition, we find that a nuclear receptor, retinoid X receptor α (RXRα), can regulate Aβ generation and that down-regulation of RXRα significantly increases Aβ secretion. We also show that (R)-flurbiprofen can interfere with the interaction between RXRα and 9-cis-retinoid acid, and that 9-cis-retinoid acid decreases (R)-flurbiprofen’s reduction of Aβ secretion. Moreover, the modulation effect of (R)-flurbiprofen on Aβ is abolished upon RXRα down-regulation. Together, these results suggest that RXRα can regulate Aβ generation and is also required for (R)-flurbiprofen-mediated Aβ generation.
Abbreviations used:
-
- 9-cis-RA
-
- 9-cis-retinoic acid
-
- Aβ
-
- β-amyloid
-
- AD
-
- Alzheimer’s disease
-
- ApoE
-
- apolipoproteins
-
- APP
-
- amyloid precursor protein
-
- BACE1
-
- β-secretase
-
- COX
-
- cyclooxygenase
-
- IFNγ
-
- interferon γ
-
- LBD
-
- ligand-binding domain
-
- LXR
-
- liver X receptor
-
- NSAID
-
- non-steroidal anti-inflammatory drug
-
- PPARγ
-
- peroxisome proliferator-activated receptor γ
-
- PS1
-
- presenilin 1
-
- RXRα
-
- retinoid X receptor α
-
- TNFα
-
- tumor necrosis factor α
Alzheimer’s disease (AD), the most common form of dementia in the elderly population, is characterized by extracellular neuritic plaques composed of fibrillar β-amyloid (Aβ) peptide and intracellular neurofibrillary tangles containing hyperphosphorylated Tau (Lee et al. 2001; Hardy and Selkoe 2002). Multiple lines of evidence suggest that overproduction/aggregation of Aβ in the brain is a major cause of AD pathogenesis (Hardy and Selkoe 2002). Aβ is derived from its precursor protein β-amyloid precursor protein (APP) by sequential cleavages by β-secretase (BACE1) and γ-secretase (Cole and Vassar 2007; Zhang and Xu 2007). γ-cleavage of APP generates Aβ40 and Aβ42 peptides, of which the latter is more amyloidogenic and neurotoxic (Hardy and Selkoe 2002).
Epidemiological studies indicate that long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) is associated with a reduced risk of AD development (Weggen et al. 2001; Eriksen et al. 2003). Studies in both cells and animals suggest that certain NSAIDs can specifically reduce Aβ42 levels independent of cyclooxygenase (COX) activity (Weggen et al. 2001; Eriksen et al. 2003), probably through direct interaction with, and inhibition of, the γ-secretase complex (Kukar and Golde 2008). In addition, NSAIDs may suppress the inflammatory reaction (i.e. microglia and astrocyte activation) associated with Aβ plaques (Lim et al. 2000; Sastre et al. 2008). For example, indomethacin, a COX-1 and COX-2 nonselective inhibitor, can significantly reduce microglia activation surrounding the intraparenchymal Aβ deposit that develops after an Aβ infusion in the lateral ventricle of rats (Netland et al. 1998).
Among NSAIDs, flurbiprofen raises particular interest because of its multiple interactions with key AD hallmarks: the R-enantiomer of flurbiprofen racemate, (R)-flurbiprofen, and its nitric oxide-releasing derivatives, HCT1026 and NCX2216, have been found effective in reducing AD amyloid pathology. Moreover, HCT1026 and NCX2216 can influence the cellular component of neuroinflammation (i.e., microglia activation) in some experimental settings (Gasparini et al. 2005). Chronic administration of (R)-flurbiprofen has also been found to attenuate learning impairments in AD mouse models (Eriksen et al. 2003; Kukar et al. 2007). However, the result from a phase III clinical trial using (R)-flurbiprofen for AD treatment by Myriad Genetics has recently turned out negative. The detailed mechanism underlying the (R)-flurbiprofen–mediated effect on Aβ generation/AD risk remains largely undetermined.
Several NSAIDs, including indomethacin and ibuprofen, can activate peroxisome proliferator-activated receptor γ (PPARγ), whose activation has been proposed to repress some microglial functions and contribute to the delay of AD progression (Bernardo et al. 2005). This suggests that PPARγ and related nuclear receptors might be involved in an NSAID-mediated beneficial effect on AD. Indeed, it has been found that activation of liver X receptor (LXR) can stimulate Aβ degradation (Xiong et al. 2008). The levels of several nuclear receptors are altered in AD brains and their agonists can reduce β- and γ-secretase activities responsible for Aβ generation (Jiang et al. 2008). However, while HCT1026, a flurbiprofen derivative that releases nitric oxide, activates PPARγ, flurbiprofen itself does not possess such PPARγ agonistic properties (Bernardo et al. 2005). Recently we found that the R-enantiomer of one NSAID, (R)-etodolac, can bind to the retinoid X receptor RXRα, another nuclear receptor family member (Kolluri et al. 2005). Hence, in the present study, we investigated whether RXRα is involved in (R)-flurbiprofen –mediated Aβ generation.
Materials and methods
Reagents and antibodies
(R)-flurbiprofen, 9-cis-retinoic acid (9-cis-RA), tumor necrosis factor α (TNFα), and interferon γ (IFNγ) were purchased from Sigma (St Louis, MO, USA). Lipofectamine 2000 reagent and Lipofectamine RNAi MAX reagent (Invitrogen, Carlsbad, CA, USA), rabbit anti-RXRα polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-cleaved Notch polyclonal antibody (Cell Signaling Technology, Boston, MA, USA), mouse anti-APP monoclonal antibodies 6E10 (Covance, Emeryville, CA, USA) and 22C11 (Roche, Nutley, NJ, USA), mouse anti-Myc monoclonal antibody 9E10 (Sigma) and mouse anti-α-tubulin monoclonal antibody (Sigma) were purchased from commercial resources. Mouse monoclonal anti-Abeta1-12 antibody, rabbit anti-BACE1 and anti-presenilin 1 (PS1) N-terminal fragment polyclonal antibodies were generated in our laboratory (Thinakaran et al. 1998; Yan et al. 2001). Rabbit anti-Aβ40 antibody (FCA3340) was kindly provided by Dr. F. Checler (Barelli et al. 1997).
Cell culture
N2a neuroblastoma cells were cultured as previously described (Bulloj et al. 2008). N2a cells stably transfected with human APP695 (N2a695 cells) were maintained in N2a culture media supplemented with 400 μg/mL G418 (Sigma).
Aβ assays
After treatments, conditioned media and cell lysates were assayed by ELISA for Aβ38, Aβ40 and Aβ42 using commercial kits (MSD, Gaithersburg, MD, USA), following the manufacturer’s protocols. Alternatively, Aβ in conditioned media and in cell lysates was immunoprecipitated and immunoblotted with 6E10 antibody or mouse monoclonal anti-Abeta1-12 antibody, and densitometry was carried out to quantify Aβ levels.
Biotinylation
To study cell surface proteins, cells were pre-incubated for 20 min in cold phosphate-buffered saline containing Mg2+/Ca2+ and then incubated with 0.5 mg/mL Sulfo-NHS-LC-Biotin (Thermo Scientific, Rockford, IL, USA) in phosphate-buffered saline containing Mg2+/Ca2+ for 30 min at 4°C. Excess biotin was quenched with 0.1 M glycine for 20 min. Cell lysates were affinity precipitated with streptavidin beads (Thermo Scientific) overnight. Samples were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted with anti-APP antibody (22C11) to detect cell surface APP.
Immunocytochemistry
N2a695 cells treated with or without (R)-flurbiprofen were immunostained with rabbit anti-Aβ40 antibody and then incubated with fluorescently labeled secondary antibody and 4′,6-diamidino-2-phenylindole. Specimens were examined and images were collected using a fluorescent microscope (Zeiss, Thornwood, NY, USA).
Regulation of RXRα levels
To over-express RXRα, N2a695 cells were transfected with a control vector (pBluescript) or an RXRα expression vector (Zeng et al. 2006), using the Lipofectamine 2000 reagent. To down-regulate RXRα, a SMARTpool of siRNAs specific for RXRα or green fluorescent protein (as a control) were purchased from Dharmacon (Chicago, IL, USA) and transfected into cells using the Lipofectamine RNAi MAX reagent (Invitrogen).
In vitro BACE1 activity assay
After over-expressing or down-regulating RXRα, cells were lysed and the lysates were assayed for BACE1 activity using a kit from Calbiochem (San Diego, CA, USA), following the manufacturer’s protocol.
Notch cleavage assay
Cells were first transfected with a Myc-tagged Notch construct NΔE. After equal splitting, cells were subjected to modulation of RXRα levels. Cell lysates were then analyzed by immunobloting with the anti-Myc antibody 9E10 and an antibody specifically recognizing the cleaved Notch fragment, Notch intracellular domain (NICD).
Aβ degradation assay
N2a naïve cells were subjected to modulation of RXRα levels. These cells were then treated with Aβ-containing media collected from N2a695 cells for the indicated times. Conditioned media were collected and assayed for residual Aβ40 levels by ELISA.
Ligand binding assay
The human RXRα ligand-binding domain (LBD) (223–462), prepared as a polyhistidine-tagged fusion protein in pET15b (Novagen, Madison, WI, USA) (1 μg), was incubated with [3H]-radiolabeled 9-cis-RA ligand in the presence of different concentrations of unlabeled 9-cis-RA or (R)-flurbiprofen at 4°C for 14 h. The RXRα LBD was captured by nickel-coated beads. Bound radiolabeled ligand was measured with a scintillation counter.
Results
(R)-flurbiprofen reduces Aβ secretion but increases the level of intracellular Aβ
We first studied the effects of (R)-flurbiprofen treatment on Aβ generation. As expected and consistent with previous studies, we found that in cells treated with (R)-flurbiprofen, the levels of secreted (extracellular) Aβ species (including Aβ40, 42 and 38) were all significantly reduced compared to those in untreated cells (Fig. 1a). Surprisingly, we found that (R)-flurbiprofen treatment significantly increased the levels of intracellular Aβ species (Fig. 1a). Immunoprecipitation-western blot analyses revealed that (R)-flurbiprofen treatment significantly reduced the level of extracellular Aβ but increased the level of intracellular Aβ (Fig. 1b). In addition, cells treated with (R)-flurbiprofen showed much stronger immunostaining of Aβ40 within the cell body than the control (Fig. 1c). Biotinylation analyses showed that cell surface levels of APP were not affected by (R)-flurbiprofen treatment (Fig. 1b), implying that the effects of (R)-flurbiprofen treatment on extracellular Aβ and intracellular Aβ levels were not attributable to altered trafficking of APP to the cell surface.
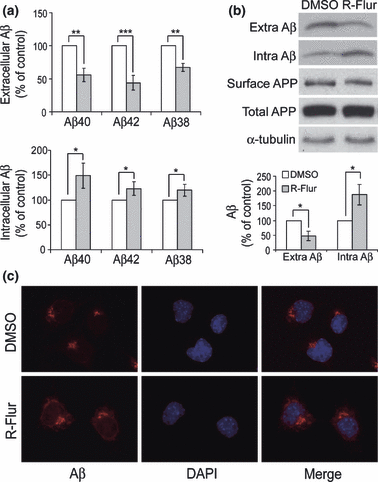
(R)-flurbiprofen treatment reduces the level of extracellular Aβ but increases that of intracellular Aβ. N2a695 cells were treated with 150 μM (R)-flurbiprofen (R-Flur) for 24 h. (a) Aβ40, 42, and 38 in conditioned media (extracellular) and in cell lysates (intracellular) were subjected to ELISA analyses. (b) Total Aβ in conditioned media (Extra) and in cell lysates (Intra) were immunoprecipitated and immunoblotted with 6E10 antibody, and their levels quantified by densitometry for comparison. Cell lysates were also analyzed for the total levels of APP and α-tubulin. In some experiments, cells were first subjected to biotinylation. Biotinylated proteins were affinity precipitated using streptavidin-conjugated beads and analyzed with an APP antibody to recognize cell surface APP. Data represent mean ± SD from three experiments. *p < 0.05, **p < 0.01, ***p < 0.001, (R)-flurbiprofen vs. control (DMSO) treatments (Student’s t-test). (c) N2a695 cells treated with or without (R)-flurbiprofen were immunostained with an anti-Aβ40 antibody and a fluorescently labeled secondary antibody (in red) and 4′,6-diamidino-2-phenylindole (in blue). Images were collected using a fluorescent microscope.
RXRα regulates Aβ secretion
To study whether RXRα regulates Aβ generation, we either over-expressed or down-regulated (by RNAi) the level of RXRα in N2a695 cells. As shown in Fig. 2(a), down-regulation of RXRα significantly increased the level of secreted (extracellular) Aβ, whereas over-expression of RXRα slightly reduced Aβ secretion (Fig. 2a). However, the level of cell surface APP was not affected by RXRα modulation (Fig. 2a). In addition, RXRα had no effect on the levels of total APP, BACE1, and PS1, an important component of the γ-secretase complex (Fig. 2a). We also found that RXRα did not affect the in vitro activity of BACE1 (Fig. 2b). As Notch is another major substrate of γ-secretase, we investigated Notch cleavage and found that the cleavage of Notch by γ-secretase for Notch intracellular domain generation was not affected by RXRα (Fig. 2c). Moreover, we found that RXRα had no effect on degradation of extracellular Aβ (Fig. 2d).
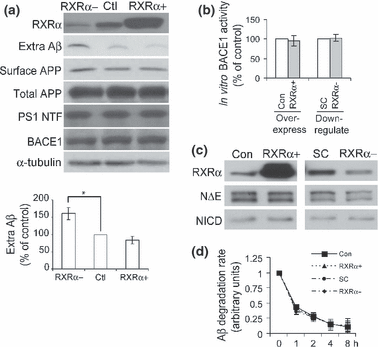
RXRα regulates Aβ secretion. (a) N2a695 cells were first transfected with scramble siRNA (in Ctl and RXRα+) or RXRα siRNA (in RXRα-) for 48 h, then transfected with the control vector (in RXRα- and Ctl) or RXRα vector (in RXRα+) for 24 h. The media were changed to Opti-MEM for another 4 h incubation, and conditioned media were collected and used for detection of secreted Aβ by immunoprecipitation and immunoblot with 6E10 antibody. Aβ levels were quantified by densitometry for comparison. Cells were lysed and analyzed for total levels of RXRα, APP, BACE1, PS1 N-terminal fragment (NTF) and α-tubulin with respective antibodies. In some experiments, cells were first subjected to biotinylation, affinity precipitated using streptavidin-conjugated beads, and then analyzed with an APP antibody to recognize cell surface APP. Data represent mean ± SD from three experiments. *p < 0.05, (Student’s t-test). (b) Cells were transfected with control vector (Con) or RXRα for over-expression, or with scramble siRNA (SC) or RXRα siRNA for down-regulation. Cell lysates were then assayed for the in vitro activity of BACE1. (c) Cells were first transfected with Notch NΔE vector. Equally split cells were then subjected to over-expressing or down-regulating RXRα as in (b). Cell lysates were analyzed for the levels of RXRα, Notch NΔE, and NICD. (d) After over-expressing or down-regulating RXRα, N2a naïve cells were treated with Aβ-containing media collected from N2a695 cells for the indicated times. Conditioned media were collected and assayed for residual Aβ40 levels by ELISA.
RXRα is required for (R)-flurbiprofen-mediated Aβ generation
We next studied whether RXRα was involved in (R)-flurbiprofen-mediated Aβ generation. We found that (R)-flurbiprofen treatment dramatically reduced the levels of extracellular Aβ in both control cells (Fig. 3a, lanes 3 vs. 4) and RXRα-over-expressing cells (Fig. 3a, lanes 5 vs. 6). However, (R)-flurbiprofen treatment did not reduce Aβ levels in RXRα-knock-down cells (Fig. 3a, lanes 1 vs. 2). ELISA results showed that the levels of extracellular Aβ40 and 42 were significantly reduced, and the levels of intracellular Aβ40 and 42 were significantly increased by (R)-flurbiprofen treatment in the presence of RXRα (control and over-expressing), but not when RXRα was knocked down (Fig. 3b and c). To exclude the possibility that (R)-flurbiprofen still reduces Aβ secretion in RXRα knock-down cells but the effect is overridden by dramatically increased Aβ secretion upon RXRα knock-down, we also treated cells with TNFα/IFNγ, which has been found to increase Aβ secretion (Blasko et al. 1999). We found that although TNFα/IFNγ treatment significantly increased the levels of Aβ40 and 42 secretion, beyond that of RXRα knock-down cells, additional (R)-flurbiprofen treatment was still able to reduce secreted Aβ40 and 42 levels in TNFα/IFNγ-treated cells (Fig. 3b).
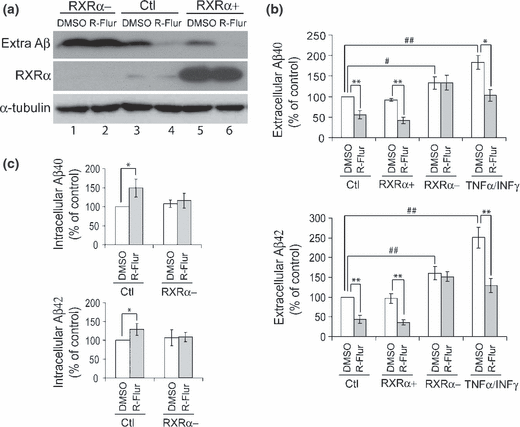
The effect of (R)-flurbiprofen on Aβ secretion is dependent on the presence of RXRα. N2a695 cells were sequentially transfected as described in Fig. 2 for over-expressing and knocking-down RXRα. Cells were then treated with 150 μM (R)-flurbiprofen (R-Flur) for 24 h. (a) Total Aβ in conditioned media was detected by immunoprecipitation and western blot with mouse monoclonal anti-Abeta1-12 antibody. Cell lysates were analyzed for the levels of RXRα and α-tubulin, using respective antibodies. (b) Aβ40 and Aβ42 in conditioned media were assayed by ELISA. In some experiments, cells were co-treated with (R)-flurbiprofen and 30 ng/mL TNFα and 1 ng/mL IFNγ for 24 h. (c) Aβ40 and Aβ42 in cell lysates were assayed by ELISA. Data represent mean ± SD from four experiments. *p < 0.05, **p < 0.01, (R)-flurbiprofen vs. DMSO treatments. #p < 0.05, ##p < 0.01, RXRα modulation (or TNFα/IFNγ treatments) vs. control (Ctl) in DMSO treated cells (Student’s t-test).
(R)-flurbiprofen interferes with the binding of 9-cis-RA to RXRα
To investigate the correlation between (R)-flurbiprofen and RXRα, we carried out an RXRα ligand competition assay with [3H]9-cis-RA, which is known to directly bind the RXRα homodimer LBD (Zhang et al. 1992; Kolluri et al. 2005). Our results showed that the binding between [3H]9-cis-RA and RXRα was competitively inhibited by both unlabeled (R)-flurbiprofen and 9-cis-RA. (R)-flurbiprofen inhibited the binding of [3H]9-cis-RA to RXRα LBD with an IC50 value of ≈75 μM (Fig. 4).
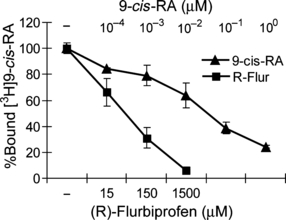
(R)-flurbiprofen interferes with the binding of 9-cis-RA to RXRα. Human RXRα LBD was incubated with 7.5 nM [3H]9-cis-RA in the presence of different concentrations of unlabeled 9-cis-RA or (R)-flurbiprofen at 4°C for 14 h. [3H]9-cis-RA bound to RXRα was measured after capturing the RXRα LBD by nickel-coated beads. Data represent the average of total bound cpm ± SEM.
9-cis-RA interferes with (R)-flurbiprofen’s reduction of Aβ secretion
Having found that (R)-flurbiprofen can competitively inhibit the binding of 9-cis-RA to RXRα, we asked whether 9-cis-RA affects (R)-flurbiprofen’s reduction of Aβ. A previous study showed that treatment with 9-cis-RA at 10 μM concentration could significantly reduce β- and γ-secretase activities (Xiong et al. 2008). However, we found that treatment with 9-cis-RA at a lower concentration (100 nM) slightly increased Aβ40 and 42 secretion (Fig. 5). Therefore, we used a 100 nM concentration in our experiments. In control cells, (R)-flurbiprofen treatment significantly reduced extracellular Aβ40 and 42 levels by approximately 40% and 50%, respectively. However, in the presence of 9-cis-RA, (R)-flurbiprofen treatment only reduced extracellular Aβ40 and 42 levels by approximately 20% and 23%, respectively, suggesting that 9-cis-RA interferes with (R)-flurbiprofen’s reduction of Aβ secretion.
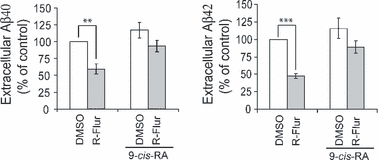
9-cis-RA inhibits (R)-flurbiprofen-mediated reduction of Aβ secretion. N2a695 cells were treated with DMSO (as control) or 150 μM(R)-flurbiprofen (R-Flur), in the absence/presence of 100 nM 9-cis-RA for 24 h. Aβ40 and Aβ42 in conditioned media were assayed by ELISA. Data represent mean ± SD from four experiments. **p < 0.01, ***p < 0.001 (Student’s t-test).
Discussion
Several reports have suggested that NSAIDs, including (R)-flurbiprofen, can reduce Aβ generation (Weggen et al. 2001; Eriksen et al. 2003). However, these studies either investigated only extracellular (secreted) Aβ, or did not differentiate between changes in extracellular and intracellular Aβ. As the level of extracellular Aβ is dramatically higher than that of intracellular Aβ, any change in intracellular Aβ will be very likely masked by the change in extracellular Aβ. Therefore, we compared the changes in extracellular and intracellular Aβ resulting from (R)-flurbiprofen treatment. In contrast with the significant reductions in extracellular Aβ levels found in previous studies, we found that (R)-flurbiprofen treatment resulted in a significant increase of intracellular Aβ levels (Fig. 1). (R)-flurbiprofen has been tested for its potential in the treatment of AD but a recent phase III clinical trial by Myriad Genetics failed to show any beneficial effects in AD patients. Although there may be multiple reasons for the failure of the (R)-flurbiprofen clinical trial, our results provide a possible explanation: i.e., an increase in intracellular Aβ, which is believed to be more closely correlated to AD progression (Gouras et al. 2000), may counteract any beneficial effect from a reduction of total Aβ levels.
Retinoid X receptor α is a ligand-activated nuclear receptor that acts as a transcription factor in multiple important cellular processes, including memory (Chen et al. 1998; Zetterstrom et al. 1999; Luria and Furlow 2004). Some RXRα ligands have been found to rescue memory deficits in rats treated with scopolamine (Shudo et al. 2004). docosahexaenoic acid, which is essential for brain maturation and spatial learning, may also influence neural function through activation of an RXR signaling pathway (de Urquiza et al. 2000). Moreover, RXRα gene variants have been recently linked to AD risk: a three marker haplotype (TGC) consisting of rs3118570, rs1536475 and rs3132293 in the RXRα gene decreases the risk of AD (Kolsch et al. 2009), suggesting that altered RXRα function may be involved in AD pathogenesis.
We found that modulation of RXRα levels directly affects Aβ generation and down-regulation of RXRα dramatically increased the level of extracellular Aβ (Fig. 2a). Interestingly, over-expression of RXRα only slightly reduced Aβ secretion, implying that the effect of RXRα on Aβ secretion is saturable.
Our results showed that the protein level and in vitro activity of BACE1, the protein level of PS1 and Notch cleavage by the PS1/γ-secretease, and the degradation of extracellular Aβ were not affected by RXRα. Therefore, how exactly RXRα regulates Aβ generation and contributes to AD pathology remains speculative. One theory is that RXRα can affect cholesterol metabolism and thus substrate accessibility of APP by secretases, because of the fact that cholesterol metabolism has a well established link to AD (Xiong et al. 2008; Kolsch et al. 2009) and cholesterol-composed lipid rafts may be a major site for Aβ generation (Ma 2007). RXRs have been found to be key regulators of cholesterol metabolism through ATP-binding cassette transporter A1 and apolipoproteins (ApoE) that control the release of cellular cholesterol into plasma high-density lipoprotein (Ghose et al. 2004; Wang et al. 2005). Altered cholesterol levels may also affect APP trafficking and thus Aβ production. Although our results showed that cell surface level of APP is not affected by RXRα (Fig. 2a), we can not exclude the possibility that intracellular trafficking of APP to major sites for Aβ generation, such as the Golgi/TGN and endosomes (Zhang and Xu 2007), is affected by RXRα. In addition, expression of ATP-binding cassette transporter A1 is directly stimulated by LXR/retinoid X receptor (RXR) activation (Nishimaki-Mogami et al. 2008). The LXR/RXR heterodimer is also considered a transcription factor for ApoE, which is closely linked to AD (Ripolles Piquer et al. 2006). One recent study also showed that ApoE can activate LXR to stimulate Aβ degradation (Jiang et al. 2008). Another possibility is RXRα’s anti-inflammatory features. Retinoids have been demonstrated to suppress pro-inflammatory cytokine production by immune cells in vitro and these effects are induced by ligand-mediated activation of the retinoid receptors, including retinoic acid receptor and RXR (Mou et al. 2004).
In addition, we found that although both down-regulation of RXRα and TNFα/IFNγ treatment significantly increased the levels of secreted Aβ, co-treatments with (R)-flurbiprofen only rescued the levels of extracellular Aβ in TNFα/IFNγ-treated cells but not in RXRα knock-down cells (Fig. 3b). These results suggest that RXRα is involved in (R)-flurbiprofen-mediated Aβ secretion. As we found that (R)-flurbiprofen can interfere with the binding of 9-cis-RA to RXRα (Fig. 4) and previously showed that another NSAID, R-etodolac, can bind to RXRα (Kolluri et al. 2005), one possibility is that (R)-flurbiprofen also directly binds to RXRα and affects the function and stability of RXRα in favor of Aβ reduction. This notion may be supported by the finding that a low concentration of 9-cis-RA (100 nM) can attenuate (R)-flurbiprofen’s reduction of Aβ secretion (Fig. 5), implying competition between 9-cis-RA and (R)-flurbiprofen for RXRα binding. However, although the low concentration of 9-cis-RA (100 nM) in our experiments had no effect on Aβ secretion, it has previously been found that 9-cis-RA can significantly reduce β- and γ-secretase activities when used at a high concentration (10 μM) (Xiong et al. 2008). Therefore, one possibility is that both binding of RXRα to (R)-flurbiprofen and to 9-cis-RA can reduce Aβ generation but that the effect of (R)-flurbiprofen is more profound than that of 9-cis-RA, so that the net effect of competition of 9-cis-RA for (R)-flurbiprofen-RXRα binding is an attenuation of the reduction in Aβ secretion. Together, our results demonstrate a novel finding that (R)-flurbiprofen treatment reduces the level of extracellular Aβ but increases that of intracellular Aβ. They also suggest that RXRα regulates Aβ generation and that the reduction of Aβ secretion by (R)-flurbiprofen requires the involvement of RXRα.
Acknowledgement
We thank Dr. F. Checler for providing the rabbit anti-Aβ40 antibody (FCA3340). This work was supported in part by National Institutes of Health grants (R01 NS046673, R01 AG030197 and R01 AG021173 to H.X., and R01 CA109345 to X.Z.), and grants from the Alzheimer’s Association (to H.X.), the American Health Assistance Foundation (to H.X.), the National Natural Science Foundation of China (30672198 and 30840036 to Y.-w.Z.), the National S&T Major Project (2009ZX09103-731 to Y.-w.Z.), and the Natural Science Funds for Distinguished Young Scholar of Fujian Province (2009J06022 to Y.-w.Z.). Y.-w.Z. is supported by the Program for New Century Excellent Talents in Universities (NCET) and the Program for New Century Excellent Talents in Fujian Provincial Universities (NCETFJ).




