Familial paroxysmal exercise-induced dystonia: atypical presentation of autosomal dominant GTP-cyclohydrolase 1 deficiency
Abstract
Paroxysmal exercise-induced dystonia (PED) is one of the rarer forms of paroxysmal dyskinesia, and can occur in sporadic or familial forms. We report a family (male index case, mother and maternal grandfather) with autosomal dominant inheritance of paroxysmal exercise-induced dystonia. The dystonia began in childhood and was only ever induced after many minutes of exercise, and was never present at rest, or on initiation of movements. In addition, family members suffered restless legs syndrome (RLS), depression, and adult-onset Parkinsonism. The index case had low cerebrospinal fluid neurotransmitters and pterins. The PED and RLS stopped on initiation of l-Dopa therapy. Both live family members were found to have a nonsense mutation (p.E84X) in exon 1 of the GTP-cyclohydrolase 1 (GCH-1) gene. We propose that GCH-1 mutations should be considered a genetic cause of familial PED, especially if additional clinical features of monoaminergic deficiency are present in affected individuals.
List of Abbreviations
-
- PED
-
- Paroxysmal exercise induced dystonia
-
- RLS
-
- Restless legs syndrome
Paroxysmal dyskinesias can be classified into paroxysmal kinesigenic dyskinesia, paroxysmal non-kinesigenic dyskinesia, paroxysmal exercise-induced dyskinesia (PED), and paroxysmal hypnogenic dyskinesia.1,2 PED is rare and may occur as a familial autosomal dominant form with variable penetrance.1,3–6 Alternatively, PED can occur as a sporadic form.2,7
The onset of PED is usually between 1 and 16 years of age. The legs are typically involved, and dystonia is often the dominant or sole movement disorder.1,2,6,7 The attacks usually occur after minutes or hours of exercise. The episodes characteristically last for 5 to 30 minutes, and between attacks the examination is normal. We report an unusual family with three generations affected by PED. Some family members suffered restless legs syndrome (RLS), depression, and adult-onset parkinsonism. The dystonia was only ever induced by many minutes of exercise. We present evidence that this family has a dopa-responsive syndrome secondary to a GTP-cyclohydrolase 1 (GCH-1) mutation. GCH-1 mutations should, therefore, be considered a genetic cause of familial PED.
Case report
The family tree is presented in Figure 1. The family gave full informed consent to publish the report and video.
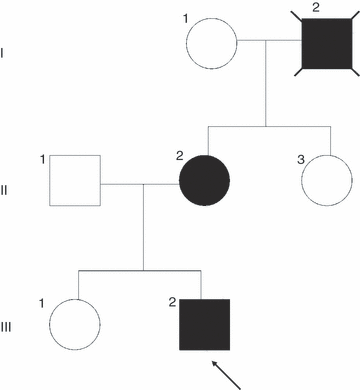
Family tree demonstrating autosomal dominant inheritance. Case III:2 is arrowed. All affected members are represented by black.
Case I:2
The index case’s maternal grandfather had exercise-induced foot posturing of unknown onset, similar to cases II:2 and III:2 (history from II:2). He developed restless leg symptoms and depression in adult life. He further developed young onset Parkinsonism in his 40’s which was atypical according to his treating physician. He died of pancreatic cancer when he was 66 years old.
Case II:2
The 41-year-old mother of the index case had a normal developmental history and no childhood medical problems. Her baseline motor and cognitive abilities were normal. Her paroxysmal attacks began at 15 years of age, and were only induced by significant or prolonged exercise such as walking, running or swimming. The attacks were never induced by standing up from a chair, or a short sprint. The shortest ever duration of exercise before onset was 2 minutes. The average duration of exercise before PED onset was 5 minutes. An attack was typically heralded by a twitching sensation of the feet and legs. Then her large toe would curl upwards, and her other toes would curl downwards. Her ankle would then invert and plantar flex. The dystonia was painful, caused skin abrasions, and made her stop activity. If she started walking again too soon, the dystonia would worsen. The whole event would last between 5 and 30 minutes. The attacks were always confined to one leg, and in the previous 10 years only the right leg had been affected. The attacks had occurred on almost every day from the age of 15 years, and were only ever precipitated by exercise. They were more common during stressful periods and when walking barefoot on rocks or sand. In addition, the attacks were more likely to occur in the evening.
In her 20s, she developed symptoms typical of RLS. She experienced an abnormal sensation in her legs as she was falling asleep, which was so bad she felt like ‘chopping her legs off’. It would last for an hour, and was relieved by leg movements. The RLS symptoms fluctuated in intensity since her 20s but occurred almost every night. She had to get out of bed on average two times per night to pace around. There were no daytime RLS symptoms. The RLS was exacerbated by stress and during pregnancy. In addition, she suffered from depression (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria) since the onset of the PED, including severe post-partum depression. She tried a number of anti-depressants, and has benefited from fluoxetine. She also has occasional classical migraine with visual aura. Her examination was normal, specifically there was no evidence of dystonia at rest, on posture, or on initiation of movements. There was no abnormality of tone or parkinsonism.
Case III:2 (index case)
The index case had a normal antenatal, perinatal, and early developmental history. His baseline motor, cognitive, and social interaction abilities were normal. He is a keen sportsman. His first attack occurred at the age of 8 years whilst walking home from school with his mother. His mother recognized the attack immediately as identical to her events. The attacks only occurred after many minutes or even hours of exercise. The attacks were never induced by getting up from a chair, or a short sprint. The shortest ever duration of exercise before onset was 3 minutes of strenuous rugby training. The usual duration of exercise before PED onset was 5 minutes. He has never had a sensory aura. During an attack, his toes and ankle would plantar flex associated with ankle inversion. The PED usually affected the left or right foot but could be bilateral. It never affected the arms or generalized. The dystonia was painful, and was partly relieved by active ankle dorsiflexion. The event lasted at least 3 minutes. The events occurred at least 2 to 3 times per week, all during exercise. There were no other neurological or psychiatric symptoms. Examination was normal, specifically there was no movement disorder at rest, on posture or on sudden movements.
Investigation
Induction of paroxysmal exercise induced dystonia
Both live family members (II:2 and III:2) were exercised with moderate paced walking barefoot (Video S1 supporting information). After 2 minutes of walking, case II:2 had a paroxysmal exercise induced dystonic event. The event was painful and relieved by active dorsiflexion. After 8 minutes of walking, case III:2 had a paroxysmal event affecting the left foot of similar characteristics to his mother.
CSF neurotransmitters and CSF examination of Case III:2
Cerebrospinal fluid (CSF) for microscopy, protein, lactate and glucose was normal. CSF glucose was 2.9mmol/L with a CSF/plasma ratio of 0.59.
CSF neurotransmitters were performed using a standard protocol.8 CSF homovanillic acid was 0.13μmol/L (normal 0.33-0.67), CSF 5-hydroxy indole acetic acid 0.06μmol/L (normal 0.11–0.22), CSF biopterin 10.2nmol/L (normal 25–45), CSF neopterin 4.9nmol/L (normal 7–29). All neurotransmitter values were low, suggestive of an autosomal dominant GCH-1 neurotransmitter disorder.8
Treatment trial
Case III:2
A treatment trial of 10mg/kg/day carbamazepine for 2 months produced no benefit. Subsequently, a treatment trial of l-Dopa (Madopar) at 62.5mg (50mg of active l-Dopa) given at 8am and midday stopped the events on the day of commencement. He has subsequently had no further dystonic events for 6 months on l-Dopa (Madopar) 125mg twice a day (6mg/kg/day).
Case II:2
After starting l-Dopa (Madopar) at 125mg (100mg of active l-Dopa) given at 8am and midday, the events have stopped. She has been able to take a 25 minute walk in the evening without an event for the first time in 25 years. Her restless legs symptoms are currently in remission.
Genetic screening
Patients gave informed consent for blood to be taken for DNA analysis. Sequencing of disease genes was carried out by standard polymerase chain reaction Sanger sequencing. Primer sequences are available on request. In view of the previous association of PED with Glucose transporter 1 (GLUT1) genetic mutations, we excluded a mutation in the GLUT1 gene.4
GTP-cyclohydrolase 1 mutation screening showed a heterozygous exon 1 c.411G>T mutation leading to a premature stop codon (p.E84X). The same mutation was found in both II:2 and III:2. The sequencing was repeated to confirm the results. The mutation has not been identified in over 200 UK control individuals. The mutated amino acid is highly conserved throughout species, suggesting an important role in the function of GCH1.
Discussion
The dystonia in this family was localized to the lower limb, and despite many years of the disease was only ever exercise-induced, and was never present at rest, on posture, or on initiation of movements. This family is therefore reminiscent of previous families with PED.1,3
Suls et al.4 have reported four families with PED, some with coexistent epilepsy who have genetic mutations in SLC2A1, the glucose transporter 1 gene (GLUT1). A further report by Weber et al.5 screened five families with PED, and identified GLUT1 mutations in three of the five families.
The family in this report was negative for GLUT1 mutations, but had a typical neurotransmitter profile and stop mutation in GCH-1 which provides a compelling argument that at least in some families PED is caused by mutations in GCH-1.
The fact that GCH-1 mutations cause PED is probably not surprising. GCH-1 mutations cause dopa-responsive dystonia (DRD) which often presents in childhood and typically involves the lower limbs.9 Unlike in this family, autosomal dominant GCH-1 deficiency is often phenotypically heterogenous both within families and between families.9,10 Factors that influence the phenotype include the age at onset, pathophysiological differences, and the specific mutation and its effect on the degree of tetrahydropterin and tyrosine hydroxlase deficiency.9,11 One interesting and unanswered question is why do members of this family have such a specific and persistent dystonic phenotype despite CSF neurotransmitters typical of ‘classical DRD’. Possible explanations may include a specific effect of this GCH-1 mutation (p.E84X) on the clinical phenotype (such as a destabilizing effect), or the presence of an associated gene that modifies the dystonia phenotype in this family.9,11
Segawa et al.9 have separated the DRD phenotypes into postural dystonia and action dystonia types, with differing pathophysiological pathways. PED would be an example of action dystonia, which is proposed to be associated with deficiency of tyrosine hydroxylase in the nigrostriatal dopaminergic neuron with consequent hypofunction of the subthalamic nucleus.9
Other than the association with PED, this report is notable because of the association with RLS. Case II:2 had symptoms that exacerbated in sleep and fulfill diagnostic criteria for RLS.12 Many patients with RLS gain symptomatic benefit from dopaminergic therapy. Trender-Gerhard et al.13 have recently reported restlessness as a common symptom in long term follow-up of DRD patients, although only 4/34 patients had exacerbation at night and would fulfill a diagnosis of RLS.
In conclusion, we provide an argument that GCH-1 screening should be undertaken in families with PED, especially if GLUT1 negative and additional features of monoamine deficiency are present such as parkinsonism (dopamine), migraine (serotonin), and depression (noradrenaline and serotonin).6,9,14,15
This report highlights the need to consider a dopa-responsive condition in an increasingly broad clinical spectrum of disease. GCH-1, along with GLUT1 should now be considered a genetic cause of familial PED.
What this paper adds
- •
GTP-cyclohydrolase 1 deficiency is a genetic cause of familial paroxysmal exercise induced dystonia.
- •
Restless legs syndrome is associated with GTP-cyclohydrolase 1 deficiency.




