Regulation of synaptic vesicle accumulation and axon terminal remodeling during synapse formation by distinct Ca2+ signaling
Abstract
The synaptic vesicle accumulation and subsequent morphological remodeling of axon terminals are characteristic features of presynaptic differentiation of zebrafish olfactory sensory neurons. The synaptic vesicle accumulation and axon terminal remodeling are regulated by protein kinase A and calcineurin signaling, respectively. To investigate upstream signals of presynaptic differentiation, we focused on Ca2+ signaling as Ca2+/calmodulin is required for the activation of both calcineurin and some adenylyl cyclases. We here showed that application of Ca2+/calmodulin inhibitor or olfactory sensory neuron-specific expression of calmodulin inhibitory peptide suppressed both synaptic vesicle accumulation and axon terminal remodeling. Thus, the trigger of presynaptic differentiation could be Ca2+ release from intracellular stores or Ca2+ influx. Application of a phospholipase C inhibitor or olfactory sensory neuron-specific expression of inositol 1,4,5-trisphosphate (IP3) 5-phosphatase suppressed synaptic vesicle accumulation, but not morphological remodeling. In contrast, application of a voltage-gated Ca2+ channel blocker or expression of Kir2.1 inward rectifying potassium channel prevented the morphological remodeling. We also provided evidence that IP3 signaling acted upstream of protein kinase A signaling. Our results suggest that IP3-mediated Ca2+/calmodulin signaling stimulates synaptic vesicle accumulation and subsequent neuronal activity-dependent Ca2+/calmodulin signaling induces the morphological remodeling of axon terminals.
Abbreviations used:
-
- dnPKA
-
- dominant-negative form of mouse type I regulatory subunit of PKA
-
- EGFP
-
- enhanced green fluorescent protein
-
- EM
-
- embryo medium
-
- ER
-
- endoplasmic reticulum
-
- EYFP
-
- enhanced yellow fluorescent protein
-
- hpf
-
- hours post-fertilization
-
- IP3
-
- inositol 1,4,5-trisphosphate
-
- IP3 5-Ppase
-
- IP3 5-phosphatase
-
- NFAT
-
- nuclear factor of activated T cells
-
- omp
-
- olfactory marker protein gene
-
- PKA*
-
- constitutively active form of the mouse PKA α catalytic subunit
-
- PKA
-
- protein kinase A
-
- PLC
-
- phospholipase C
-
- UAS
-
- Gal4 upstream activating sequence
-
- VAMP2
-
- vesicle-associated membrane protein 2
-
- WP
-
- calmodulin inhibitory peptide
The formation and refinement of synaptic connections are key steps of neural development to establish elaborate brain networks. The presynaptic axon terminal is hallmarked by a large number of synaptic vesicles orderly clustered around the active zone where synaptic vesicles undergo exocytosis to release neurotransmitters (Dresbach et al. 2001). Characteristic features of pre-synaptic differentiation during synaptogenesis include active zone formation, synaptic vesicle accumulation in the axon terminal, and change in cytoskeletal and membranous organizations (e.g., see Kullberg et al. 1977; Hamori and Somogyi 1983; Yoshihara et al. 1997; Dent et al. 1999). The assembly of these synaptic specializations orderly proceeds after axodendritic contacts in cultured hippocampal neurons (Friedman et al. 2000). Furthermore, subsequent refinement of neural connections often includes activity-dependent addition or elimination of synaptic arbors (Goda and Davis 2003). To systematically clarify the signaling for pre-synaptic differentiation, we have employed the olfactory sensory neuron-specific gene manipulation system in combination with in vivo imaging of transparent zebrafish embryos (Yoshida and Mishina 2003). The olfactory sensory proto-map is formed as a stereotyped pattern of glomerular arrangement in the olfactory bulb within 3 days post-fertilization (Dynes and Ngai 1998). During pre-synaptic differentiation of zebrafish olfactory sensory neurons, synaptic vesicles visualized with vesicle-associated membrane protein 2 (VAMP2)-enhanced green fluorescent protein (EGFP) fusion protein markedly accumulate in axon terminals between 36 and 60 h post-fertilization (hpf), while the morphological remodeling of axon terminals from complex shapes with filopodia to simple shapes without filopodia proceed between 60 and 84 hpf (Yoshida and Mishina 2005). Correspondingly, odor responses in the olfactory bulb become detectable at 60–72 hpf (Li et al. 2005). We showed that protein kinase A (PKA)-cAMP response element-binding protein signaling regulates synaptic vesicle accumulation in the axon terminals of zebrafish olfactory sensory neurons and calcineurin-nuclear factor of activated T cells (NFAT) signaling controls morphological remodeling of the axon terminals (Yoshida and Mishina 2005). Then, the question arises what is the upstream mechanisms that direct PKA-cAMP response element-binding protein and calcineurin-NFAT signaling.
In this study, we focused on Ca2+ signaling as Ca2+/calmodulin is required for the activation of calcineurin (Klee et al. 1979) and can also activate some types of adenylyl cyclases (Mons et al. 1999). We demonstrated that inhibition of calmodulin suppressed both VAMP2-EGFP punctum formation and morphological remodeling of axon terminals. However, application of a phospholipase C (PLC) inhibitor or olfactory sensory neuron-specific expression of inositol 1,4,5-trisphosphate (IP3) 5-phosphatase (IP3 5-Ppase) selectively suppressed VAMP2-EGFP punctum formation. On the other hand, application of a voltage-gated Ca2+ channel blocker or expression of Kir2.1 inward rectifying potassium channel prevented morphological remodeling. These results suggest that IP3-mediated Ca2+/calmodulin signaling is essential for synaptic vesicle accumulation in zebrafish olfactory sensory neurons in vivo, whereas neuronal activity-dependent Ca2+/calmodulin signaling triggers axon terminal remodeling.
Materials and methods
Animals
Zebrafish AB strain was used. Zebrafish embryos were raised at 28.5°C in embryo medium (EM) containing 17 mM NaCl, 0.27 mM CaCl2, 0.66 mM MgSO4 and 0.4 mM KCl.
Construction of zebrafish olfactory sensory neuron-specific expression vectors
The 1.4-kb olfactory marker protein gene (omp) promoter-driven zebrafish GAP43-EGFP (Pomp-GG), rat VAMP2-EGFP (Pomp-VG) and bovine tau-EGFP (Pomp-TG) expression vectors were previously described (Yoshida and Mishina 2005). Two oligonucleotides, 5′-CATGAGAAGAAAGTGGCAGAAAACAGGGCATGCGGTACGAGCTATTGGTCGACTGTCTTCTTAGT-3′ and 5′-CTAGACTAAGAAGACAGTCGACCAATAGCTCGTACCGCATGCCCTGTTTTCTGCCACTTTCTTCT-3′, were annealed to generate the calmodulin inhibitory peptide coding sequence. The 1.3-kb coding sequence of the zebrafish Kir2.1 gene was cloned by RT-PCR with primers, 5′-CCATGGGAAGTGTGCGGGCCAAC-3′ and 5′-TCTAGATTATATTTCAGATTCCCGCCGTAG-3′, using mRNAs prepared from adult zebrafish brains as a template. The 1.2-kb coding sequence of rat IP3 5-Ppase was a gift from Dr. M Iino (Furutani et al. 2006). The caCN coding sequence of Pomp-GG-caCN, Pomp-VG-caCN and Pomp-TG-caCN (Yoshida and Mishina 2005) were replaced with the coding sequences of calmodulin inhibitory peptide, zebrafish Kir2.1 and rat IP3 5-Ppase to yield Pomp-GG-WP, Pomp-GG-Kir2.1 and Pomp-GG-5-Ppase, Pomp-VG-WP, Pomp-VG-Kir2.1 and Pomp-VG-5-Ppase, and Pomp-TG-WP, Pomp-TG-Kir2.1 and Pomp-TG-5-Ppase, respectively.
The caCN coding sequence of Pomp-caCN (Yoshida and Mishina 2005) was replaced with the 0.7-kb coding sequence of the Gal4-VP16 fusion gene isolated from tub-GVP-Uunc (Köster and Fraser 2001) to yield Pomp-GVP. The 0.5-kb DNA fragment of 13-mer of Gal4 upstream activating sequence (UAS) sites was amplified with primers, 5′-GCGGCCGCTGTCCTCCGGGCTG-3′ and 5′-ACATGTCACAGATCCTGC-3′, using tub-GVP-Uunc as a template and cloned into pCRII-TOPO vector (Invitrogen, Carlsbad, CA, USA) to yield pCRII-U13. The 0.5-kb NotI-BspLU11I fragment carrying a 13-mer of UAS from pCRII-U13 and the 1.4-kb BspLU11I-SalI fragment carrying the VAMP2-EGFP followed by SV40 polyadenylation signal sequence from Pomp-VG were ligated into the NotI-SalI sites of pBluescript II SK+ (Stratagene, La Jolla, CA, USA) to yield U13-VG. The coding sequence of VAMP2-EGFP in U13-VG was replaced by the 1.1-kb coding sequence of a dominant-negative form of mouse type I regulatory subunit of PKA (Clegg et al. 1987) (dnPKA), the 1.1-kb coding sequence of constitutively active form of the mouse PKA α catalytic subunit gene (Orellana and McKnight 1992) (PKA*), and the 1.2-kb coding sequence of IP3 5-Ppase to yield U-dnPKA, U-PKA* and U-5-Ppase, respectively. The 0.7-kb AgeI-ScaI fragment carrying 5-mer of UAS sites followed by VAMP2 from U13-VG was ligated with the 4.0-kb AgeI-SmaI fragment from Pomp-VG to yield U-VG. A linker sequence, 5′-CTCGAGGCCTAGCGCTGGCGCCCGGGCCC-3′, was inserted between the XhoI and ApaI sites of U-VG to yield U-VG-linker. The 2.1-kb MluI-BssHII fragment from U-5-Ppase was blunted and inserted into the AatI site of U-VG-linker to yield U-VG-U-5-Ppase. The 6.5-kb NotI fragment from Pomp-GVP was inserted into the NotI site of U-PKA* and U-dnPKA to yield Pomp-GVP-U-PKA* and Pomp-GVP-U-dnPKA, respectively. The PKA* coding sequence of Pomp-GVP-PKA* was replaced with the coding sequence of DsRed-monomer from pDsRed-monomer-C1 (Clontech, Palo Alto, CA, USA) and that of enhanced yellow fluorescent protein (EYFP) from pEYFP-N1 (Clontech) to yield Pomp-GVP-U-RFP and Pomp-GVP-U-YFP, respectively. The VAMP2-EGFP coding sequence of U-VG-U-5-Ppase was replaced with the coding sequence of enhanced cyan fluorescent protein from pECFP-N1 (Clontech) to yield U-CFP-U-5-Ppase. The IP3-5-Ppase coding sequence of U-CFP-U-5-Ppase was replaced with the coding sequences of EYFP and DsRed-monomer to yield U-CFP-U-YFP and U-CFP-U-RFP, respectively.
Microinjection of DNA into zebrafish embryos
Expression vectors were linearized by SpeI or SacII and dissolved in 100 mM KCl containing 0.05% phenol red. About 0.2–0.5 nL of the DNA solution at the concentrations of 50–100 ng/μL was injected into the cytoplasm of 1- to 4-cell embryos.
Microscopy
Zebrafish embryos expressing reporter genes in the olfactory placode at 28–29 hpf were screened by a fluorescent microscope. At 36, 60 and 84 hpf, the embryos were mounted in 1% low-melting point agarose in EM containing 0.02% 3-aminobenzoic acid ethyl ester (tricaine, Sigma, St. Louis, MO, USA) with their head toward the glass bottom of a dish. Embryos were imaged by Radiance 2100 Confocal Scanning System (Bio-Rad, Hercules, CA, USA) using Nikon 60× water lens [numerical aperture, 1.00]: zoom setting, 5.0; Z-step, 1–2 μm; image size, 512 × 512 pixels.
Drug treatments
Zebrafish embryos stably carrying omp promoter-driven tau-EGFP transgene or those injected with Pomp-GG, Pomp-TG or Pomp-VG were dechorionated and soaked in EM containing 1% ethanol or 0.1% dimethyl sulfoxide with either 1 μM calmidazolium chloride (Calbiochem, La Jolla, CA, USA), 1 μM U-73122 (Calbiochem), 1 μM U-73433 (Calbiochem) or 10 μM nifedipine (Sigma) from 30 hpf to 84 hpf. Treated embryos were mounted in 1% low-melting point agarose in EM at 36, 60 and 84 hpf for microscopy.
Image processing
All quantitative measurements were made on the computer screen using the NIH Image 1.62 in a blind manner with respect to drug treatment, expression vectors injected and developmental stages. For measurements of axon terminal morphology and VAMP2-EGFP puncta, z series optical sections were projected by the brightest point method and smoothened by the rank filter method. The outline of an axon terminal was traced from the first branch point or from the point where axonal width was three or more times wider than that of the axon shaft. The trace and the enclosed region were defined as the perimeter and area of the axon terminals, respectively. The complexity was given by dividing the square of the perimeter by the area. For the quantitative measurements of VAMP2-EGFP punctum, the z-projected images with VAMP2-EGFP signals in the linear range of fluorescent intensity below saturation (ranging from 68 to 255 in the NIH Image software) were used. The VAMP2-EGFP punctum was defined as an area larger than five pixels where the intensity of VAMP2-EGFP signals was five or more times stronger than that of non-varicose and non-punctate axonal shaft on the same axon. Axons longer than 10 μm were used for measurements. Data represent mean ± SEM. Statistical significance was evaluated by one-way or two-way anova. When the interaction was significant, unpaired t-test or Fisher’s post hoc test was employed.
Results
Ca2+/calmodulin inhibitor suppressed both synaptic vesicle accumulation and axon terminal remodeling
We hypothesized that Ca2+/calmodulin signaling regulates the axon terminal differentiation of zebrafish olfactory sensory neurons. To test the hypothesis, we first examined the effect of calmidazolium, an inhibitor of calmodulin, on synaptic vesicle accumulation and axon terminal remodeling. Zebrafish embryos at 30 hpf were soaked in the medium containing calmidazolium and were incubated in the presence of the drug thereafter. Treatment of zebrafish embryos with 1 μM calmidazolium caused no apparent changes in gross morphology as well as axon extension estimated by terminal reach values that are distances between center of the nasal pit and axon terminals of individual olfactory sensory neurons (Yoshida et al. 2002) (Fig. S1). We thus examined the effect of calmidazolium at the concentration of 1 μM in subsequent experiments.
Zebrafish embryos were injected with omp promoter-driven VAMP2-EGFP expression vector (Pomp-VG, Fig. 1a) to monitor the synaptic vesicle accumulation. VAMP2-EGFP punctate areas in the axon terminal of olfactory neurons in mock-treated embryos markedly increased between 36 and 60 hpf, then slightly increased during next 24 h (Fig. 1b) as described (Yoshida and Mishina 2005). VAMP2-EGFP punctate areas in the axon terminals of calmidazolium-treated embryos were smaller than those of mock-treated embryos at 60 and 84 hpf (t-test, p = 0.0021 and 0.0089, respectively) (Fig. 1c and d). These results suggest that the calmodulin inhibitor suppresses the accumulation of synaptic vesicles in axon terminals.

Effects of calmodulin inhibitors on the VAMP2-EGFP punctum formation in axon terminals of olfactory sensory neurons. (a) Omp promoter-driven expression vectors for VAMP2-EGFP (Pomp-VG, top) and for both VAMP2-EGFP and calmodulin inhibitory peptide (Pomp-VG-WP, bottom) in olfactory sensory neurons. Black boxes, the omp promoter; a crosshatched box, the 3′ downstream sequence of the omp gene; hatched boxes, SV40 polyadenylation signal sequence; lines, pBluescript II SK+. (b, c) Representative VAMP2-EGFP signals in olfactory neuron axon terminals in mock (b)- and calmidazolium (c)-treated embryos injected with Pomp-VG vector at 36 (left), 60 (middle) and 84 hpf (right) are shown in the upper panels. The threshold images of VAMP2-EGFP signals are on the bottom. Arrowheads point axonal shafts. Bar = 5 μm. (d) Areas of VAMP2-EGFP puncta in axon terminals of olfactory sensory neurons in mock (open bars)- and calmidazolium (gray bars)-treated embryos at 36, 60 and 84 hpf. n = 38–55. Two-way anova: drug treatment effect, F(1,240) = 13.4, p < 0.001; age × drug treatment interaction, F(2,240) = 3.6, p = 0.029. (e) Areas of VAMP2-EGFP puncta in axon terminals of olfactory sensory neurons in Pomp-VG (open bars)- and Pomp-VG-WP (gray bars)-injected embryos at 36, 60 and 84 hpf. n = 38–44. Two-way anova: expression vector effect, F(1,244) = 9.6, p = 0.0022; age × expression vector interaction, F(2,244) = 5.5, p = 0.0048. *p < 0.05; **p < 0.01.
Zebrafish embryos were next injected with omp promoter-driven GAP43-EGFP expression vector (Pomp-GG, Fig. 2a) and the labeled axon terminals of olfactory sensory neurons were imaged by a confocal microscope at 60 and 84 hpf to monitor axon terminal remodeling. Area, perimeter and complexity values significantly decreased from 60 to 84 hpf in mock-treated embryos (t-test, p < 0.001, = 0.0028 and = 0.010, respectively) (Fig. 2b and d) as described (Yoshida and Mishina 2005). On the other hand, perimeter and complexity values were unchanged during development in calmidazolium-treated embryos (t-test, p = 0.23 and 0.59, respectively), while area values were slightly decreased in calmidazolium-treated embryos (t-test, p = 0.040) (Fig. 2c and d). At 84 hpf, area, perimeter and complexity values were all significantly larger in calmidazolium-treated embryos than in mock-treated embryos (t-test, p < 0.001 for all parameters). These results suggest that suppression of Ca2+/calmodulin by calmidazolium prevented the remodeling of axon terminal from large and complex shapes to small and simple shapes between 60 and 84 hpf.
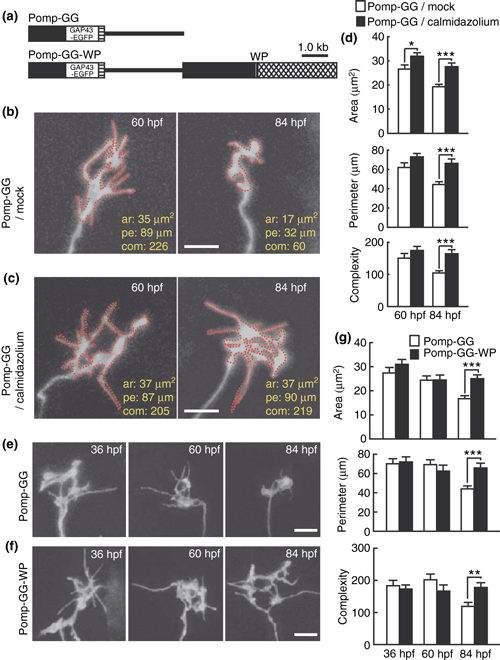
Effects of calmodulin inhibitors on the axon terminal morphology of olfactory sensory neurons. (a) Omp promoter-driven expression vectors for GAP43-EGFP (Pomp-GG, top) and for both GAP43-EGFP and calmodulin inhibitory peptide (Pomp-GG-WP, bottom) in olfactory sensory neurons. Black boxes, the omp promoter; a crosshatched box, the 3′ downstream sequence of the omp gene; hatched boxes, SV40 polyadenylation signal sequence; lines, pBluescript II SK+. (b, c) Representative images of the axon terminal of olfactory sensory neurons in mock (b)- and calmidazolium (c)-treated embryos injected with Pomp-GG vector at 60 (left) and 84 hpf (right). Bar = 5 μm. The red dotted lines represent the outline of axon terminal for the measurement of axon terminal morphology. The values of area (ar), perimeter (pe) and complexity (com) are indicated in the bottom right of each image. (d) Area (top), perimeter (middle) and complexity (bottom) values of axon terminals of olfactory sensory neurons in mock (open bars)- and calmidazolium (filled bars)-treated embryos injected with Pomp-GG vector at 60 and 84 hpf. n = 32–46. (e, f) Representative GAP43-EGFP signals in axon terminals of olfactory sensory neurons in zebrafish embryos injected with Pomp-GG (e) or Pomp-GG-WP (f) at 36 (left), 60 (middle) and 84 hpf (right). Bar = 5 μm. (g) Area (top), perimeter (middle) and complexity (bottom) values of axon terminals of olfactory sensory neurons in zebrafish embryos injected with Pomp-GG (open bars) and Pomp-GG-WP (filled bars) expression vectors at 36, 60 and 84 hpf. n = 33–44. Two-way anova: expression vector effect, F(1,227) = 8.1 and p = 0.0047, F(1,227) = 5.9 and p = 0.0033, and F(1,227) = 3.0 and p = 0.050 for axon terminal area, perimeter and complexity, respectively; age × expression vector interaction, F(2,227) = 2.8 and p = 0.060, F(2,227) = 4.8 and p = 0.0088, and F(2,227) = 5.3 and p = 0.0056 for axon terminal area, perimeter and complexity, respectively. *p < 0.05; **p < 0.01; ***p < 0.001.
Olfactory sensory neuron-specific expression of calmodulin inhibitory peptide suppressed both synaptic vesicle accumulation and axon terminal remodeling
To restrict the effect of calmodulin suppression in olfactory sensory neurons, we employed the double-cassette vector strategy in living zebrafish embryos (Yoshida and Mishina 2003). The amino acid sequence of zebrafish calmodulins is identical to that of human calmodulins (Friedberg and Taliaferro 2005). Thus, we used the calmodulin-binding domain of mammalian smooth muscle myosin light chain kinase as a calmodulin inhibitory peptide (WP) (Török and Trentham 1994). The omp promoter-driven expression vector Pomp-VG-WP (Fig. 1a) was injected into zebrafish embryos to express VAMP2-EGFP and WP in the same olfactory neurons. Olfactory sensory neurons in zebrafish embryos injected with Pomp-VG served as controls. VAMP2-EGFP punctate areas in the axon terminals of embryos injected with Pomp-VG-WP were significantly smaller than those of mock-treated embryos at 60 and 84 hpf (t-test, p = 0.0014 and 0.047) (Figs 1e and S2[link]). These results suggest that the inhibition of calmodulin in olfactory sensory neurons suppressed synaptic vesicle accumulation in the axon terminals.
We then examined the axon terminal remodeling using double-cassette vector Pomp-GG-WP for GAP43-EGFP and WP (Fig. 2a). The axon terminals of olfactory sensory neurons in control embryos injected with Pomp-GG were remodeled to simple shapes from 60 to 84 hpf (t-test; p < 0.001 for axon terminal area, perimeter and complexity) (Fig. 2e and g) as previously reported (Yoshida and Mishina 2005). In contrast, the axon terminals of olfactory sensory neurons in embryos injected with Pomp-GG-WP showed no morphological changes between 60 and 84 hpf (t-test; p = 0.83, 0.63 and 0.63 for axon terminal area, perimeter and complexity, respectively) (Fig. 2f and g). Area, perimeter and complexity values were significantly larger in Pomp-GG-WP-injected embryos than in control Pomp-GG-injected embryos at 84 hpf (t-test, p < 0.001, < 0.001 and = 0.0013, respectively) (Fig. 2g). These results suggest that the inhibition of calmodulin in olfactory sensory neurons suppressed the morphological remodeling of their axon terminals.
Pharmacological inhibition of phospholipase C perturbed synaptic vesicle accumulation but not axon terminal remodeling
The finding that calmodulin is essential for both synaptic vesicle accumulation and axon terminal remodeling raises a question whether a sole Ca2+ signaling that activates calmodulin orchestrates the two representative events of presynaptic differentiation or different Ca2+ sources are responsible for respective events. Major Ca2+ sources would be Ca2+ release from intracellular stores or Ca2+ influx from the extracellular fluid. Ca2+ release from the endoplasmic reticulum (ER) is triggered by IP3, which is produced by large PLC family isozymes including PLC-β, PLC-γ and PLC-δ (Berridge 1984) by hydrolysis of the phosphotidylinositol 4,5-bisphosphate. Thus, we examined the effect of pharmacological suppression of PLCs on VAMP2-EGFP punctum formation. Zebrafish embryos at 30 hpf were soaked in the medium containing 1 μM U-73122, a potent PLC inhibitor with broad specificity toward all PLCs (Smith et al. 1990; Knox et al. 2004) or U-73433, an inactive analogue of U-73122, and were incubated in the presence of the drug thereafter. The drug treatment caused no apparent changes in gross morphology of zebrafish embryos (Fig. 3a). Treatment with U-73122 suppressed VAMP2-EGFP punctum formation in the axon terminals at 60 hpf [post hoc test, p < 0.05 (vs. mock treatment) and p < 0.05 (vs. U-73433 treatment)] (Fig. 3b). These results suggest that suppression of PLCs by U-73122 prevented the synaptic vesicle accumulation at 60 hpf, suggesting that IP3 signaling is important for synaptic vesicle accumulation. On the other hand, the treatment with U-73122 had little effect on the complexity value of Pomp-GG injected embryos at 84 hpf (Fig. 3c) suggesting no apparent effect of PLC inhibition on the axon terminal remodeling.
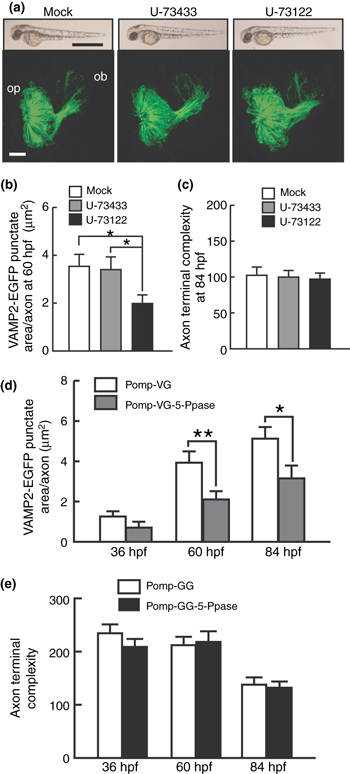
Effect of phospholipase C inhibitor and IP3 5-Ppase expression on the VAMP2-EGFP punctum formation and axon terminal remodeling of olfactory sensory neurons. (a) Lateral views of whole zebrafish bodies (upper panels) and frontal views of the olfactory placode and bulb (lower panels) of mock (left)-, U-73433 (middle)- or U-73122 (right)-treated embryos carrying the omp promoter-driven tau-EGFP transgene at 60 hpf. For the lower panels, dorsal is to the top and medial is to the right. Bar = 1 mm for upper panels and 20 μm for lower panels. op, olfactory placode; ob, olfactory bulb. (b) VAMP2-EGFP punctate areas of axon terminals of olfactory sensory neurons in mock (open bar)-, U-73433 (gray bar)- and U-73122 (black bar)-treated zebrafish embryos at 60 hpf. n = 40–50. One-way anova: F(2,131) = 3.6, p = 0.030. (c) Complexity values of axon terminals of olfactory sensory neurons in mock (open bar)-, U-73433 (gray bar)- and U-73122 (black bar)-treated zebrafish embryos at 84 hpf. n = 35–37. One-way anova: F(2,107) = 0.079, p = 0.92. (d) Areas of VAMP2-EGFP puncta in axon terminals of olfactory sensory neurons in Pomp-VG (open bars)- and Pomp-VG-5-Ppase (gray bars)-injected embryos at 36, 60 and 84 hpf. n = 36–45. Two-way anova: expression vector effect, F(1,234) = 12.7, p < 0.001; age × expression vector interaction, F(2,234) = 1.4, p = 0.25. (e) Complexity values of axon terminals of olfactory sensory neurons in zebrafish embryos injected with Pomp-GG (open bars) and Pomp-GG-5-Ppase (filled bars) expression vectors at 36, 60 and 84 hpf. n = 32–39. Two-way anova: expression vector effect, F(1,211) = 0.99, p = 0.32; age × expression vector interaction, F(2,211) = 0.59, p = 0.56. *p < 0.05; **p < 0.01.
Olfactory sensory neuron-specific expression of IP3 5-Ppase suppressed synaptic vesicle accumulation
We further examined the effect of IP3 5-Ppase, which selectively hydrolyzes IP3 to inhibit Ca2+ release from ER (Majerus 1992; Laxminarayan et al. 1994). Olfactory sensory neuron-specific expression of IP3 5-Ppase using Pomp-VG-5-Ppase significantly suppressed the formation of VAMP2-EGFP puncta (Figs 3d and S3[link]). VAMP2-EGFP punctate areas in the axon terminals of embryos injected with Pomp-VG-5-Ppase were significantly smaller than those of control embryos injected with Pomp-VG at 60 and 84 hpf (t-test, p = 0.0095 and 0.026) (Fig. 3d). On the other hand, expression of IP3 5-Ppase did not affect the morphological remodeling of olfactory neuron axon terminals (Figs 3e and S3[link]). These results suggest that the inhibition of Ca2+ release from ER in olfactory sensory neurons selectively suppressed VAMP2-EGFP punctum formation in the axon terminals.
Voltage-gated Ca2+ channel blocker suppressed axon terminal remodeling
As the suppression of IP3-mediated Ca2+ release from ER exerted little effect on axon terminal remodeling, we next examined the effects of pharmacological inhibition of Ca2+ influx. When bath-applied after 30 hpf, L-type Ca2+ channel blocker nifedipine at the concentration of 10 μM caused no apparent changes in gross morphology of zebrafish embryos (Fig. 4a). The nifedipine treatment had little effect on VAMP2-EGFP punctate area at 60 hpf during early stage of synapse formation (t-test, p = 0.12) (Fig. 4b). On the other hand, the complexity values at 84 hpf were significantly larger in nifedipine-treated embryos than in mock-treated embryos (t-test, p < 0.001) (Fig. 4c). These results suggest that neuronal activity-induced Ca2+ signaling regulates axon terminal remodeling.
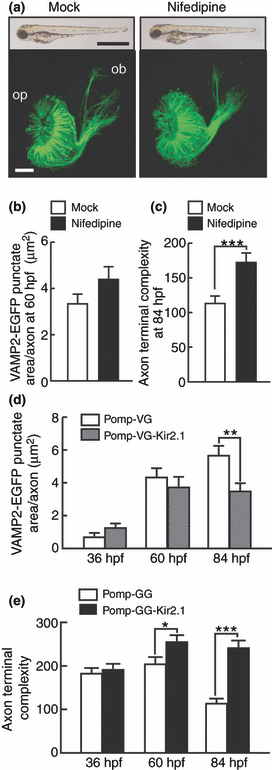
Effect of Ca2+ channel blocker and Kir2.1 expression on the VAMP2-EGFP punctum formation and axon terminal remodeling of olfactory sensory neurons. (a) Lateral views of whole zebrafish bodies (upper panels) and frontal views of the olfactory placode and bulb (lower panels) of mock (left)- or nifedipine (right)-treated embryos carrying the omp promoter-driven tau-EGFP transgene at 84 hpf. For the lower panels, dorsal is to the top and medial is to the right. Bar = 1 mm for upper panels and 20 μm for lower panels. op, olfactory placode; ob, olfactory bulb. (b) VAMP2-EGFP punctate areas of axon terminals of olfactory sensory neurons in mock (open bar)- and nifedipine (black bar)-treated zebrafish embryos at 60 hpf. n = 37. (c) The complexity values of axon terminals of olfactory sensory neurons in mock (open bar)- and nifedipine (black bar)-treated zebrafish embryos at 84 hpf. n = 40–49. (d) Areas of VAMP2-EGFP puncta in axon terminals of olfactory sensory neurons in Pomp-VG (open bars)- and Pomp-VG-Kir2.1 (gray bars)-injected embryos at 36, 60 and 84 hpf. n = 31–48. Two-way anova: expression vector effect, F(1,236) = 4.4, p = 0.037; age × expression vector interaction, F(2,236) = 3.8, p = 0.024. (e) Complexity values of axon terminals of olfactory sensory neurons in zebrafish embryos injected with Pomp-GG (open bars) and Pomp-GG-Kir2.1 (filled bars) expression vectors at 36, 60 and 84 hpf. n = 34–47. Two-way anova: expression vector effect, F(1,239) = 36.3, p < 0.001; age × expression vector interaction, F(2,239) = 9.6, p < 0.001. *p < 0.05; **p < 0.01; ***p < 0.001.
Olfactory sensory neuron-specific expression of Kir2.1 channel prevented axon terminals from morphological remodeling
It is known that expression of inward rectifying potassium channel Kir2.1 results in a significant hyperpolarization and inhibits neuronal firing and calcium spiking (Yu et al. 2004; Hua et al. 2005). We thus examined the effect of blocking of neuronal firing that triggers Ca2+ influx by olfactory sensory neuron-specific expression of Kir2.1. VAMP2-EGFP punctate areas in the axon terminals of embryos injected with Pomp-VG-Kir2.1 were comparable to those of control Pomp-VG-injected embryos at 36 hpf (t-test, p = 0.090) and 60 hpf (t-test, p = 0.46) (Figs 4d and S4[link]). On the other hand, at 84 hpf, VAMP2-EGFP punctate areas were smaller in Pomp-VG-Kir2.1-injected embryos (t-test, p = 0.005). The axon terminals of olfactory sensory neurons in embryos injected with Pomp-GG-Kir2.1 showed no morphological changes between 60 and 84 hpf (t-test, p = 0.52) (Figs 4e and S4[link]). The complexity values were significantly larger in Pomp-GG-Kir2.1-injected embryos than in control Pomp-GG-injected embryos at 60 and 84 hpf (t-test, p = 0.021 and p < 0.001, respectively). These results suggest that the activity blockade of olfactory sensory neurons exerted little effect on the accumulation of synaptic vesicles in the axon terminal at the early stage of synapse formation but strongly prevented the morphological remodeling of axon terminals and suppressed synaptic vesicle accumulation at the late stage of synapse formation.
IP3 signaling is upstream of PKA signaling
Present investigation together with previous results (Yoshida and Mishina 2005) suggests that VAMP2-EGFP punctum formation in the axon terminal of olfactory sensory neurons requires both PKA and IP3-mediated Ca2+ signaling. To examine the interaction of two signaling genetically, we manipulated both IP3 signaling and PKA signaling in olfactory sensory neurons employing Gal4-UAS system (Scheer and Campos-Ortega 1999). We first constructed omp promoter-driven Gal4-VP16 vector (Pomp-GVP, Fig. 6a), Pomp-GVP linked with Gal4-binding UAS-driven DsRed (Pomp-GVP-U-RFP), Pomp-GVP linked with UAS-driven EYFP (Pomp-GVP-U-YFP), and UAS-driven ECFP linked with UAS-driven EYFP (U-CFP-U-YFP) or UAS-driven DsRed (U-CFP-U-RFP) (Fig. 5a). ECFP positive cells in embryos coinjected with Pomp-GVP-U-RFP and U-CFP-U-YFP were both DsRed and EYFP positive (Fig. 5b and c). Moreover, DsRed intensity in the olfactory neurons of embryos injected with Pomp-GVP-U-RFP and U-CFP-U-YFP, with Pomp-GVP-U-YFP and U-CFP-U-DsRed, and with Pomp-CFP-RFP carrying the omp promoter-driven ECFP linked with the omp promoter-driven DsRed were comparable (Fig. 5c). Thus, the Gal4-UAS system enabled the co-expression of ECFP, EYFP and DsRed in the same olfactory sensory neurons. Expression levels of these molecules by Gal4-UAS vectors were comparable to those by omp promoter-driven double-cassette vectors.
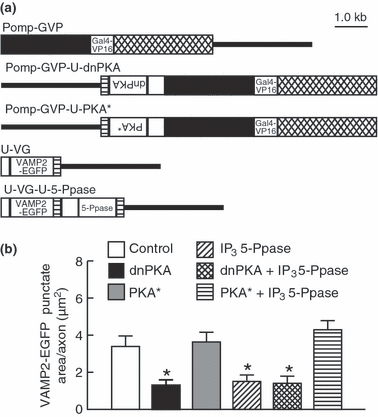
Interaction between IP3 signaling and PKA signaling for the VAMP2-EGFP punctum formation in axon terminals of olfactory sensory neurons. (a) Omp promoter-driven Gal4 activator-effector constructs and UAS-mediated effector-reporter constructs coinjected for the expression of VAMP2-EGFP alone (Pomp-GVP and U-VG), VAMP2-EGFP and dnPKA (Pomp-GVP-U-dnPKA and U-VG), VAMP2-EGFP and PKA* (Pomp-GVP-U-PKA* and U-VG), VAMP2-EGFP and IP3 5-Ppase (Pomp-GVP and U-VG-U-5-Ppase), VAMP2-EGFP, dnPKA and IP3 5-Ppase (Pomp-GVP-U-dnPKA and U-VG-U-5-Ppase), and VAMP2-EGFP, PKA* and IP3 5-Ppase (Pomp-GVP-U-PKA* and U-VG-U-5-Ppase). White boxes, 5 or 13 UAS sites; black boxes, the omp promoter; crosshatched boxes, the 3′ downstream sequence of the omp gene; hatched boxes, SV40 polyadenylation signal sequence; lines, pBluescript II SK+. (b) Areas of VAMP2-EGFP puncta in axon terminals of olfactory sensory neurons expressing VAMP2-EGFP alone (open bar), VAMP2-EGFP and dnPKA (filled bar), VAMP2-EGFP and PKA* (gray bar), VAMP2-EGFP and IP3 5-Ppase (right-bound hatched bar), VAMP2-EGFP, dnPKA and IP3 5-Ppase (crosshatched bar) and VAMP2-EGFP, PKA* and IP3 5-Ppase (hatched bar) at 60 hpf. n = 41–55. One-way anova: F(5,295) = 11.5, p < 0.001. *p < 0.01 or < 0.05 compared to control axon expressing VAMP2-EGFP alone, axon expressing VAMP2-EGFP and PKA*, and that expressing VAMP2-EGFP, IP3 5-Ppase and PKA*. Other comparisons showed no statistical significance.
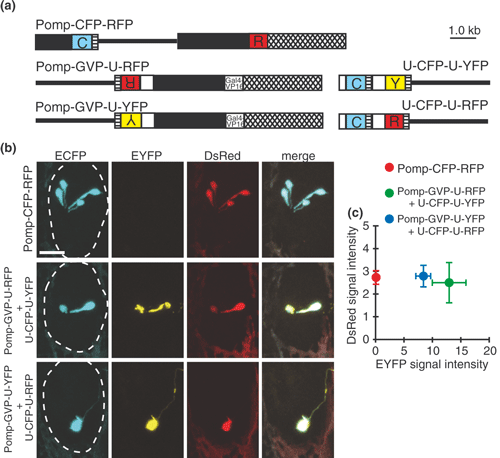
Coexpression of three genes in the olfactory sensory neurons by Gal4-UAS system. (a) Omp promoter-driven double-cassette vector for ECFP and DsRed (Pomp-CFP-RFP), omp promoter-driven Gal4 activator-reporter constructs (Pomp-GVP-U-RFP and Pomp-GVP-U-YFP) and UAS-mediated reporter constructs (U-CFP-U-YFP and U-CFP-U-RFP). White boxes, 5 or 13 UAS sites; black boxes, the omp promoter; crosshatched boxes, the 3′ downstream sequence of the omp gene; hatched boxes, SV40 polyadenylation signal sequence; lines, pBluescript II SK+; C, ECFP; R, DsRed; Y, EYFP. (b) Representative ECFP (left row, cyan), EYFP (second row, yellow) and DsRed (third row, red) signals in the olfactory placode of embryos injected with Pomp-CFP-RFP (top), with Pomp-GVP-U-RFP and U-CFP-U-YFP (middle), and with Pomp-GVP-U-YFP and U-CFP-U-RFP (bottom) at 60 hpf. These fluorescent signals were merged on the right. Olfactory placodes are enclosed with dashed lines. Bar = 20 μm. (c) EYFP and DsRed fluorescent signal intensities of ECFP-positive olfactory sensory neurons injected with Pomp-CFP-RFP (red circle), with Pomp-GVP-U-RFP and U-CFP-U-YFP (green circle), and with Pomp-GVP-U-YFP and U-CFP-U-RFP (blue circle). n = 12–24.
We then constructed Pomp-GVP linked with UAS-driven dominant negative PKA (Pomp-GVP-U-dnPKA) or UAS-driven constitutively active PKA (Pomp-GVP-U-PKA*), UAS-driven VAMP2-EGFP (U-VG), and U-VG linked with UAS-driven IP3 5-Ppase (U-VG-U-5-Ppase) to express two effector molecules in a single olfactory sensory neuron labeled with a reporter (Fig. 6a). Olfactory sensory neurons coinjected with Pomp-GVP and U-VG served as controls. Both expression of dnPKA by co-injecting Pomp-GVP-U-dnPKA with U-VG and expression of IP3 5-Ppase by co-injecting Pomp-GVP with U-VG-U-5-Ppase, suppressed VAMP2-EGFP punctum formation at 60 hpf [post hoc test, p < 0.01 (vs. control) and p < 0.05 (vs. control)] (Fig. 6b). Expression of both IP3 5-Ppase and dnPKA by co-injecting Pomp-GVP-U-dnPKA and U-VG-U-5-Ppase also suppressed VAMP2-EGFP punctum formation (post hoc test, p < 0.01 vs. control). However, VAMP2-EGFP punctate areas in axon terminals were comparable among olfactory neurons expressing dnPKA, IP3 5-Ppase, and both. Moreover, VAMP2-EGFP punctate area of olfactory sensory neurons injected with both Pomp-GVP-U-PKA* and U-VG-U-5-Ppase was comparable to that of controls, suggesting that suppressive effect of IP3 5-Ppase on VAMP2-EGFP punctate area was rescued to the level of controls by additive expression of PKA*. These results suggest that IP3-mediated Ca2+ signaling regulates synaptic vesicle accumulation upstream of PKA.
Discussion
Axons of zebrafish olfactory sensory neurons begin to extend toward the olfactory bulb around 22 hpf, reach the target sites and stop the extension at about 50 hpf (Dynes and Ngai 1998; Yoshida et al. 2002). Then a stereotyped pattern of glomerular arrangement in the olfactory bulb – the anatomical basis for an olfactory sensory map – is manifested between 48 and 84 hpf (Wilson et al. 1990; Dynes and Ngai 1998). The contacts between olfactory neuron axons and dendrites of postsynaptic cells in the olfactory bulb are detectable from ∼50 hpf and synaptic vesicles visualized with VAMP2-EGFP markedly increase in the axon terminal by 60 hpf (Yoshida and Mishina 2005). Consistently, odor responses in the olfactory bulb become detectable at 60–72 hpf (Li et al. 2005). On the other hand, the morphological remodeling of axon terminals from large and complex shapes to small and simple ones proceeds between 60 and 84 hpf (Yoshida and Mishina 2005). Moreover, the stretch of axon terminals decreases to the size comparable to the diameter of glomeruli during the remodeling, suggesting the refinement of synaptic connections of olfactory sensory neurons with postsynaptic neurons in the olfactory bulb (Yoshida and Mishina 2005). Thus, the early stage of synapse formation between olfactory neuron axons and dendrites of post-synaptic cells in the olfactory bulb is characterized by synaptic vesicle accumulation in the axon terminals, and the late stage by axon terminal remodeling. In this study, we showed that Ca2+/calmodulin is required for both of the characteristic developmental changes of the axon terminals during synapse formation. However, PLC inhibitor or olfactory sensory neuron-specific expression of IP3 5-phosphatase suppressed synaptic vesicle accumulation, but not morphological remodeling. On the contrary, voltage-gated Ca2+ channel blocker or olfactory sensory neuron-specific expression of Kir2.1 hardly affected the synaptic vesicle accumulation at 60 hpf but severely inhibited the axon terminal remodeling. Thus, the marked increase in VAMP2-EGFP punctate area in the axon terminal at the early stage of synaptogenesis depends on IP3 signaling rather than neuronal activities. On the other hand, the morphological remodeling of axon terminals at the late stage of synapse formation requires neural activity and is independent of IP3 signaling.
As our previous results show that PKA signaling regulates synaptic vesicle accumulation in the axon terminals of zebrafish olfactory sensory neurons, an intriguing question arises whether IP3 and PKA signaling work in the same signaling pathway or in parallel. Expression of IP3 5-phosphatase, dominant negative PKA or both suppressed VAMP2-EGFP puncta formation to similar degree. Suppression of VAMP2-EGFP puncta formation by IP3 5-phosphatase was rescued by expression of constitutively active PKA. These results suggest that IP3 signaling and PKA signaling act sequentially to control synaptic vesicle accumulation and PKA regulates synaptic vesicle accumulation in the downstream of IP3-mediated Ca2+ release from ER. As type 3 adenylyl cyclase is enriched in rodent olfactory neurons (Bakalyar and Reed 1990; Xia and Storm 1997; Mons et al. 1999), Ca2+/calmodulin-stimulated adenylyl cyclases may transduce IP3-mediated Ca2+ signal to PKA signal.
As we previously demonstrated that calcineurin and NFAT regulates the axon terminal remodeling, our present results suggest that neural activity-induced Ca2+ entry through voltage-gated Ca2+ channels activates calcineurin-NFAT signaling. Neuronal activity-dependent activation of calcineurin-NFAT signaling through voltage-gated Ca2+ channels is reported in cultured neurons including cerebellar granule cells (Benedito et al. 2005) and hippocampal pyramidal cells (Graef et al. 1999). So far, variety of activity-dependent refinement of neural circuitry including at vertebrate neuromuscular junctions, cerebellar climbing fiber synapses and retinotectal synapses are suggested to be driven by post-synaptic cells, though molecular mechanisms are largely unknown (Goda and Davis 2003). In zebrafish olfactory system, the axon terminal remodeling was suppressed by olfactory sensory neuron-specific expression of Kir2.1 or calmodulin inhibitory peptide. Thus, pre-synaptic Ca2+ signaling generated by spontaneous firing appears to induce axon terminal remodeling. In fact, spontaneous firing was observed in frog olfactory sensory neurons (Rospars et al. 1994) and the suppression of neuronal activity in mouse olfactory sensory neurons by expression of Kir2.1 caused diffuse axon projection around glomeruli (Yu et al. 2004). Thus, present investigation implies that neural activity-induced Ca2+ signaling in axon terminals may regulate autonomously their morphological refinement.
In the present study, we have shown that Ca2+ signaling plays a key role in the regulation of presynaptic differentiation of zebrafish olfactory sensory neurons. However, distinct Ca2+ sources are required for the synaptic vesicle accumulation and axon terminal remodeling (Fig. 7). At the early stage of synapse formation, IP3-mediated Ca2+/calmodulin signaling induces synaptic vesicle accumulation in the axon terminals. At the late stage of synapse formation, activity-dependent Ca2+/calmodulin signaling stimulates morphological remodeling of the axon terminals. We recently demonstrated that zebrafish orthologue of human IL1 receptor accessory protein-like 1, mutations in which are associated with X-linked mental retardation and autism (Carriéet al. 1999; Piton et al. 2008), plays a role in both synaptic vesicle accumulation and the morphological remodeling during pre-synaptic differentiation of olfactory sensory neurons (Yoshida and Mishina 2008). An intriguing possibility is that IL1 receptor accessory protein-like 1 may mediate upstream signals to induce axon terminal differentiation during synapse formation.
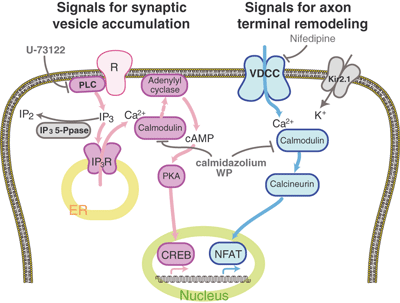
Distinct Ca2+ signaling for synaptic vesicle accumulation and axon terminal remodeling during presynaptic differentiation of zebrafish olfactory sensory neurons. IP3-mediated Ca2+/calmodulin signaling and following PKA signaling induce synaptic vesicle accumulation in the axon terminals at the early stage of synapse formation (shown in warm colors). Ca2+/calmodulin-stimulated adenylyl cyclases may transduce IP3-mediated Ca2+ signal to PKA signal. On the other hand, activity-dependent Ca2+/calmodulin signaling and following calcineurin signaling stimulate morphological remodeling of the axon terminals at the late stage of synapse formation (shown in cold colors). IP3 5-phosphatase, Kir2.1 and drugs that interfere synaptic vesicle accumulation or axon terminal remodeling are shown in gray.
Acknowledgements
We are grateful to Dr. Y. Masuda, A. Uotsu and A. Kakihara for technical assistance, Dr. M. Iino for rat IP3 5-phosphatase cDNA, Dr. S. E. Fraser for tub-GVP-Uunc and Y. Nakajima for help in manuscript preparation. This work was supported in part by research grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan.




