Mitochondrial diseases in childhood: a clinical approach to investigation and management
Abstract
Mitochondrial diseases are a common cause of inherited neurological disorders in children. Although dysfunction of the central nervous system is prominent, multisystem involvement also occurs. Diagnosis relies on characteristic clinical features, an understanding of mitochondrial genetics, and a logical, informed approach to investigations. There is a significant body of recent literature on advances in mitochondrial genetics and the investigation of mitochondrial diseases. However, to our knowledge there remains a paucity of published information on the management of these disorders. Management of the complex constellation of neurological and multisystem clinical features is challenging, and is reliant on a multidisciplinary approach. The care of the child and family is dependent on clear communication between health professionals from primary, secondary, and tertiary care as well as specialist input from quaternary services. The aim of this review is to provide paediatric neurologists, paediatricians, and allied health professionals with a structured approach to the diagnosis and management of children with suspected or confirmed mitochondrial disease.
List of Abbreviations
-
- ATP
-
- Adenosine triphosphate
-
- COX
-
- Cytochrome c oxidase
-
- MELAS
-
- Mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes
-
- mtDNA
-
- Mitochondrial DNA
-
- nDNA
-
- Nuclear DNA
-
- POLG1
-
- Polymerase gamma gene
-
- SNHI
-
- Sensorineural hearing impairment
Mitochondrial diseases in children are increasingly recognized. They have been reported in an ethnically diverse population in the UK as the most common inherited neurometabolic disorder of childhood.1 True prevalence is difficult to ascertain, as mitochondrial disease is almost certainly underreported. However, epidemiological evidence suggests a birth prevalence of 1 in 7634 live births and a lifetime risk of developing mitochondrial disease of approximately 1 in 5000.2,3 Individual mutations are reported at much higher carrier frequencies, approaching 1 in 400, but only a small proportion of these individuals will develop disease.4,5 Although definitive treatment for these conditions remains elusive, the diagnosis is important for prognosis, genetic counselling, and supportive management of subsequent impairment. The aim of this review is to provide clinicians with a pragmatic clinical approach to diagnosis and management of these conditions in children.
Key concepts in mitochondrial pathophysiology
Historically, mitochondria have been considered as discrete, isolated intracellular organelles primarily responsible for the production of adenosine triphosphate (ATP). Although their essential role in synthesizing ATP is in no doubt, recent evidence suggests that their morphology is much more dynamic than previously suspected, with frequent fission and fusion events forming a diffuse reticular network of mitochondria within the cell.6 In the inner mitochondrial membrane, ATP is produced by oxidative phosphorylation. This process involves the transfer of electrons along the mitochondrial respiratory chain, four multi-subunit complexes (I–IV), through a series of oxidation and reduction reactions that ultimately result in the consumption of oxygen at complex IV. Essential to this electron transport are two ‘electron carriers’, ubiquinone (coenzyme Q10) and cytochrome c, which shuttle between the complexes. A consequence of this electron transfer is the extrusion of protons into the intermembrane space at complexes I, III, and IV. The proton gradient generated is then dissipated through complex V (ATP synthase), resulting in the condensation of adenosine diphosphate and inorganic phosphate to form ATP.7
Mitochondrial genetics
The mitochondrial genome is a small, circular double-stranded DNA molecule that encodes 13 polypeptides. These include the mitochondrial respiratory chain complexes and the RNA involved in intramitochondrial translation of these polypeptides. The mitochondrial genome is polyploidy, with multiple copies of mitochondrial DNA (mtDNA) within each cell. This results in complex transmission of mutations and genotypic–phenotypic variation in the presentation of mitochondrial diseases.
Complexes I, III, IV, and V are under the influence of both the mitochondrial and nuclear genomes, whereas complex II is entirely encoded by nuclear DNA (nDNA; Table I).7 Approximately 70 to 75% of the mitochondrial disorders that present in childhood are due to mutations in nDNA and follow Mendelian inheritance patterns, most often autosomal recessive. The nDNA mutations may be structural (i.e. coding for respiratory complexes) or non-structural, affecting mtDNA replication and repair, metabolism, and mitochondrial integrity.8 Of these latter, ‘non-structural’ genes, mutations in the mitochondrial polymerase gamma (POLG1) are by far the most common. Specific mutations in POLG1 cause a severe childhood disorder characterized by hepatic failure, developmental regression, and refractory epilepsy (Alpers–Huttenlocher syndrome) and are associated with depletion of mtDNA in the brain and liver. In adult life, other mutations in the same gene cause multiple deletions of mtDNA and a very different syndrome associated with chronic progressive external ophthalmoplegia, ptosis, myopathy, and ataxia. This variation in the genotype–phenotype relationship is a recurring theme in mitochondrial genetics.
| Complex | I | II | III | IV | V | |
|---|---|---|---|---|---|---|
| Enzyme activity | NADH–CoQ reductase | Succinate CoQ reductase | CoQ–cytochrome c reductase | Cytochrome c oxidase | ATP synthase | |
| Number | 39 | 4 | 10 | 10 | 12 | |
| Nuclear DNA encoded subunits | Nuclear mutations | NDUFS 1–8 NDUFV 1–2 B17.2L | SDH A–D | UQCRB BCS1L | SURF1 SCO1–2 COX10, 15 LRPPRC | ATPAF2 |
| Mitochondrial DNA encoded subunits | Number | 7 | 0 | 1 | 3 | 2 |
| Subunits | MTND1–6 MTND4L | MTCYB | MTCO1–3 | MTATP6 MTATP8 | ||
- NADH, nicotinamide adenine dinucleotide; CoQ, coenzyme Q.
Large-scale single deletions, rearrangements, and more than 250 different point mutations have been identified in mtDNA and account for 25 to 30% of paediatric mitochondrial disease.9,10 Although large-scale single deletions or rearrangements of mtDNA are rarely transmitted (estimated 1 in 24 risk)11 the same is not true for point mutations in mtDNA, where potentially all offspring may be affected.
Homoplasmy, heteroplasmy, and threshold
These concepts are unique to mitochondrial genetics and are directly relevant to the diagnostic process. Homoplasmy describes the situation where all the copies of mtDNA within the cell are identical. When only a proportion of the mtDNA molecules are affected by a mutation, then two species of mtDNA coexist, a situation known as heteroplasmy. This explains why disease does not occur in everyone with mtDNA mutations; in certain mutations, a ‘threshold’ level of mutated mtDNA is required before function is compromised and clinical consequences become apparent. This threshold may vary between individuals, tissue types, and specific mtDNA mutations. The concept of threshold does not pertain to homoplasmic mutations where some individuals have 100% mutated mtDNA and yet do not demonstrate clinical disease. This suggests other genotypic or environmental factors influence the expression of mitochondrial disease.
Mutations in mtDNA are almost universally maternally inherited, although a single case of paternal inheritance has been described.12 Interestingly, there may be a significant variability between siblings in the proportion of mtDNA they inherit: this is a direct consequence of a process known as the mitochondrial ‘genetic bottleneck’.13 Some mitochondria will contain higher levels of mutated mtDNA and these may, through chance, segregate together in a particular cell. When this process happens in primordial germ cells (a time when mtDNA replication is relatively slow), the resulting primary oocytes will harbour a range of levels of mutated mtDNA. The number of abnormal mitochondria incorporated into each oocyte during cell division may result in either an affected or an unaffected individual.
Spectrum of mitochondrial disease
Mitochondrial diseases are a heterogenous group of disorders in which the process of ATP production is disrupted. Cells with high-energy requirements such as neurons, skeletal and cardiac muscle are particularly vulnerable to this limited ATP supply and feature prominently in the various mitochondrial phenotypes. Classification relies on accurate clinical, biochemical, and genetic information and may be based on either genotype or phenotype, but there is significant overlap (Table II). For example, Leigh syndrome may occur because of several different nuclear or mitochondrial DNA mutations; whereas mutations in a particular gene, such as POLG1, may cause multiple clinical syndromes.14
| Syndrome | Common genotype | Age at onset | Clinical features |
|---|---|---|---|
| Mitochondrial DNA mutations | |||
| Pearson syndrome | Large-scale deletion/rearrangement | Early childhood | Sideroblastic anaemia, pancytopenia, exocrine pancreatic failure, renal tubular defects. Survivors develop Kearn–Sayres syndrome. |
| Kearn–Sayres syndrome (KSS) | Large-scale deletion/rearrangement | Later childhood | Progressive ophthlamoplegia, pigmentary retinopathy, raised cerebrospinal fluid protein, cerebellar ataxia, cardiac conduction defects. Less common features include SNHI, myopathy, dysphagia, diabetes mellitus, hyperparathyroidism, dementia. |
| Chronic progressive external ophthlamoplegia (CPEO) | Large-scale deletion/rearrangement. Point mutation mtDNA (m.3243A>G) | Adolescence/early adult | External ophthalmoplegia and bilateral ptosis. Other features may include proximal myopathy, cardiac conduction defects, dysphagia. |
| Maternally inherited Leigh syndrome (MILS) | Point mutation mtDNA | Infancy and early childhood | Sub-acute or acute encephalopathy, lactic acidosis, seizures, stepwise neurodevelopmental regression, cerebellar and brainstem dysfunction. |
| Mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS) | Point mutation mtDNA (>80%MTTL1 gene) | Child or adulthood | Stroke-like events: clinical and radiological signs, seizures. Other features include diabetes mellitus, cardiomyopathy, SNHI, pigmentary retinopathy, cerebellar ataxia. |
| Myoclonic epilepsy with ragged red fibres (MERRF) | Point mutation mtDNA (MTTK) | Child or adult | Myoclonus, seizures, cerebellar ataxia and myopathy; other features: dementia, optic atrophy, bilateral SNHI, peripheral neuropathy, spasticity, multiple lipomata. |
| Neurogenic weakness with ataxia and retinitis pigmentosa (NARP) | Point mutation mtDNA (MTATP6/MTATP8) | Late child or adult | Peripheral neuropathy, ataxia, pigmentary retinopathy. |
| Leber’s hereditary optic neuropathy (LHON) | Point mutation mtDNA (MTND1/MTND4/MTND6) | Adult (third to fourth decade) | Most common mtDNA disorder. Optic neuropathy with sub-acute bilateral deterioration in vision. Occasional extra-ocular manifestation: cardiac conduction defects, dystonia with associated basal ganglia degeneration. Ninety-five per cent of patients harbouring one of three common point mutations: m.11778G→A, m.3460G→A or m.14484T→C. |
| Nuclear gene mutations | |||
| Multisystem neonatal onset disease with lactic acidaemia | COX10, COX15, SCO2, SCO1 | Neonatal | Encephalopathy, myopathy, renal tubular dysfunction, hepatic dysfunction with lactic acidosis. |
| GRACILE syndrome | BCS1L | Infancy | Growth retardation (may be intrauterine), amino aciduria, cholestasis, iron overload, and lactic acidosis. |
| Alpers–Huttenlocher syndrome/hepatoencephalopathy | POLG1, DGOUK, MPV17, EFG1 | Infancy and early childhood | Encephalopathy, aggressive seizures, spasticity, hepatic dysfunction. |
| Myopathy. Myoencephalopathy with or without renal tubulopathy | TK2 RRM2B | Infancy and early childhood | Progressive myopathy, ophthalmoplegia renal tubular defects, hepatic dysfunction. |
| Sporadic Leigh syndrome | Point mutation mtDNA; SURF1; NDUFS2 | Infancy and early childhood | Developmental delay and stepwise regression associated with acute episodes of encephalopathy. Dystonia, brainstem dysfunction, lactic acisosis. |
| Barth syndrome | TAZ (G4.5) | Infancy/early childhood | Cardiomyopathy, skeletal myopathy, growth failure, hypotonia, and neutropenia. |
| Mitochondrial neurogastrointestinal encephalopathy (MNGIE) | ECGF1 | Late childhood/adolescence | Chronic progressive ophthalmoplegia, ptosis, gastrointestinal dysmotility, leukoencephalopathy, peripheral neuropathy, and myopathy. |
- SNHI, sensorineural hearing impairment; mtDNA, mitochondrial DNA.
Clinical presentation
Mitochondrial disease may present at any age, with a spectrum of symptoms and signs, to several medical specialties (Fig. 1). Myopathy is the feature that mitochondrial disease is probably best known for; most often this presents as part of a multisystem disease, only rarely being reported as a lone clinical manifestation.15
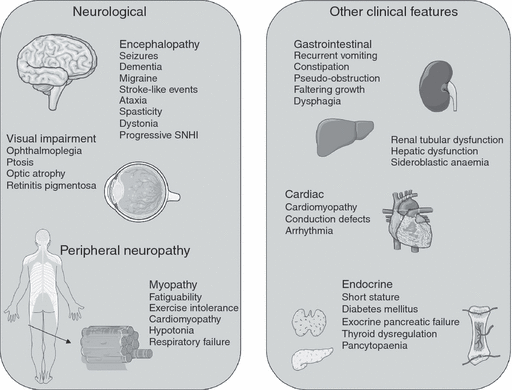
Clinical presentations of mitochondrial diseases. SNHI, sensorineural hearing impairment.
The most common childhood-onset mitochondrial disease is Leigh syndrome. This presents in infancy or early childhood and is characterized by developmental delay and regression. Most commonly, the child has a stepwise regression associated with an acute encephalopathic illness and a combination of signs, which may include dystonia, hypotonia, ataxia, nystagmus, optic atrophy, dysphagia, and central respiratory dysfunction. There is often a partial recovery between acute episodes. Magnetic resonance imaging (MRI) demonstrates necrotic lesions in the brainstem and basal ganglia. In a Spanish case series of 51 children with mitochondrial respiratory chain disorders, 18% presented clinically with Leigh syndrome but 31% demonstrated phenotypes not previously reported in association with mitochondrial disease.16 Some infants present with a severe multisystem disease associated with lactic acidaemia, hypotonia, weakness, failure to thrive, and intestinal dysmotility. In others, the multisystem involvement is only apparent after many years.
A single organ may be predominantly affected, for example the eye in Leber’s hereditary optic neuropathy. In others, a characteristic combination of unrelated organs is the clue to the diagnosis. For example, the pancreas and cochlea may present as diabetes mellitus and deafness, a phenotype associated with point mutations and deletions in mtDNA.
The diagnosis of mitochondrial disease, therefore, should be considered in all children who present with neurodevelopmental regression or acute/sub-acute neurological signs and symptoms (e.g. encephalopathy, stroke like events, epilepsy) in association with other system disorders, including eye disorders sensorineural hearing impairment (SNHI), and cardiovascular or gastrointestinal disturbance. Clinicians should have a high index of clinical suspicion, in particular when there is a history of neurodevelopmental deterioration associated with intercurrent illness. Clinical and laboratory diagnostic criteria have been developed to assist diagnosis of mitochondrial disease10 and applied to children.17,18 The clinician must remain vigilant to other diagnoses. Both congenital disorders of glycosylation and guanidoacetate methyltransferase deficiency may present with multisystem disease that includes neurological or neurodevelopmental impairment. Secondary respiratory chain deficiencies may also be present in guanidoacetate methyltransferase deficiency.19
Prognosis
Although the prognosis for mitochondrial disease is dependent on the underlying diagnosis, there is large phenotypic variation. In a case series of 113 paediatric patients with confirmed mitochondrial disease, the most significant prognostic factor for mortality was cardiac involvement (in 40%). Those children with cardiomyopathy had an 18% survival rate compared with those with no cardiac involvement, who had 95% survival at 16 years.20
Investigation of mitochondrial diseases
There are no standard guidelines for the investigation of mitochondrial disease.21 Investigation is determined by clinical presentation and exclusion of a broad differential diagnosis. No single investigation, except identification of the specific gene mutation, is sensitive or specific to mitochondrial disease. Discussion with a clinician who has expertise in mitochondrial disease is advised in order to guide investigation, in particular with respect to laboratory facilities and handling of specimens.
Clinical and laboratory investigation
Biochemical investigations
Although high serum and cerebrospinal fluid lactate supports a diagnosis of mitochondrial disease, these lack sensitivity and specificity; some patients with confirmed mitochondrial diseases have normal serum and cerebrospinal fluid lactate.21–25 Measurement of serum lactate may be confounded by difficulties with venepuncture in young children, and borderline to high levels should be viewed accordingly and repeated. Some mitochondrial diseases are more likely to demonstrate high lactate (neonatal renal Fanconi syndrome), whereas other conditions (chronic progressive external ophthlamoplegia) rarely ever demonstrate elevated lactate.23 As with lactate, organic acid abnormalities associated with mitochondrial disease are variable and lack sensitivity and specificity.26 Elevation of urinary fumarate and malate have been identified in mitochondrial disease.27 Ethyl-malonic acid and methyl-glutaconic acid may be elevated but tend to be high in infants owing to renal immaturity.21 However, some organic-acid profiles, together with the appropriate clinical features, are helpful in making a diagnosis. Elevation of 3-methyl-glutaconic acid in a young male with hypotonia, cardiomyopathy, and neutropenia is indicative of Barth syndrome; this should be confirmed by analysis of cardiolipins on a blood spot followed by genetic studies of G4.5 (TAZ).
Imaging
Computed tomography imaging is appropriate in an acute setting, to rule out raised intracranial pressure in a child who presents with seizures or encephalopathy and can identify basal ganglia calcification that can occur in mitochondrial disease. MRI, however, is the most likely available imaging modality to assist in the diagnosis of mitochondrial disorders.
MRI findings are heterogeneous; they depend on metabolic brain defects, age of the patient, and stage and severity of the disease.28 Specific MRI findings have been identified in patients with syndromic phenotypes. These often include involvement of cortical and sub-cortical grey matter, basal ganglia, dentate, brainstem grey matter and collicula and, less commonly, the thalami. Abnormalities of the white matter are characteristic of some forms of mitochondrial disease, for example patients with Kearns–Sayre syndrome or mitochondrial neurogastrointestinal encephalopathy. MRI lesions associated with the stroke-like episodes of mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS) may be permanent or reversible. Lesions are typically asymmetrical, multifocal, and high signal, often occurring in the occipital and parietal lobes. They mimic ischaemic infarcts but do not correspond to vascular territory nor is there an abnormal magnetic resonance angiogram. The oedema may resolve or leave an area of scarring or atrophy. In Alpers–Huttenlocher syndrome, MRI findings include atrophy of the grey and white matter, with cortical thinning and delayed myelination. A disproportionate involvement of the occipital lobes has also been reported as characteristic.28–31
Where available, proton magnetic resonance spectroscopy can be a useful adjunct in making a diagnosis, demonstrating an impairment of oxidative metabolism as an accumulation of lactate, present as a doublet peak at 1.3 parts per million. An accumulation of N-acetyl-aspartate is also commonly seen.28 Although 31P-magnetic resonance spectroscopy of brain, and more particularly skeletal muscle, is proving a useful research tool in investigating energy metabolism in patients with mitochondrial disease, its sensitivity as a diagnostic test remains relatively poor.32
Neurophysiological testing
The electroencephalogram in mitochondrial disease. The range of abnormalities seen on electroencephalogram (EEG) in mitochondrial disease is almost as broad as the range of clinical phenotypes. With the exception of Alpers–Huttenlocher syndrome, the changes are not pathognomonic, and the use of EEG is more in directing management than in reaching a diagnosis. In Alpers–Huttenlocher syndrome the EEG typically shows high amplitude and slow activity, with superimposed polyspikes of lower amplitude found predominantly over posterior regions. Between regions of slow wave activity, there is relative flattening of the EEG.33
During episodes of encephalopathy in MELAS, generalized slow wave discharges are seen on the EEG. When complicated by a stroke-like episode, focal epileptiform discharges appear in the region of the lesion. Except for the unusual location (typically posterior brain regions), there is nothing to distinguish it from other causes of a focal epileptic discharge.34 Patients with the myoclonic epilepsy with ragged red fibres syndrome typically show progressive slowing of background rhythms with symmetrical posterior polyspike and wave complexes often related to myoclonic jerks. Photosensitivity is common. In some patients with a severe progressive syndrome, a progression to severe encephalopathy with repeated episodes of focal motor status is seen. In these patients the EEG shows multi-focal quasi-periodic sharp waves or generalized polyspike and slow wave complexes, again with photic enhancement.35 These EEG features follow a similar progression to that seen in other causes of progressive myoclonus.
Neuropathy in mitochondrial disease. In contrast to the wide range of EEG abnormalities, the findings on peripheral neurophysiology are relatively straightforward. Most patients have a length-dependent axonal type neuropathy, affecting motor and sensory fibres. Studies of nerve conduction show reduced amplitude motor and sensory responses with relatively preserved conduction velocity and evidence of neurogenic change in distal muscles on electromyography. This is particularly evident in neurogenic weakness with ataxia and retinitis pigmentosa.36 An exciting recent development is the finding that one form of hereditary neuropathy, Charcot–Marie–Tooth type 2A, has been shown to be due to mitochondrial dysfunction. This dominantly inherited, axonal motor and sensory neuropathy is caused by mutations in mitofusin-2, a nuclear gene that regulates mitochondrial fusion and fission and tethers mitochondria to the endoplasmic reticulum.37,38
Tissue biopsy
Measurement of the activity of the respiratory chain enzymes in tissue samples (skeletal muscle, liver, and cardiac muscle) can guide molecular diagnostic testing according to the respiratory chain complex affected. Selecting the appropriate tissue (Fig. 2) in which to investigate mitochondrial disease is of the utmost importance if a diagnosis is to be confirmed. An affected, accessible tissue such as muscle is usually best in the index case, but in cases of hepatocerebral depletion, where sequencing of POLG1 is negative, then liver biopsy may be more appropriate. When the clinical features of MELAS or maternally inherited diabetes and deafness suggest that the m.3243A>G mutation is responsible, this can be investigated with a sample of urinary sediment (epithelial cells). The same technique can be applied for non-invasive screening of maternal relatives. Blood is not an appropriate tissue with which to perform this analysis (or that of many other mtDNA mutations and deletions) because the level of mutant heteroplasmy is often very low and may not be detected.
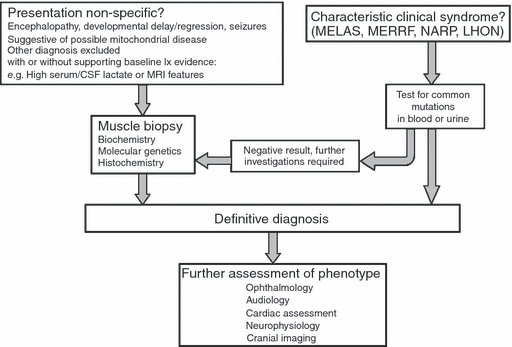
Investigation of mitochondrial diseases. MELAS, mitochondrial encephalopathy with lactic acidosis and stroke-like episodes; MERRF, myoclonic epilepsy with ragged red fibres; NARP, neurogenic weakness with ataxia and retinitis Diamentosa; LHON, Leber’s hereditary optic neuropathy.
Skin biopsy. This is the least invasive method of tissue sampling. Skin fibroblasts may be stored indefinitely to provide a renewable source of DNA from the patient for future diagnostic testing. Clinicians must be aware that, unfortunately, owing to mitochondrial heteroplasmy, enzyme activity may be normal in cultured skin fibroblasts yet abnormal in skeletal muscle, producing false negative results.
Muscle biopsy. Histological evidence of mitochondrial proliferation in the sub-sarcolemmal space on staining for nicotinamide adenine dinucleotide tetrazolium reductase (NADH-TR) and succinate dehydrogenase activities is a common finding in childhood mitochondrial disease, but classically described ragged red fibres are unusual.20 Sequential staining of a sample for cytochrome c oxidase (COX) and succinate dehydrogenase activity can identify individual muscle fibres with COX-deficient activity. This histology can identify mitochondrial disorders in muscle heteroplasmy, where individual abnormal fibres may be seen despite normal serum biochemical analysis of COX activity.
Enzyme activity for the respiratory chain complexes can be assessed in mitochondria isolated from muscle tissue or cultured cells by spectrophotometry. Complexes are studied in isolation or in linked assays I+III or II+III. Complex V (ATP synthase) activity can be assayed on fresh skeletal muscle, but given the distance that many muscle biopsies travel to diagnostic laboratories, this test is often not possible. Isolated or multiple deficiencies of the mitochondrial respiratory chain complexes can be identified by using spectrophotometry, but it is worth noting that poorly prepared samples or deficiencies of other enzymes, such as pyruvate dehydrogenase, may lead to secondary respiratory chain deficiencies.
Coenzyme Q10 may be measured in blood; however, quantification in muscle biopsy is more sensitive and particularly associated with complexes I+III and II+III dysfunction. This may guide further diagnostic investigation for mitochondrial disease and treatment, in particular, a trial of supplementation.39
Management of mitochondrial diseases
Acute management
Acute presentations of paediatric mitochondrial disease include encephalopathy, seizures, and stroke-like events. The mainstay of management is supportive and specific to the presenting symptoms and signs. In an acute deterioration the child with known mitochondrial disease may require repeat imaging, EEG, or biochemical investigations to identify disease progression, predict prognosis, and determine acute management. Supportive treatment includes aggressive seizure management, sedation, and intensive care. Lactic acidosis is often corrected with simple fluid resuscitation though bicarbonate may sometimes be helpful. Although dichloroacetate has been demonstrated to reduce serum lactate, its use has not been associated with improved neurological outcome,40 and the association with peripheral neuropathy41,42 precludes daily administration in children.
Infection should be sought and treated promptly as it may be a precipitating factor for the acute deterioration or a treatable differential diagnosis. Stroke-like events should be managed supportively and evidence of focal seizure activity sought on EEG. Prompt anticonvulsant treatment may reduce the extent and duration of functional impairment associated with these ‘metabolic infarcts’. There is some preliminary evidence in small-scale trials that acute intravenous infusion of L-arginine may be of benefit in reducing the severity of stroke-like episodes and that chronic daily administration may decrease the frequency of adverse events.43 Therapy with l-arginine may prove beneficial but further study is required in larger cohorts. Given the cardiomyopathy and arrhythmias associated with many mitochondrial diseases, the possibility of cardio-embolic stroke should not be dismissed, and MRI with magnetic resonance angiography should be performed to exclude a true vascular event requiring urgent intervention.
Less acute presentations of mitochondrial disease in children, such as developmental delay, regression, seizures, or SNHI, allow more time for clinical assessment, planned investigation, and therapeutic input. Most children present with a variety of non-specific features including failure-to-thrive, hypotonia, recurrent vomiting, and abnormal movements or posture.
Long-term management
When a diagnosis of mitochondrial disease is made, or suspected, the clinician should be vigilant for asymptomatic involvement of other organ systems. This is important as prophylactic treatment with angiotensin-converting enzyme inhibitors and β-blockers may prevent progression of cardiomyopathy, while early intervention with hearing aids can promote language development. Early assessment of eye movements, ptosis, and visual acuity by an experienced ophthalmologist can be helpful in planning surgery, providing suitable aids, and registering the patient as visually impaired.
Central to the long-term management of mitochondrial disease is the understanding that the symptoms and signs are progressive and that the disease course is unpredictable. Level of functioning may vary on a daily basis. Carers, teachers, therapists, and the affected child often need continually to adjust their approach, treatment strategies, and individual management aims. Clinicians should discuss this with the child and their family and communicate effectively with allied health professionals. Educational placement and requirements will need regular review.
Neurological complications
Postural support and management of motor disorders. Progressive proximal myopathy involving both hip and shoulder girdle muscles is the most common form of myopathy. This may result in a waddling gait and excessive lumbar lordosis (or scoliosis) and has an impact on posture management and mobility, particularly regarding stairs. Another feature of muscle involvement is rhabdomyolysis. This is often provoked by unaccustomed exercise and accompanied by muscle pain and stiffness with typically dark urine secondary to myoglobinuria. Repeated or severe episodes may compromise renal function, and attacks may be life-threatening.
Dyskinesia, dystonia, and spasticity also contribute to postural difficulties. Oral antispasticity agents can be used and intrathecal baclofen may be considered.
Postural management and physiotherapy is paramount. Goals will vary depending on the severity and aetiology of the motor disorder. In a non-ambulant child, adequate 24-hour postural support for seating and sleep position is essential to maintain hip and spine integrity and for activities of daily living such as feeding, access to play, and education.
In an ambulant child, maintenance of walking may be a priority in early life. However, if progression of myopathy or spasticity is such that the child’s participation is impaired, wheelchair mobility may significantly improve quality of life. Environmental impact should also be considered, especially with a change in physical demands, for example transition between primary and secondary education. Limited walking ability may not be adequate for access, even if the child’s clinical condition has not deteriorated.
Maintenance of joint mobility in ambulant and non-ambulant children is also essential. Orthotic support and stretching will be required, with regular assessment and physiotherapy. Rigid orthoses may be uncomfortable and poorly tolerated in children with dystonia rather than spasticity.
The ability in adult life to stand and transfer has significant implications for independent living, removing the need for hoisting. This requires not only weight-bearing ability but also adequate range of movement at the ankles, knees, and hips. Early referral to spinal or orthopaedic surgeons is advised if there is evidence of hip dysplasia or scoliosis.
Epilepsy. Epilepsy is a common presentation, either as a mitochondrial epileptic encephalopathy or in association with a specific syndrome, for example myoclonic epilepsy with ragged red fibres syndrome. The epilepsy phenotype is variable: often there is a combination of generalized and focal seizures including myoclonic seizures, tonic-clonic seizures, or infantile spasms.44 Seizures are sometimes refractory to multiple anticonvulsants, especially if they are early in onset, as in Alpers–Huttenlocher syndrome.
Sodium valproate should be avoided for risk of hepatic dysfunction, particularly in children with POLG1 mutations or Alpers–Huttenlocher syndrome,45 but also in other mitochondrial disorders. Carbamazepine or levetiracetam are reasonable first-line anticonvulsants.
The introduction of a ketogenic diet has been reported as safe and effective; however, close clinical supervision is required owing to the potential risk of metabolic decompensation.46
Hearing. Hearing impairment has been reported in 42% of children with mitochondrial disorders and is progressive.47 In this case series, all the children in whom SNHI was identified had onset of other neurological symptoms at the same age or before hearing impairment was identified. The severity or rate of progression was not related to other disease symptoms. The underlying pathophysiology is a combination of both cochlear and central auditory pathway dysfunction. Higher-frequency sounds are initially lost. This is believed to be because the outer hair cells of the cochlear have higher metabolic demand, hence they are more susceptible to mitochondrial dysfunction.47 Investigation includes assessment of hearing including audiogram, oto-acoustic emissions, and auditory brainstem responses. MRI identifies central lesions in the auditory pathway. Local practice ensures audiological assessment at presentation and reassessment of hearing according to clinical concern. Carers and professionals should be aware of the potential for deterioration in hearing and any concerns should trigger prompt assessment. Simple amplification with hearing aids is often sufficient to manage hearing loss. Cochlear implantation has been successful in the management of individuals with cochlear dysfunction who have not responded to amplification devices.48
Vision. Ocular complications of mitochondrial disease include optic neuropathy, retinal dystrophy, ophthalmoplegia, ptosis, and cortical visual impairment. Clinical experience of cortical visual impairment after encephalopathy is seen commonly in Leigh syndrome. Assessment of functional vision in a child with cortical visual impairment is challenging, especially if the child has communication, motor, or other cognitive impairment. Assessment should be multidisciplinary and include an ophthalmologist, optician, orthoptist, and a specialist teacher for the visually impaired. There should be adequate time for detailed clinical evaluation and the interpretation should be discussed with respect to the child’s cognitive, communication, and motor abilities. Educational advice should be clear in terms of level of visual acuity, a field defect, or ophthalmoplegia, especially in relation to seating position in the classroom. Advice on promoting cortical visual function by use of multi-sensory toys and reinforcement with auditory and tactile stimulation should also be specified.
Non-neurological complications
Cardiology. Cardiomyopathy has a significant impact on the prognosis of children with mitochondrial respiratory chain disease. Symmetrical, biventricular, hypertrophic cardiomyopathy is predominant, although some children develop dilated cardiomyopathy.20,49 The cardiomyopthy may precede the diagnosis of mitochondrial disease. Conduction defects are common and may be the cause of mortality before being symptomatic. Successful use of pacemaker devices and cardiac transplantation has been reported.49
Barth syndrome is an X-linked recessive condition caused by mutations in the taffazin gene (TAZ, G4.5),50 which results in an overall reduction in the concentration and altered composition of cardiolipin, the mitochondrial phospholipids.51 Patients have variable clinical features but cardiomyopathy, skeletal myopathy, growth failure, hypotonia, and neutropenia are prominent. As a mitochondrial disease, it is rather unusual in that aggressive early management may not only avoid cardiac transplantation, but also improve the long-term outcome and survival into adult life.52
Referral for cardiology assessment should be made at presentation and follow-up arranged accordingly. Cardiac symptoms should be enquired about at every review. No specific treatment or clinical guidelines have been recommended in the management of cardiomyopathy in mitochondrial disease.53
Nutrition and gastrointestinal complications. Nutritional complications occur in up to 15% of patients with mitochondrial disease.54 They may be a direct consequence of mitochondrial dysfunction within the gastrointestinal tract (e.g. mitochondrial neurogastrointestinal encephalopathy, or Pearson syndrome)53,54 where neuromuscular dysfunction affects gut peristalsis, or secondary to central neurological impairment.54,55 Maintaining nutrition in children with mitochondrial disease can be a significant challenge.
Bulbar dysfunction may be due to an upper motor neuron problem or to myopathy. It can be acute or slowly progressive. In an acute encephalopathy or intensive-care setting, supplemental enteral tube feeds or even parenteral nutrition may be required. Parenteral nutrition can be associated with liver dysfunction or diabetes. During initial recovery, swallowing may improve sufficiently for oral feeding; however, ongoing speech and language therapy and dietetic assessment is required. Slowly progressive dysphagia may not be easily demonstrated on clinical assessment. Videofluoroscopy is a helpful adjunct although the study may not represent the usual feeding pattern. Specific enquiry about the duration of feeds and any tiring towards the end of meals is essential. Feeding strategies should be sensitive to the wishes of the child and family but should ensure adequate nutrition to meet energy requirements. Early supplemental gastrostomy feeding is advocated to maintain calorie intake.53 Dysphagia may result in recurrent aspiration; management includes gastrostomy insertion with consideration of fundoplication if there is clinical gastro-oesophageal reflux. Dysmotility may result in gastro-oesophageal reflux, delayed gastric emptying, constipation, and pseudo-obstruction; these should be managed symptomatically.
Respiratory management. The aetiology of respiratory complications of mitochondrial disease is multifactorial. Predisposition to infection and respiratory compromise are the two main complications. Posture (including spinal deformity), proximal myopathy, and movement disorders secondary to neurological impairment may result in a restrictive pattern of respiratory compromise. Respiratory function should be monitored if there is significant myopathy or respiratory muscle weakness. Central respiratory dysfunction occurring in Leigh syndrome may result in recurrent apnoeas. A careful history will identify nocturnal hypoventilation secondary to myopathy, posture, or central respiratory dysfunction (irregular breathing patterns, sighs, hiccoughs, and witnessed apnoea). Such symptoms warrant close monitoring and investigation including liaison with a respiratory paediatrician. Sleep studies, overnight saturation monitoring, or airway examination under anaesthetic may be indicated. Some patients require nocturnal ventilatory support. Reduced ability to clear secretions and poor cough increase susceptibility to infection. History of recurrent respiratory infection should alert the clinician to risk of aspiration. Pneumococcal vaccination, annual influenza vaccination, and prompt treatment with antibiotics and chest physiotherapy if signs of infection are present are essential. Recurrent chest infections may benefit from prophylactic antibiotics. Cardiomyopathy may present as respiratory compromise.
Multisystem management. Some mitochondrial diseases (e.g. Pearson, Kearns–Sayre, and Barth syndromes) may present with endocrinopathies, short stature, or renal or haematological abnormalities.53–58 Regular clinical examination with attention to growth parameters is required. Baseline investigation including electrolytes, liver function tests, and haematological indices are usually completed at presentation and repeated according to clinical progress. Management of identified complications should be discussed with appropriate specialists. Exogenous insulin is usually required in the management of diabetes mellitus. The use of growth hormone therapy in short stature or demonstrated growth hormone deficiency in mitochondrial disease has been debated. Case reports demonstrate variable response and there has been concern about deterioration in clinical condition.59,60
Other considerations
General anaesthesia. General anaesthesia is often required in the investigation and management of children with mitochondrial disorders. Increased metabolic demand associated with general anaesthesia theoretically poses a risk in children with mitochondrial disease, and case studies of adverse reactions with metabolic decompensation after general anaesthesia have been reported.61 Patients with myopathy sometimes have increased sensitivity to neuromuscular blocking agents, and those with cardiomyopathy may be at risk of conduction abnormalities. Many children with suspected mitochondrial disease will have a brief general anaesthesia for muscle biopsy during investigation, but significant anaesthetic complications are not commonly reported. A recent case review study of 38 children with mitochondrial disease who underwent 58 general anaesthesias was reassuring. Only three adverse events were reported, of which only one was a metabolic decompensation and occurred 12 hours after the general anaesthesia. The authors suggest clinical assessment, care with preoperative fasting, and routine use of lactate-free intravenous fluids perioperatively, but that there need not be any other variations from usual anaesthetic practices.62
Vitamin supplementation. A variety of vitamin supplements have been used in mitochondrial disease. The physiological basis is to remove toxic metabolites or promote ATP production by using electron-transport chain mediators, bypassing the metabolic defect. Multiple case reports and small randomized controlled trials have been published; although evidence of benefit is generally lacking for any supplementation, there have been no significant harmful effects from treatment.63 Frequently used supplements include coenzyme Q10. This has demonstrated a reduction of lactate and pyruvate, with clinical benefit reported in some small trials, but has not shown sustained improvement in long-term outcome.53 However, patients with primary ubiquinone deficiency do appear to benefit from high-dose (5mg/kg/d) ubiquinone-replacement therapy64 as do those with Friedreich ataxia when this therapy is combined with vitamin E supplementation.65 Riboflavin has also been shown to be of benefit in some individuals with complex I and complex II deficiencies.66,67 A trial of supplementation for 3 to 6 months is often prescribed then discontinued if there are adverse effects or no clinical benefit.
Coordination of clinical care
As for all children with complex neurodisability, many professionals are involved in coordinating care. Communication is essential, which can be challenging in the management of mitochondrial disease, where there may be input from quaternary, tertiary, secondary, and primary care. In the UK there are three quaternary centres (Newcastle, Oxford, and London) funded by the National Commissioning Group, that offer clinical and diagnostic support (Fig. 3).

Coordination of care for children with mitochondrial diseases. NCG, National Commissioning Group.
Community, therapy, and educational support
Service provision remains predominantly from primary and secondary care. Accurate information about the child’s diagnosis, prognosis, and current level of functioning must be communicated from the tertiary or quaternary service effectively, including training and support for local professionals. After acute admission, either at diagnosis or a significant deterioration in condition, to a tertiary centre, liaison with local services must be considered early to allow adequate discharge planning. Funding, equipment, home adaptation, therapy, carer support, and assessment for educational provision may take several months to organize.
Speech and language therapy
The child may have both communication difficulties and dysphagia. Cognitive function may be well preserved despite significant oromotor dysfunction, motor impairment, or SNHI. Provision of low- and high-technology communication aids may be required. Training for the child and their carers in using these is essential. For a child with SNHI, training in Makaton or British Sign Language may be required. Choosing appropriate communication aids may be further complicated by visual and motor impairment. Oral motor skills and swallowing must be regularly reviewed. Feeding advice, including postural management at meal times and food textures to be offered, should be regularly updated for carers and professionals, especially in respite and school settings.
Physiotherapy
Postural management has previously been discussed. Both muscle strengthening exercises and stretching to maintain joint range of movement are key goals. There has been specific consideration in mitochondrial disease to the concept of ‘mitochondrial training’. Endurance training can maximize the numbers of functional mitochondria in skeletal muscle. Small case series have demonstrated the benefit of resistance training on increasing muscle strength and improving muscle oxidative capacity in patients with mitochondrial myopathy caused by heteroplasmic large-scale deletions of mtDNA.68–70
Occupational therapy
The occupational therapist must be prepared for prompt alteration of provision at short notice. Discharge planning and home adaptation are key, as is ensuring adaptations to allow the child to have access to school. Training carers in equipment and planning activities of daily living in the home and educational setting is always required. Discharge planning from tertiary to local care relies upon good communication between therapists and social services, especially for financial support.
Educational support
Individualized educational support with awareness of functional ability from a cognitive, physical, and sensory perspective is mandatory. Where resources allow, detailed neuropsychology input is valuable. Carers and teachers must be aware of specific management plans for the child if they become acutely unwell.
Family support
Stressors on the family should not be underestimated. Not only are there emotional demands of having a child diagnosed with a chronic condition, there are also significant practical implications: travelling long distances between home, school, and hospitals, caring for siblings, loss of financial earnings, and learning to care for a physically and cognitively disabled child, who may have dual sensory impairment. If there has been a significant neurological deterioration, the family may grieve the loss of their child as they previously knew him/her. The burden of outpatient appointments and professionals making assessments in the home or school setting is considerable. Nurse specialists and community nursing staff play a central part in the family’s care. Social services, dieticians, and respite carers may all also be involved. Local sensory support services should offer advice to education and carers (see Table SI, supplementary material published online). Allocation of a key worker can help to prioritize and coordinate appointments and facilitate communication between professionals.
With the heterogeneity of mitochondrial disease, variable clinical presentation, unpredictable course, and (often unknown) prognosis, it would be impossible to suggest standard advice. Care packages, therapy, and educational support must be individualized and subject to frequent review.
End-of-life issues are always complex. Particular challenges in mitochondrial disease include unknown definitive diagnosis, variable phenotypic prognosis, and episodes of significant acute deterioration with a variable outcome. Decisions should be made in accordance with national guidelines for withholding and withdrawal of care in children after extensive discussion with all clinicians, therapists, and family members.71 Decisions about appropriate levels of care must be regularly reviewed.
Genetic counselling
Genetic advice depends on the underlying genetic diagnosis. In the 75% of children who have nDNA defects, families can be counselled for a recurrence risk of 1:2 or 1:4, according to dominant or recessive inheritance. In mtDNA mutations, however, counselling becomes more complex. Deletions in mtDNA generally occur de novo. Point mutations and duplications are more commonly transmitted and inherited maternally; however, owing to heteroplasmy, the mother may not display clinical symptoms and may have a low mutation rate in her blood. There will be a variable amount of abnormal mtDNA transmission, resulting in heterogeneity of both the genotype and phenotype between siblings. Some mitochondrial mutations have been identified where the risk of affected offspring is related to the percentage of mutated mtDNA in maternal blood.72
Prenatal diagnosis also becomes complex in the context of heteroplasmy. Chorionic villous sampling is unreliable for heteroplasmic mtDNA mutations.73 Oocyte donation was the only definitive method available in the UK to prevent transmission, but pre-implantation diagnosis of embryos after in vitro fertilization has very recently become available. Nuclear transfer of an adult somatic cell into an enucleated oocyte has been successful in animal studies and may eventually be extended to humans.74
Conclusion
This review summarizes the clinical presentation, investigation, and management of mitochondrial disorders, an increasingly recognized and heterogenous group of childhood diseases. Understanding mitochondrial genetics and the principles of investigation allows a pragmatic approach to investigation and diagnosis. To our knowledge, literature on the management of mitochondrial disease in childhood is limited and this review reflects local clinical experience. All professionals and carers should appreciate the variation in prognosis, clinical phenotype, and the potential for significant clinical change without warning in children with a mitochondrial disease.
What this paper adds
- •
A current overview of paediatric mitochondrial disease from a clinical perspective
- •
A structured approach to the investigation of suspected childhood mitochondrial disease
- •
Current advice on management of children with mitochondrial disease from a nationally commissioned mitochondrial disease centre




