Transcription factor specificity protein 1 (Sp1) is the main regulator of nerve growth factor-induced sphingosine kinase 1 gene expression of the rat pheochromocytoma cell line, PC12
Abstract
Sphingosine kinase (SPHK) is known to exert an anti-apoptic role in various cells and cell lines. We previously reported that human brain is rich in SPHK1 (Murate et al. 2001). After showing a high expression of SPHK1 in rat brain, we examined the gene expression mechanism using nerve growth factor (NGF) -stimulated rat PC12 cells. With RT–PCR, we found that both rat brain and PC12 utilized exon 1d mostly out of eight untranslated first exons. NGF induced an increase in SPHK enzyme activity and protein about double those in PC12 cells, and NGF-induced SPHK1 mRNA was three times higher than in the control. The minimal 5′ promoter was determined, and TrkA specific inhibitor K252a inhibited the NGF-induced promoter activity of SPHK1. The truncation or mutation of putative transcription factor-binding motifs revealed that one specificity protein 1 (Sp1) binding motif of the 5′ region of exon 1d is prerequisite. Electrophoresis mobility shift assay confirmed the promoter analysis, indicating increased Sp1 protein binding to this motif after NGF treatment. Chromatin immunoprecipitation assay also showed the binding of Sp1 and the promoter region in vivo. These results suggest the signal transduction pathway from NGF receptor TrkA to transcription factor Sp1 protein binding to the promoter Sp1-like motif in NGF-induced rat SPHK1 gene expression.
Abbreviations used
-
- AP-2
-
- activator protein 2
-
- CHIP
-
- chromatin immunoprecipitation
-
- EMSA
-
- electrophoresis mobility shift assay
-
- ERK
-
- extracellular signal-regulated kinase
-
- hASMase
-
- human acid sphingomyelinase
-
- NGF
-
- nerve growth factor
-
- PMA
-
- phorbol 12-myristate 13-acetate
-
- RACE
-
- rapid amplification of 5′ cDNA ends
-
- Sp1
-
- specificity protein 1
-
- S1P
-
- sphingosine-1-phosphate
-
- SPHK
-
- sphingosine kinase
-
- TrkA
-
- high affinity NGF receptor
Sphingolipid metabolites, including ceramide, sphingosine and sphingosine-1-phosphate (S1P), play essential roles in cell growth, survival and death (Hannun 1996; Spiegel and Merrill 1996). Ceramide and sphingosine are generally thought of as pro-apoptic factors, whereas S1P generated by sphingosine kinase (SPHK) is anti-apoptic. (Edsall et al. 1997; Olivera et al. 1999). Recently, two isoforms of SPHKs were cloned (Kohama et al. 1998; Liu et al. 2000; Melendez et al. 2000; Nava et al. 2000). SPHK1 is thought to affect cell survival, growth and motility (Edsall et al. 1997, 2001; Ruis et al. 1997; Olivera et al. 1999), whereas SPHK2 is reported to induce cell growth arrest or cell death in some cell lines (Liu et al. 2003; Igarashi et al. 2003). Several stimuli activate SPHKs, thereby increasing the intracellular concentration of S1P (Edsall et al. 1997; Ruis et al. 1997; Kleuser et al. 1998; Meyer zu Heringdorf et al. 1999; Choi et al. 1996).
SPHK activity has been found in rat kidney, spleen, liver, and brain, bovine kidney and brain, human platelets and placenta and porcine platelets (Yatomi et al. 1997; Edsall and Spiegel 1999; Gijsbers et al. 1999). Along with others, we reported the tissue localization of SPHK1 in human tissues, mouse tissues, and rat cerebellum (Murate et al. 2001; Fukuda et al. 2003; Terada et al. 2004).
Although the mechanism underlying the activation of SPHK1 remains largely unknown, previous reports favored the idea of a post-translational or transcriptional regulation for the agonist-induced stimulation of SPHK (Melendez and Khaw 2002; Pitson et al. 2003; Pitson et al. 2005). A recent report showed that SPHK1 is translocated rapidly from cytosol to membrane for the activation by extracellular signal-regulated kinase (ERK) by nerve growth factor (NGF) stimulation in PC12 cells (Toman et al. 2004). We have previously reported the transcriptional control of human SPHK1 in the phorbol 12-myristate 13-acetate (PMA)-induced differentiation of a human leukemia cell line, MEG-O1 (Nakade et al. 2003), However, transcriptional control of SPHK1 in the neuronal cells has not yet been studied in the neuronal cells. The biological activity of NGF, one of the major neurotrophic factors, is mediated by the interaction of two receptors, i.e. high-affinity p140trkA and low-affinity p75 (Nakagawara 2001). PC12 cells express both types of NGF receptors. Stimulation of TrkA activates the SPHK/S1P pathway leading to the survival of PC12 cells (Edsall et al. 1997).
In the present study, we have examined the regulatory mechanism of SPHK gene expression in NGF-stimulated PC12 cells and have demonstrated the important role of NGF-inducible binding of specificity protein 1 (Sp1) transcription factor, and Sp1-like motif within the 5′ promoter region of the exon 1d of SPHK1 gene.
Materials and methods
Animals, cell line and reagents
Wistar rats were used to prepare tissue RNA and protein samples. The rat pheochromocytoma cell line, PC12, was cultured in 10% fetal calf serum in Dulbecco's Modified Eagle's medium (Sigma, St. Louis, MO, USA) at 37°C in 5% CO2 (Yoshimura et al. 1998). Rabbit anti-mouse SPHK1 antibody was prepared as described previously (Murate et al. 2001). NGF was purchased from Sigma, and K252a from Alomone Laboratories (Jerusalem, Israel). pBluescriptII KS(+) vector was purchased from Invitrogen (Carlsberg, CA, USA). pGL3 basic vector for luciferase assay was from Promega (Madison, WI, USA). Sp1 expression vector was kindly provided by Dr G. Suske (Institut für Molekularbiologie und Tumorforshung Phillipps-Universitat Marburg, Marburg, Germany). Anti-Sp1 (SC-59), anti-activator protein 2 (AP-2) antibodies (SC-184) and normal rabbit IgG were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), and anti-β-actin antibody (#AANO2) from Cytoskeleton, Inc. (Denver, CO, USA).
Sphingosine kinase enzyme activity
The enzyme activity of SPHK was measured according to the procedure of Olivera et al. 1999 with minor modifications (Murate et al. 2001). Briefly, 50 µg protein was used for the enzyme assay. The reaction mixture (200 µL) consisted of 20 mm Tris-HCl, pH 7.4, 2.5 mm MgCl2, 0.25 mm EDTA, 5% glycerol, 1.2 mm dithiothreitol, 1 mm Na3VO4, and 15 mm NaF, 20 µm sphingosine with 0.25% Triton X-100, and 50 µg enzyme. The reaction (30 min at 37°C) was started by the addition of 20 µL [γ-32P]ATP (2 µCi) and 10 mm ATP complex, and terminated by the addition of 20 µL 1 N HCl, followed by 0.8 mL of chloroform/methanol/HCl (100:200:1). After vigorous vortexing, 240 µL chloroform and 240 µL 1 m KCl were added, and the suspension centrifuged at 2000 g for 10 min at room temperature. The organic phase containing the lipids was removed, concentrated, and applied to a silica gel 60 TLC plate, which was developed in butan-1-ol/acetic acid/water (3:1:1, v/v). The S1P spot was visualized by autoradiography, and quantified using a densitometer (Densitograph Atto, Tokyo, Japan).
Western blotting
Western blotting was performed using anti-SPHK1 antibody (Murate et al. 2001) and anti-Sp1 antibody, respectively. Five or 10 µg of protein were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and electro-transferred onto Hybond-P membranes (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK). A positive signal was detected with an ECL Plus western blotting system (Amersham Pharmacia Biotech).
Semi-quantitative reverse transcription–polymerase chain reaction
For RNA preparation, cells were harvested, and total RNA was extracted using an RNA STAT-60 kit (Tel-Test, Inc., Friendswood, TX, USA). Semi-quantitative RT–PCR was performed according to the method described previously (Nakade et al. 2003). The first-strand cDNA was prepared with 5 µg of RNA using the Super Script First-Strand System (Invitrogen). In the preliminary experiments, the relative amounts of cDNA and the range of PCR cycles that permit the linear amplification of SPHK1 and β-actin were determined. The primer sequences were: SPHK1 exon 1a, sense 5′-ACCAAGGCATGTATTGCAGTGACGC-3′, antisense 5′-CAGTGTGCAGCTGCTTATCGGTGTT-3′; SPHK1 exon 1d, sense 5′-AGTTGGTGAGGATCGTGG-3′, antisense 5′-AGCTGCTTATCGGTGTTG-3′; and β-actin, sense 5′-TTCTACAATGAGCTGCGTGTGGC-3′, antisense 5′-CTCATAGCTCTTCTCCAGGGAGGA-3′. The PCR conditions for rat SPHK1 (rSPHK1) were 96°C for 15 s, 59°C for 30 s, followed by 72°C for 30 s. The number of cycles was 36, 38 and 40. The PCR conditions for β-actin were 96°C for 2 min followed by 19, 21 and 23 cycles at 96°C for 15 s, 58°C for 30 s, and 72°C for 30 s. Using the NIH image (version 6), each band was surveyed for its relative band intensity. Within the range of the linear PCR amplification, the relative expression of the SPHK1 message was evaluated by calculating the band intensity ratio of SPHK1/β-actin.
Rapid amplification of 5′ cDNA ends
RNA ligase-mediated rapid amplification of 5′-cDNA ends (5′-RACE) was performed according to the instruction manual with a GeneRacer™ kit (Invitrogen) to determine the transcription initiation point of rSPHK1 in PC12 cells. The reverse SPHK1 gene-specific primer and reverse SPHK1 gene-specific nested primer were 5′-GGCGCTGCTAGAGGTAACGG-3′, and 5′-TCGCCCTCTCCACCTCCCT-3′, respectively. Cloned PCR products were analyzed for their DNA sequences and the 5′-transcription initiation point was determined.
Transfection and luciferase assay
The vector containing rat SPHK1 gene and its 5′ promoter region was the kind gift of Dr K. Shiota (Tokyo University, Tokyo, Japan). The 877 bp (BglII and PstI digested) fragment derived from this original vector was introduced to the BamHI and PstI sites of pBluescriptII KS(+) vector using the Ligation high kit (Toyobo, Osaka, Japan). The introduced DNA fragment was digested by MluI (in rSPHK1 genome) and HindIII [in pBluescriptII KS(+) vector], and again introduced to the MluI and HindIII sites of pGL3 basic vector. This construct was tentatively named rSPHK exon 1d/−604 (+1 denotes the transcription initiation point of exon 1d). A further 5′ DNA fragment was added to this construct to form rSPHK exon 1d/−3942. The required DNA fragment of the 5′ promoter region was digested by KpnI and MluI, and ligated to the KpnI and MluI sites of rSPHK exon 1d/−604. This construct was named rSPHK exon 1d/−3942. The deletion mutants were prepared by the conventional enzyme-based digestion method. rSPHK exon 1d/−3942 was digested by either KpnI/StuI or KpnI/BstEII. Each fragment was blunted and self-ligated. The final constructs were designated as rSPHK exon 1d/−2008 and rSPHK exon 1d/−294, respectively. To circumvent several putative transcription factor-binding motifs within this region, further deletion mutants were constructed using PCR-based methods. According to the DNA sequence of the 5′ promoter region of rat SPHK1 exon 1d, the following primer sets were prepared. The upper primer for producing rSPHK exon 1d/−119 was 5′-GGGGGTACCGCATTTTCACGCGGAGTT-3′, and that for producing rSPHK exon 1d/−64 was 5′-GGGGGTACCATGCTAGCCTCTCCAATCCC-3′. A single underline denotes the KpnI enzyme site. The lower primer was GL primer 2 of the pGL3 basic vector (Promega). The PCR products were digested by KpnI and NcoI, purified, and ligated to the pGL3 basic vector to produce, respectively, the truncated promoters, rSPHK exon 1d/−119 (including AP-2 and 2 Sp1 binding motifs) and rSPHK exon 1d/−64 (deleted AP-2 and 2 Sp1 binding motifs). To introduce a mutation to the AP-2 and Sp1 binding motifs, the following primer sets were prepared, and PCR was performed using rSPHK exon 1d/−119 as the template. The upper primer for the mutated AP-2-binding motif was 5′-GGGGTACCGCATTTTCACGCGGAGTTCGCAATATTAGTGGGGAGCGGTG-3′, that for the mutated distal Sp1-binding motif was 5′-GGGGGTACCGCATTTTCACGCGGAGTTCGCGCCCGAATTAGTGAATTGCGGT-3′, and that for mutated proximal Sp1-binding motif was 5′-GGGGGTACCGCATTTTCACGCGGAGTTCGCCCGAAGGGGTGGGGAGCGGTGGGACTATTATTACATTGCTAGCCT-3′. A single underline denotes the KpnI enzyme site, and a double underline indicates the mutated sequence.
In order to show that the promoter region containing AP-2 and two Sp1 motif can confer NGF responsiveness to a heterologous promoter, a fragment of human acid sphingomyelinase (hASMase) promoter (Murate et al. 2002) was used as the partner. The luciferase vector containing the promoter region (−219 bp from the transcription start point to +112 bp) of hASMase was combined to the rSPHK1 promoter by PCR based method. For the convenience of ligation, the sequences of the upstream primers used for generating the hybrid promoter of hAMSase and rSPHK1 are: hASMase (−219/+112), 5′-GGGCTCGAGCGCAGCGTTGACAGCCGCCCGCCACC-3′; rSPHK exon 1d (−280/−33), 5′-GGGGGTACCCCTGGCGGCTTCTTTTTGTCC-3′. The downstream primers are: hASMase (−219/+112), 5′-GGGAAGCTTCCAGCGGCCAGGCCCATCCAAAGG-3′; rSPHK exon 1d (−280/−33), 5′-GGGCTCGAGGGGGCTCTCATCGGGATTGG-3′. A single, double and dotted underline denote the added KpnI enzyme site, the XhoI enzyme site, and the HindIII enzyme site, respectively. Each PCR product was subcloned into the pBluescriptII KS(+) vector, and constructs were digested by XhoI/HindIII [hASMase in pBluescriptII KS(+) vector] or KpnI/XhoI [rSPHK exon 1d in pBluescriptII KS(+) vector]. Both hASMase fragment and rSPHK exon 1d promoter fragment were ligated to the KpnI and HindIII sites of pGL3 basic vector to make the hybrid promoter, or the hASMase fragment was ligated to the XhoI and HindIII sites of pGL3 basic vector to make the control hASMase reporter.
The DNA transfection was performed by the calcium precipitation method as described by Wilson et al. 1993. Briefly, 5 µg luc vector and 2 µg β-galactosidase expression vector were cotransfected to PC12 cells. After 8 h, glycerol shock was induced. The cells were transferred to the complete medium with/without 100 ng/mL NGF for 20 h. Luciferase and β-galactosidase activities were measured, and the relative promoter activity was expressed as luc/β-galactosidase activity. When examining the effect of K252a, it was added 1 h before NGF stimulation.
Electrophoresis mobility shift assay
Nuclear extract was prepared from PC12 cells treated with/without 100 ng/mL NGF for the times indicated. Cells were subjected to a preparation of nuclear extract using NE-PER® nuclear and cytoplasmic extraction reagents (Pierce, Rockford, IL, USA). For the preparation of Sp1 over-expressed nuclear extract, PC12 cells were transfected with 20 µg of Sp1 expression vector (as described above) by the calcium precipitation method. After 24 h, nuclear extracts were prepared similarly. Biotin-labeled probes containing the rat SPHK1 exon 1d type promoter fragment were synthesized and annealed. Electrophoresis mobility shift assay (EMSA) was performed with 2 µg nuclear extract and biotin-labeled double strand DNA probes (50 fmol) with a LightShift™ Chemiluminescent EMSA kit (Pierce) according to the manufacturer's protocol. Binding reactions were loaded onto a 6% polyacrylamide gel (8 × 8 × 0.1 cm) in 0.5 × TBE buffer (0.089 M Tris-borate, 0.089 M boric acid, 10 mM EDTA) and electrophoresed at 100 V for 3.5 h. Biotin-labeled double stranded DNAs were transferred to a positive-charge nylon membrane (Hybond-N, Amersham Pharmacia Biotech). Biotin-labeled DNA was then integrated with streptavidin–horseradish peroxidase conjugate. In some experiments, probes with mutations or deletions of the putative transcription factor binding motif were used. The sense oligomer of the AP-2 mutated probe was 5′-GTTCGCAATTATTAGTGGGGAGCGGTGGGACCACGCCCCCATGCT-3′, that of the AP-2 deleted probe was 5′- GCGGAGTTGTTGGGGTGGGGAGCGGTGGGACCACGCCCCCATGCT-3′, that of the distal Sp1 mutation was 5′-GTTCGCCCGAATTAGTGAATTGCGGTGGGACCACGCCCCCATGCT-3′, that of the distal Sp1 deletion was 5′-GCGGAGTTGTTCGCCCGAAGAGCGGTGGGACCACGCCCCCATGCT-3′, that of the proximal Sp1 mutation was 5′-GTTCGCCCGAAGGGGTGGGGAGCGGTGGGACTATTATTACATGCT-3′, and that of the proximal Sp1 deletion was 5′- GTTCGCCCGAAGGGGTGGGGAGCGGTGGGGCTAGCCTCTCCAATC-3′. A double underline denotes the mutated sequence. For the supershift experiment, anti-AP-2 or anti-Sp1 antibody was used. The antibody was mixed with nuclear extract for 15 min at room temperature before mixing with the labeled probe.
Chromatin immunoprecipitation assay
PC12 cells were crosslinked for 10 min by adding formaldehyde directly to the cell culture medium to a final concentration of 1%. Cross-linking was stopped to a final concentration of 0.125 m Glycine. Crosslinked cells were washed twice with phosphate-buffered saline, scraped and resuspended in the swelling buffer (10 mm Tris-HCl, PH 7.4, 3 mm CaCl2, 0.1% Nonidet P-40 (v/v), and protease inhibitors). After a 10-min incubation on ice, cells were vortexed and nuclei were collected by centrifugation. Isolated nuclei were resuspended in lysis buffer (50 mm Tris-HCl, pH 8.0, 1% sodium dodecyl sulfate, 10 mm EDTA, and protease inhibitors) and sonicated using eight pulses of 30 s. After centrifugation, the supernatant was diluted 10-fold with dilution buffer (16.7 mm Tris-HCl, pH 8.0, 167 mm NaCl, 1.1% Triton X-100, and protease inhibitors) and precleared with protein A-Sepharose 4B (Upstate Biotechnology, Lake Placid, NY, USA) containing 0.5 mg/mL of salmon sperm DNA. After 10 µL of protein A-Sepharose (50% slurry containing 0.5 mg/mL salmon sperm DNA) was then added to the solution again, the supernatant was divided into three aliquots. Nothing was added to one aliquot, and to the others, normal rabbit IgG or Sp1-specific antibody (final concentration: 1 ng/µL) was added and incubated 4 h at 4°C on a rotating wheel. The beads were pelleted and washed sequentially in the following buffers: buffer A [50 mm HEPES, pH 7.9, 140 mm NaCl, 0.1% (w/v) deoxycholic acid (sodium salt), 1 mm EDTA, 1% (v/v) Triton X-100 and protease inhibitors]; buffer B [50 mm HEPES, pH 7.9, 500 mm NaCl, 0.1% (w/v) deoxycholic acid (sodium salt), 1 mm EDTA, 1% (v/v) Triton X-100, and protease inhibitors], and TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) (twice). Immuno-complexes were extracted from the beads with elusion buffer [50 mm Tris-HCl, pH 8.0, 10 mm EDTA, 1% (w/v) sodium dodecyl sulfate]. Cross-linking was reversed by heating the elutes at 65°C overnight. The elutes were then digested with proteinase K at 50°C for 5 h. The solutions were extracted with phenol/chloroform/isoamyl alcohol. DNA was purified by ethanol precipitation and resuspended in 30 µL of TE buffer. The SPHK1 promoter region was amplified by PCR using primers 5′-CCTGGCGGCTTCTTTTTGTCC-3′ (forward) and 5′-GGGGCTCTCATCGGGATTGG-3′ (reverse).
Results
Rat brain expresses sphingosine kinase 1 protein
The protein level of SPHK1 in rat brain was analyzed by western blotting (Fig. 1a). Rat cerebrum, midbrain and cerebellum showed higher levels of SPHK1 protein. Heart and kidney also expressed a significant but lower level of SPHK1 protein with the longer exposure of the same membrane. This result is slightly different from that based on the mouse data reported by Fukuda et al. (2003), which showed a modest SPHK1 expression in mouse heart and kidney as well as in brain.
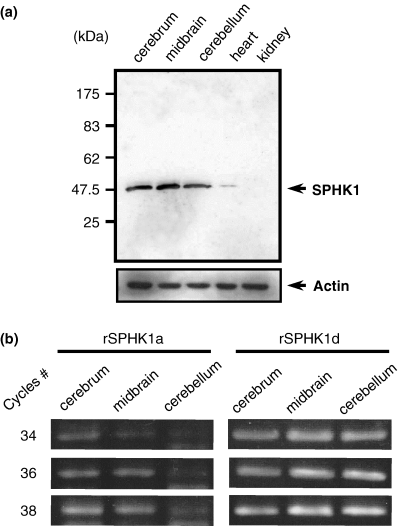
Sphingosine kinase 1 (SPHK1) protein and message in rat brain. (a) Western blotting with anti-SPHK1 antibody. Rat cerebrum, midbrain, cerebellum, heart and kidney were separated and lysed with sodium dodecyl sulfate sample buffer. Then 5 µg of each sample was loaded. After transfer to the membrane, the amount of SPHK1 protein was determined with anti-SPHK1 antibody. It is of note that the increase of protein loading or the longer exposure could also detect the positive band in the heart and kidney samples. (b) RT–PCR analysis of SPHK1 exon 1. After preparing the first strand cDNA, 34, 36 and 38 cycles of PCR were performed with primers described in Materials and methods. The expected band size of exon 1a is 350 bp, and exon 1d is 326 bp.
Rat brain and PC12 cells utilizes exon 1d more than exon 1a
The rat SPHK1 gene structure was published recently (Imamura et al. 2001). RT–PCR was applied to total RNA from the cerebrum, midbrain and cerebellum to detect the transcript of SPHK1 first exon. For the analysis of gene transcription level, we used the semiquantitative RT–PCR method as reported previously (Nakade et al. 2003). In the preliminary experiments, the relative amount of mRNA was checked by serially amplifying the β-actin message, after which the template volume for the RT–PCR of SPHK1 was adjusted between samples. Figure 1(b) shows that rat cerebrum, midbrain and cerebellum utilized exon 1d more than exon 1a. The other exons (1c, 1b, 1e, and 1f) were not detected by the present RT–PCR experiments (data not shown). PC12 cells also utilized exon 1d as the major first exon as shown in Fig. 2(d), whereas the signal of exon 1a was not detected, even after NGF stimulation under our experimental conditions (Fig. 2d).
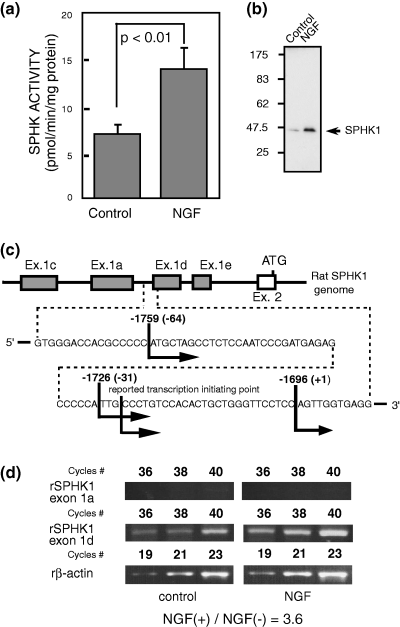
Sphingosine kinase (SPHK) enzyme activity, SPHK1 protein and message in PC12 cells. (a) PC12 cells treated with or without nerve growth factor (NGF) (100 ng/mL) for 1 day were collected. SPHK activity was measured as described in Materials and methods. The results were expressed as mean ± SD from several separate experiments. The statistical significance was analyzed with Student's t-test. (b) Western blotting using anti-SPHK1 antibody against PC12 cells treated with/without NGF (100 ng/mL) for 1 day. Four micrograms of protein was loaded per lane. (c) Rapid amplification of 5′-cDNA ends (5′-RACE). The transcription initiation points of exon 1d are shown. The first ATG of translation start codon, ATG of the second exon was regarded as +1. The number within the parenthesis is the base pair number from −1696 (our determined +1 of exon 1d), and this base pair counting was used throughout the following promoter assay. (d) Semi-quantitative RT–PCR analyses of control and NGF-treated PC12 cells were performed according to the procedure described in Materials and methods. Upper part illustrates the increase in the SPHK1 exon 1a and 1d message with serial PCR cycling, and lower part is the internal control of β-actin. The relative message level is expressed as rSPHK1/β-actin. Control sample is regarded as 1.0.
Nerve growth factor stimulates sphingosine kinase 1 gene expression in PC12 cells
NGF was reported to stimulate SPHK enzyme activity (Ruis et al. 1997). In the present study, NGF increased SPHK enzyme activity (Fig. 2a) as well as the SPHK1 protein level to about twice those in the control (Fig. 2b). It was shown that NGF induced an increase in SPHK1 gene expression (exon 1d type) in PC12 cells 3.6 times that in the control (Fig. 2d).
Determination of transcription initiation points of exon 1
Using the 5′-RACE method, we examined the transcription initiation points of exon 1d (Fig. 2c) of PC12 cells and found three points, i.e. −1759, −1726, and −1696 from the first ATG in the second exon, which were very close to the reported initiating points available online. Other initiation points around exon 1a located upstream of exon 1d were not found.
Nerve growth factor-induced sphingosine kinase 1 exon 1d promoter activity is blocked by TrkA inhibitor
The TrkA (high affinity-NGF receptor) specific inhibitor, K252a, suppressed SPHK1 message expression induced with NGF (Fig. 3a). Using the DNA fragment of the 5′ promoter region of rat SPHK1, we prepared luciferase vectors with inserts of various lengths (exon 1d/−3943, exon 1d/−2008, exon 1d/−604, exon 1d/−294, exon 1d/−119 and exon 1d/−64, in Fig. 4 as explained in the Materials and methods). Figure 3(b) shows that NGF increased SPHK1 exon 1d type promoter activity (exon 1d/−604 as shown in Fig. 4 left) more than three times as compared with non-NGF treated exon 1d/−604, which was consistent with the increase in the message level. It was shown that inhibition of the NGF receptor suppressed NGF-induced promoter activity, suggesting that the NGF-induced SPHK1 gene expression was located downstream of TrkA receptor signaling.
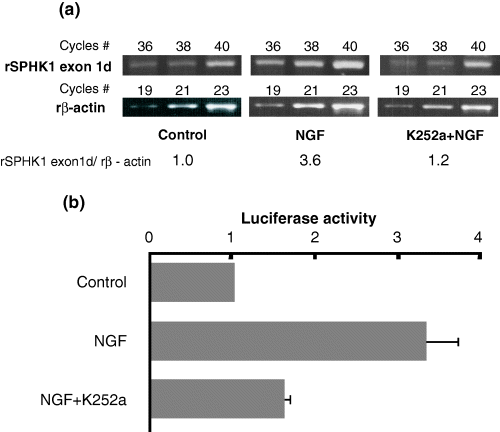
Nerve growth factor (NGF)-induced sphingosine kinase 1 (SPHK1) message and effect of TrkA specific inhibitor (K252a) on its promoter activity. (a) Semi-quantitative analysis of SPHK1 message was performed. Message levels of control and NGF-stimulated PC12 cells without or with K252a treatment are shown. Relative message level of SPHK1 was calculated with the ratio of SPHK1/β-actin as described in Fig. 2. (b) Promoter analysis of rat SPHK1 (exon 1d type). Five micrograms of the reporter construct including 604 bp 5′ of exon 1d (rSPHK exon1d/−604: left side of Fig. 4) was transfected to PC12 cells along with β-galactosidase control expression vector using calcium precipitation method. The luciferase activity assay was performed in triplicate. Relative promoter activity is expressed as the luciferase count of each pGL3 reporter/β-galactosidase expression as described in Materials and methods. Promoter activity of the control is regarded as 1.0. Data is presented as the mean ± SD from several separate experiments.
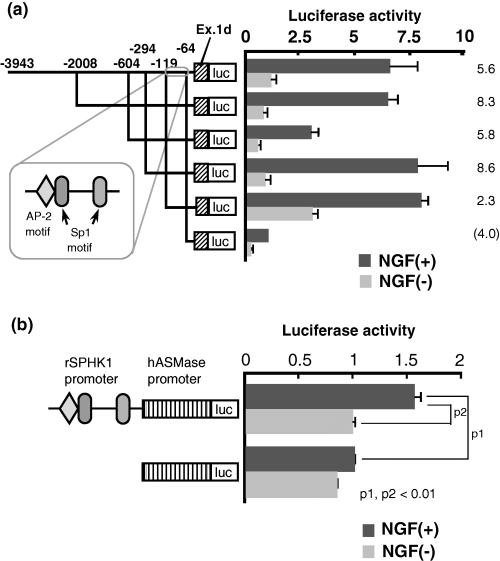
Promoter activity of rat sphingosine kinase 1 (rSPHK1) (exon 1d type) induced by nerve growth factor (NGF). (a) Deletion mutants of various lengths of 5′ promoter in SPHK1 exon 1d were prepared from the longest form of construct −2008 and designated −604, −294, −119, and −64, respectively. Putative transcription factor binding motif is illustrated as well. Each promoter activity was analyzed as described in Fig. 3. Relative luciferase activity is shown in the right panel. Solid column is NGF (+) samples and shaded column is NGF (–) samples. Mean ± SD was calculated from at least three separate experiments, each performed in triplicate. Data of NGF-induced promoter activity from the shortest promoter construct (rSPHK exon 1d/−64) is regarded as 1.0. The fold increase of each vector construct treated with NGF is also illustrated on the right side of the histogram. (b) The hybrid promoter containing human acid sphingomyelinase (hASMase: 219 bp long) and the promoter region of rat SPHK1 (248 bp long) was prepared as described in the Materials and methods. The luciferase assay was performed by transfecting this hybrid luciferase reporter or control hASMase reporter into PC12 cells. The fold increase of the hybrid promoter activity was analyzed with NGF treatment. Mean ± SD was calculated from at least three separate experiments, each performed in triplicate. The relative luciferase activity of NGF-treated hASMase promoter was regarded as 1.0. p1 and p2: statistical significances were calculated with StatView version 5 (SAS Institute Inc. Cary, NC, USA). One-way factorial anova was performed.
Determination of nerve growth factor-responsive promoter region
We tried to determine the 5′ promoter region of SPHK1 exon 1d responsible for NGF stimulation in PC12 cells. About a 4-kb length 5′ of the exon 1d was analyzed. As shown in Fig. 4(a), a 55-bp length (−119/−64) was sufficient (7.69-fold increase) for NGF-induced exon 1d transcription as compared to NGF-induced exon 1d/−64 luciferase vector. The relative ratio of luc activity (promoter activity) between NGF(+)1d/−119 luc/NGF(–)1d/−119 luc was 2.3 and was lower than that of 1d/−294 luc (8.6). However, the decrease of the ratio is due to the high luciferase activity of NGF(–) 1d/−119 luc, and the luciferase activity of NGF(+)1d/−119 itself was comparable to NGF(+)1d/−294 luc. So, we focused on this region. The decrease of promoter activity was observed in NGF-treated 1d/−604/luc vector. The presence of suppressor elements between −604 and −294 is the possible answer; however, the reason still remains to be determined. Available online data of the putative binding motif of transcription factor, TFSEARCH (http://www.cbrc.jp/research/db/TFSEARCH.html), demonstrated three putative boxes (one AP-2, and two Sp1 boxes) within this 55-bp length. By introducing a mutation into the respective transcription factor binding motifs, our analysis revealed that both the AP-2 and Sp1 motifs regulated the exon 1d expression, and that the proximal Sp1 binding motif was the prerequisite promoter motif (Fig. 5). Our experiment using the hybrid of hASMase promoter and rSPHK1 promoter (containing this 55-bp region) showed that the addition of the rat SPHK1 promoter region containing AP-2 motif and Sp1 motifs could also confer the NGF responsiveness to hASMase promoter (Fig. 4b).
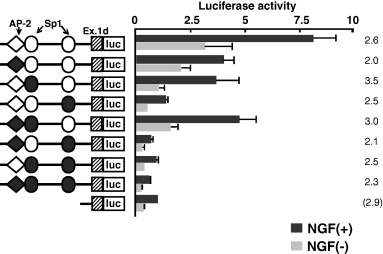
Mutation of transcription factor binding motifs affects the promoter activity. Based on results shown in Fig. 4, mutations were inserted into activator protein 2 (AP-2), distal specificity protein 1 (Sp1) and proximal Sp1, or two or three of these binding motifs of the rat sphingosine kinase (rSPHK) exon 1d/−119 (Fig. 4), respectively. Solid circles and squares denote insertion of the mutation into the respective binding motif. Mutated motifs are described in detail in Materials and methods. Relative promoter activity from nerve growth factor (NGF)-treated shortest promoter construct (rSPHK exon 1d/−64) is regarded as 1.0. The fold increase of each vector construct treated with NGF is illustrated on the right side of the histogram.
Nerve growth factor increases specificity protein 1 (Sp1) protein binding to the proximal Sp1-like motif
Figure 6(a) illustrates the oligonucleotides used for EMSA experiments. Figure 6(b) shows that our EMSA probe could detect two bands (a and b), both of which were increased time-dependently after NGF treatment. Band a was stronger than band b. Cold competitor greatly reduced bands a and b, suggesting their specificity (Fig. 6c). Figure 7(a) showed the mutated or deleted oligonucleotides used for further analysis. Mutation or deletion of the proximal Sp1 motif but not the distal Sp1 or AP-2 motifs diminished bands a and b in NGF-stimulated nuclear extract (Fig. 7b). This suggested that both bands were derived from the proximal Sp1-like motif but not from the distal Sp1-like motif (which slightly overlapped with AP-2 motif) or the AP-2 motif in NGF-induced SPHK1 gene expression. Anti-AP-2 antibody did not change the band pattern, whereas the addition of anti-Sp1 antibody greatly reduced band a intensity and diminished band b (Fig. 8a). We also analyzed changes in Sp1 protein levels during NGF stimulation. It was observed that the Sp1 level increased during the observation period (Fig. 8b).
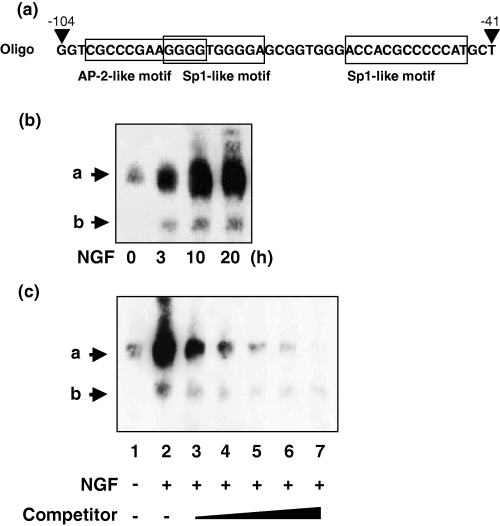
Electrophoresis mobility shift assay of nuclear extract of PC12 cells with or without nerve growth factor (NGF) treatment. (a) Illustration of probes used in the present experiment (as the sense strand). Activator protein 2 (AP-2) and specificity protein 1 (Sp1)-like motifs are boxed. (b) Time-dependent changes in band pattern after NGF treatment. PC12 cells were treated with NGF (100 ng/mL) for the times indicated. Two bands were named a and b. (c) Effect of cold competitor, which was increased from × 1.25 (lane 3) to × 20 (lane 7).
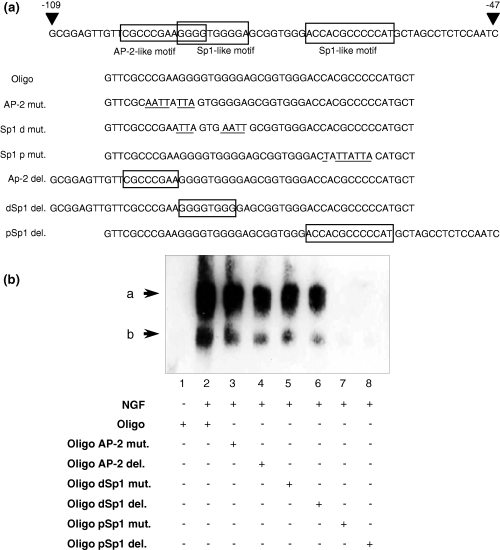
Effect of mutation and deletion within probable protein binding motif. (a) Illustration of mutated and deleted oligonucleotides used in this experiment. Mutations are the same as those used in the promoter assay described in Fig. 5. Mutated bases are underlined, and deleted areas are boxed. (b) Electrophoresis mobility shift assay (EMSA) data. Nuclear extracts prepared from PC12 cells treated with or without nerve growth factor (NGF) were reacted with normal, mutated or deleted oligos and subjected to EMSA. AP-2, activator protein 2; Oligo, oligonucleotide; Sp1, specificity protein 1; dSp1, distal Sp1; pSp1, proximal Sp1.
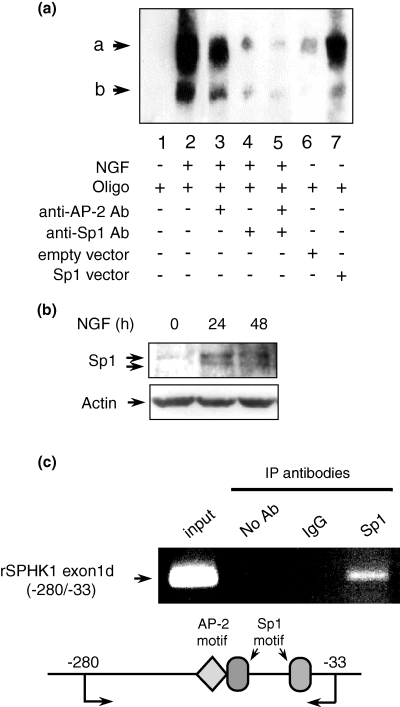
Specificity protein 1 (Sp1) protein expression in electrophoresis mobility shift assay (EMSA) and chromatin immunoprecipitation (CHIP) assay. (a) EMSA was performed with 2 µg of PC12 cell nuclear extract (lanes 1–5). Anti-Sp1 or anti-activator protein 2 (anti-AP-2) antibody was added 15 m before mixing with biotin-labeled probe (lanes 4–5). Nuclear extracts in lanes 6 and 7 were prepared from PC12 cells transiently transfected with empty vector (lane 6) or Sp1 expression vector (lane 7). (b) Changes in Sp1 protein level after nerve growth factor (NGF) treatment. Whole cell lysates were prepared from PC12 cells treated with or without NGF (100 ng/mL) for the times indicated. Ten micrograms of protein was loaded per lane. Western blotting was performed with anti-Sp1 antibody. β-Actin was shown as the internal control. (c) CHIP assay. The chromatin of PC12 cells was cross-linked with formaldehyde according to the Materials and methods. The purified chromatin was treated with normal IgG, anti-Sp1 antibody and no addition, respectively. After immunoprecipitation, DNA was extracted and PCR was performed with the primer set described in the Materials and methods. Input shows the chromatin just before immunoprecipitation. The expected band size was 248 bp. rSPHK1, rat sphingosine kinase 1.
Sp1 over-expressed nuclear extract showed an increase in both bands a and b, thus confirming the involvement of Sp1 protein (Fig. 8a, lanes 6 and 7). CHIP assay shown in Fig. 8(c) demonstrated that anti-Sp1 antibody but not normal IgG could precipitate the complex between Sp1 protein and the rSHPK1 promoter, suggesting that Sp1 protein did bind with the region containing AP-2 and Sp1 in NGF-stimulated PC12 cells in vivo.
Discussion
NGF induces a sustained elevation of sphingosine kinase activity in PC12 cells, probably due to its increased protein synthesis (Ruis et al. 1997). However, a precise study has not yet been reported. Our present study clearly demonstrated that the transcriptional control is involved in NGF-induced SPHK1 activation in PC12 cells.
Imamura et al. (2001) have reported not only the presence of six alternative first exons of rat SPHK1 but also that the CpG island located ∼800-bp upstream of SPHK1 exon 1a contains a tissue-dependent, differentially methylated region. They suggest that the tissue-specific expression of SPHK1 subtypes appears to be regulated by the level of transcription factors in combination with the methylation status of this tissue-dependent differentially methylated region. Fukuda et al. (2003) showed that SPHK1 is the major SPHK isozyme in rat brain that contributes to total SPHK activity. In the present study, we also observed, by using its specific antibody, that SPHK1 is expressed in rat brain (cerebrum, midbrain and cerebellum) (Fig. 1a).
Although the SPHK1 exon 1a transcript was also observed in rat cerebrum and midbrain, exon 1d type could be observed significantly in all rat brain tissues. PC12 cells transcribed exon 1d exclusively (1, 2). We determined the transcription initiation point of exon 1d in PC12 cells; however, the corresponding initiation point of exon 1a could not be detected under our experimental conditions (Fig. 2c). Therefore, we focused on the expression of the exon 1d type transcript.
Consistent with the data of enzyme activity and protein level in PC12 cells stimulated with NGF (Fig. 2a), the semiquantitative RT–PCR showed an increased SPHK1 gene expression by NGF stimulation. When rSPHK exon 1d/−3942 (5′ of exon 1d) was used as a luciferase construct in the promoter assay, NGF induced about a sevenfold increase in promoter activity compared with that in the control (rSPHK exon 1d/−64) (Fig. 4a). Interestingly, the increase in the SPHK1 message as well as the promoter activity were inhibited by the TrkA specific inhibitor, K252a (Figs 3a and b), suggesting that SPHK1 gene expression was regulated via a NGF-TrkA signal transduction pathway.
The reporter assay using deletion mutants identified AP-2 and two Sp1 binding motifs as the minimal regions responsive to NGF, with the latter motif being the more important. The introduction of a mutation into the binding motifs and the addition of anti-Sp1 antibody in EMSA confirmed the results obtained in the promoter assay. Heterologous promoter consisting of human acid sphingomyelinase (Murate et al. 2002) and rat SPHK1 promoter containing this Ap-2 and two Sp1 motif showed NGF response supporting the importance of this motif for the NGF responsiveness.
Transcription factor Sp1 is a zinc-finger protein that binds to GC-rich elements in constitutively activated genes (Courey and Tjian 1988). However, evidence has accumulated to indicate that Sp1 mediates the inducible regulation of genes by various stimulants (Miltenberger et al. 1995; Daniel and Kim 1996; Nakano et al. 1997; Rohlff et al. 1997). Sp1 interacts with several cellular factors (Merika and Orkin 1995; Datta et al. 1995) as well as with viral gene products (Trejo et al. 1997), indicating that the interaction of multiple proteins is one mechanism by which Sp1 can mediate signal transduction in a gene- and cell type-dependent manner despite its ubiquitous expression.
Sp1 is one member of a family of factors that bind to the Sp1 DNA motif (Kadonaga et al. 1987; Kingsley and Winoto 1992; Hagen et al. 1995). Recent studies have demonstrated the direct phosphorylation of Sp1 by ERK2 (Merchant et al. 1999; Liu et al. 2001). It has also been reported that NGF increases the Sp1 DNA binding activity (Yan and Ziff 1997), but not the Sp1 protein level in PC12 cells (Atkins et al. 2003). However, our data from western blotting showed an increase in Sp1 protein in PC12 cells after NGF treatment (Fig. 8b). The appearance of two bands that bind to the proximal Sp1-like motif might be due to some post-transcriptional modification, probably phosphorylation, of Sp1 protein after NGF treatment (Fig. 6). However, we cannot rule out the possible involvement of other transcription coactivators in the band formation in EMSA. Further investigation will be necessary to properly interpret the appearance of these two bands. CHIP assay (Fig. 8c) confirmed the in vivo binding of Sp1 protein and Ap-2 and two Sp1 motifs in vivo. Therefore, based on our present data, it may be concluded that the increase in Sp1 protein itself and also its possible phosphorylation induced the increased Sp1 binding leading to enhanced SPHK1 transcription. In our previous study with PMA-treated MEG-O1 cells (Nakade et al. 2003), AP-2-like protein was shown to bind to two AP-2 motifs after PMA stimulation, thus increasing the transcription. Sp1 protein already binds the Sp1 motif in human SPHK1 5′ promoter in control MEG-O1 cells. In PC12 cells, the binding of Sp1 protein (bands a and b) to Sp1 motifs is induced by NGF stimulation.
Taken together, the results presented in the current study revealed that NGF stimulation induced a novel Sp1 protein binding to the proximal Sp1 motif in the 5′ promoter region of exon 1d, thereby leading to an enhancement in the transcription of SPHK1 in PC12 cells.
Acknowledgements
This work we supported in part by a Grant-in-Aid for Scientific Research (C) 16590453 from the Japanese Ministry of Education, Culture, Sports, Science and Technology, and by a grant from Aichi-Cancer Center.




