Predictors of severe hepatic steatosis using abdominal ultrasound in HIV-infected patients
Abstract
Objective
Multiple factors may lead to hepatic steatosis (HS) in HIV-positive patients. HS may result in severe liver damage on its own and/or by accelerating the progression of chronic viral hepatitis B or C.
Methods
The sensitivity/specificity of liver ultrasound (US) to recognize severe HS is above 85%. A cross-sectional case–control study of all HIV out-patients who underwent liver US since 2004 was conducted at our institution.
Results
Eight hundred and thirty (36.1%) out of 2300 HIV-positive patients on regular follow-up underwent liver US during the study period. Severe HS was diagnosed in 108 (13%) patients. A total of 117 matched HIV controls lacking HS were selected randomly. In patients with severe HS, we found significantly higher values of body mass index (BMI), plasma viral load, serum glucose, alkaline phosphatase, triglycerides and low-density lipoprotein cholesterol, as well as significantly higher prevalence of diabetes, elevated alcohol consumption, lipohypertrophy and advanced liver fibrosis. Furthermore, a trend towards longer exposure to nucleoside analogues was noticed. In the multivariate analysis, only elevated alcohol consumption [odds ratio (OR) 7, P=0.013], lipohypertrophy (OR 5.3, P=0.008), plasma viral load (OR 2.1, P=0.02), BMI (OR 1.2, P=0.013) and serum glucose (OR 1.03, P=0.027) were significantly associated with severe HS.
Conclusions
Severe HS in HIV-positive patients is associated with predisposing factors that are similar to those of HIV-negative individuals. However, its characteristic association with elevated plasma viral load might suggest a direct involvement of HIV in liver fat deposition. Therefore, the benefit of controlling HIV replication with antiretroviral therapy should be balanced against its effect of occasionally inducing metabolic abnormalities and lipodystrophy.
Introduction
Hepatic steatosis (HS) is a manifestation of excessive accumulation of triglycerides in the liver. When it is accompanied by inflammation and fibrosis, there is an increased risk of progression to severe liver disease [1]. HS often accounts for unexplained liver enzyme elevations in persons with no predisposing liver conditions [2]. The prevalence of HS depends on the study population and the method used to make the diagnosis. In the general population, the prevalence of HS is 16–29% using ultrasonography (US) [3–5], 31–34% using proton magnetic resonance [6,7] and 15–39% using liver biopsy [8–10]. HS can be found in up to half of patients with chronic hepatitis C virus (HCV) infection, and is particularly prevalent in the sub-set of individuals infected with HCV genotype 3 [11,12]. In patients with HIV infection, the prevalence of HS ranges between 3 and 57% [13,14]; HS seems to be more common (40–69%) [15–18] and more severe [19] in HIV–HCV co-infected patients.
Classical risk factors for HS are linked to the host, such as alcohol abuse and metabolic abnormalities associated with insulin resistance (obesity, diabetes, dyslipidaemia) [20]. In patients co-infected with HIV and HCV, HS has been associated with higher serum HCV load and/or HCV genotype 3 as well as the use of some antiretroviral (ARV) agents [17,21,22] such as nucleoside reverse transcriptase inhibitors (NRTIs) (through mitochondrial toxicity) and protease inhibitors (which may induce insulin resistance).
Liver biopsy remains the best diagnostic tool for recognizing and measuring the extent of HS [23]. However, this invasive procedure entails some risks for the patient and is not suitable as a routine diagnostic tool for HS [24]. Furthermore, sampling error of liver biopsy can result in substantial misdiagnosis and staging inaccuracies [25,26], especially when the tissue size is relatively small [27]. Consequently, efforts have been made to identify non-invasive methods to recognize HS [28,29]. US is generally requested as the first imaging technique to assess liver parenchyma and the extent of damage in patients with potential liver illnesses. The correlation between HS suggested by US and its proof on liver biopsy remains controversial [30–32]; however, the correlation is excellent when only severe HS is assessed [33,34]. Severe HS is diagnosed using US based on four findings: bright hepatic parenchyma, enhanced liver–kidney contrast, vascular blurring and deep hepatic attenuation. The sensitivity, specificity, positive predictive value and negative predictive value of a bright hepatic ultrasound for HS are 64, 97, 96 and 65%, respectively. However, these values increase to 91, 93, 89 and 94%, respectively, for severe HS (30% or more hepatocytes with fat accumulation on liver biopsy) [34–36]. Compared with magnetic resonance, which is the most sensitive non-invasive tool for HS, the performance of US is equally useful in the diagnosis of severe HS [37]. In this study, we assessed the prevalence and predictors of severe HS in a relatively large group of HIV-infected patients who underwent hepatic US.
Patients and methods
Study population
A case–control study was performed retrospectively in HIV-positive patients on regular follow-up at the out-patient clinic of Hospital Carlos III, a reference centre for HIV/AIDS located in Madrid, Spain. All patients who underwent liver US between January 2004 and May 2007 were identified. Only US requested to provide complementary information in HIV-positive patients with persistently elevated liver enzymes and/or with chronic hepatitis of any aetiology (viruses, alcohol) was selected. US requested for acute liver events was excluded from this analysis. Three experienced radiologists carried out all the liver US examinations. Severe HS was diagnosed by the radiologists based on the consideration of characteristic imaging findings, namely bright liver pattern, liver–kidney contrast, vascular blurring and/or deep hepatic attenuation. The diagnosis was established when two or more of these findings were recognized by the radiologist. Age- and gender-matched HIV-positive individuals who underwent US and did not show any evidence of HS were selected as controls.
Liver fibrosis was measured in all cases and controls using transient elastography (Fibroscan®; Echosens, Paris, France). Using this non-invasive tool to assess hepatic fibrosis, values above 13.0 kPa correlate well with Metavir scores of F3 or more. For values above 14.5 kPa, the correlation with F4 (cirrhosis) in the liver biopsy is >95% [38]. Demographics, metabolic risk factors, alcohol consumption and use of highly active antiretroviral therapy (HAART) (months accumulated under each drug) were recorded in a case report form especially designed for this study. Elevated alcohol consumption was defined as daily intake >60 g. Lipodystrophy was defined as sub-cutaneous fat loss (lipoatrophy) and/or increased girth or buffalo hump (lipohypertrophy), reported by the patient and confirmed by the doctor in charge.
Laboratory parameters
Tests included complete blood cell counts, aspartate transaminase, alanine transaminase, bilirubin, alkaline phosphatase, γ-glutamyl transferase and a complete lipid profile. Immunological parameters such as absolute number and percentage of CD4 T-cells were also obtained. Plasma viral load was measured using a commercial bDNA assay (Versant HIV-1 RNA; Siemens Medical Systems, Erlogen, Germany). Serum HCV antibodies were screened using an enzyme immunoassay (Abbott Diagnostics, Madrid, Spain). Serum HCV-RNA was measured using a real-time polymerase chain reaction assay (Cobas TaqMan; Roche Molecular Systems, Barcelona, Spain). HCV genotyping was performed using a commercial hybridization test (LiPA HCV genotype 1.0; Siemens Medical Systems).
Statistical analyses
A descriptive analysis was carried out for all the clinical and demographic variables. Comparisons between cases and controls were performed using Student's t-test or the Mann–Whitney U-test (for continuous variables) and the Pearson χ2 test or Fisher's exact test (for categorical variables). Univariate logistic regression and stepwise forward multivariate logistic regression analyses were performed to explore which factors were associated with the presence of significant HS. Differences were deemed significant at P<0.05. All reported values were two-sided. All analyses were performed using the spss software version 14 (SPSS Inc., Chicago, IL, USA).
Results
Eight hundred and thirty (36.1%) out of 2300 HIV-positive patients on regular follow-up underwent hepatic US during the study period. Severe HS was diagnosed in 108 (13%) of patients who underwent US. In four of them, a liver biopsy could be obtained and features of HS were confirmed in all cases (although with a wide range of fat deposition). Two patients showed more than 50% of hepatocytes with fat deposition, while this level was around one third for the other two individuals. The former two patients showed significant degrees of liver fibrosis (Metavir scores F2 and F3, respectively). There was no fibrosis in the latter two. None showed signs of damage caused by elevated alcohol consumption. One hundred and seventeen patients without evidence of HS using US were selected as controls, after matching for gender and age. The main characteristics of these 225 individuals, as well as differences between groups, are depicted in Table 1. Their mean age was 43 years and 81% were men. Their mean CD4 count was 560 cells/μL and 69% had undetectable plasma viral load. Overall, 40% had chronic HCV and 12% chronic hepatitis B. Elevated alcohol consumption was admitted by 17% of individuals. Diabetes and hyperlipidaemia were present in 14 and 43% of patients, respectively. In 185 patients (82.2%), liver fibrosis could be measured in parallel with US using transient elastography. Advanced liver fibrosis (Metavir scores F3 and F4) was present in 19% of patients, and was recognized significantly more frequently in patients with HS than in controls (26 vs. 13%; P=0.015).
| All patients (n=225) | Severe HS (n=108) | Controls (n=117) | P-value | |
|---|---|---|---|---|
| Male (n, %) | 183 (81) | 92 (85) | 91 (78) | 0.17 |
| Mean age (years) ( ± SD) | 43.8 ± 7 | 44 ± 7 | 43.7 ± 7 | 0.69 |
| Mean weight (kg) ( ± SD) | 71.4 ± 13 | 76.1 ± 15 | 67.3 ± 10 | < 0.001 |
| Mean BMI ( ± SD) | 24.1 ± 4 | 24.9 ± 4 | 23 ± 3 | 0.002 |
| Elevated alcohol consumption (n, %) | 39 (17) | 27 (25) | 12 (10) | 0.003 |
| Diabetes (n, %) | 32 (14) | 24 (22) | 8 (7) | 0.001 |
| Dyslipidaemia (n, %) | 97 (43) | 49 (45) | 48 (41) | 0.59 |
| HIV risk group | 0.21 | |||
| Intravenous drug use (n, %) | 93 (41) | 38 (35) | 55 (48) | |
| Homosexual men (n, %) | 98 (44) | 50 (47) | 48 (41) | |
| Heterosexuals (n, %) | 28 (12) | 17 (16) | 11 (9) | |
| Other (n, %) | 6 (3) | 3 (3) | 3 (3) | |
| Mean plasma viral load (HIV-1 RNA copies/mL) | 161 | 234 | 98 | 0.03 |
| Mean CD4 count (cells/μL) | 560 ± 336 | 553 ± 305 | 565 ± 337 | 0.7 |
| Mean CD4 nadir (cells/μL) | 294 ± 227 | 311 ± 226 | 279 ± 228 | 0.3 |
| Lipodystrophy | ||||
| Lipoatrophy (n, %) | 30 (13) | 14 (13) | 16 (14) | 0.84 |
| Lipohypertrophy (n, %) | 40 (18) | 27 (25) | 13 (11) | 0.03 |
| HCV antibodies (n, %) | 108 (48) | 40 (37) | 68 (58) | 0.002 |
| Positive serum HCV-RNA (n, %) | 89 (40) | 32 (30) | 57 (49) | 0.003 |
| Mean HCV-RNA level (copies/mL) | 199.558 | 63.112 | 374.268 | 0.08 |
| HCV genotype (n=92) | 0.54 | |||
| 1 (n, %) | 61 (66) | 20 (62) | 41 (68) | |
| 2 (n, %) | 1 (1) | 1 (3) | 0 (0) | |
| 3 (n, %) | 18 (20) | 7 (22) | 11 (18) | |
| 4 (n, %) | 12 (13) | 4 (13) | 8 (14) | |
| Positive serum HBsAg (n, %) | 28 (12) | 15 (14) | 13 (11) | 0.54 |
| Advanced liver fibrosis (Metavir F3–F4; n=185) (n, %) | 36 (19) | 24 (26) | 12 (13) | 0.027 |
| Laboratory values (mean ± SD) | ||||
| Glucose (mg/dL) | 106 ± 20 | 111 ± 24 | 102 ± 15 | 0.001 |
| ALT (IU/L) | 59 ± 60 | 59 ± 40 | 59 ± 74 | 0.97 |
| AST (IU/L) | 50 ± 46 | 51 ± 34 | 50 ± 56 | 0.94 |
| GGT (IU/L) | 95 ± 52 | 112 ± 153 | 80 ± 150 | 0.12 |
| Total bilirubin (mg/dL) | 0.9 ± 0.8 | 1.05 ± 0.8 | 1.1 ± 0.9 | 0.32 |
| Alkaline phosphatase (U/I) | 226 ± 89 | 240 ± 98 | 213 ± 79 | 0.02 |
| Total cholesterol (mg/dL) | 183.4 ± 40 | 188 ± 45 | 179 ± 34 | 0.08 |
| Triglycerides (mg/dL) | 147 ± 107 | 173 ± 131 | 123 ± 79 | < 0.001 |
| LDL cholesterol (mg/dL) | 63 ± 39 | 71 ± 42 | 56 ± 35 | 0.04 |
| HDL cholesterol (mg/dL) | 75.8 ± 46 | 64 ± 44 | 87 ± 45 | 0.001 |
- ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, gamma glutamyl transferase; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HDL, high-density lipoprotein, HS, hepatic steatosis; LDL, low-density lipoprotein; SD, standard deviation.
Mean values of BMI, glucose, low-density lipoprotein (LDL) cholesterol and triglycerides, as well as the prevalence of elevated alcohol consumption, diabetes mellitus and lipohypertrophy, were significantly increased in patients with HS compared with controls. Interestingly, plasma viral load levels were significantly higher in patients with HS than in controls (Fig. 1). In contrast, HCV antibodies and detectable HCV viraemia were less frequent in patients with severe HS than in controls.
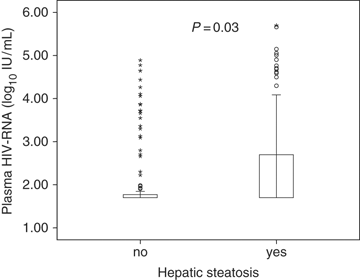
Relationship between hepatic steatosis and plasma viral load.
Prior exposure to antiretroviral therapy (ART) did not differ significantly in patients with HS and controls, as shown in Table 2. This observation was confirmed even when considering separately the length of exposure to different drug classes or to any single drug. Nevertheless, a trend towards an association between severe HS and longer exposure to ARV treatment – specifically with NRTIs – was observed.
| Drugs | Total(n=225) | Severe HS(n=108) | Controls(n=117) | P-value |
|---|---|---|---|---|
| NRTIs | 153 ± 95 | 161 ± 99 | 145 ± 97 | 0.26 |
| Zidovudine | 29 ± 31 | 33 ± 32 | 26 ± 30 | 0.10 |
| Didanosine | 23 ± 24 | 24 ± 25 | 22 ± 23 | 0.66 |
| Stavudine | 27 ± 26 | 29 ± 26 | 26 ± 26 | 0.42 |
| NNRTIs | 28 ± 27 | 27 ± 28 | 29 ± 26 | 0.49 |
| PIs | 23 ± 28 | 23 ± 24 | 26 ± 28 | 0.51 |
| All therapies | 205 ± 124 | 212 ± 126 | 198 ± 122 | 0.43 |
- HS, hepatic steatosis; NNRTIs, non-nucleoside reverse transcriptase inhibitors; NRTIs, nucleoside reverse transcriptase inhibitors; PI, protease inhibitors.
The results of the logistic regression analysis are displayed in Table 3. In the univariate analysis, increased values for BMI, glucose, LDL cholesterol, high-density lipoprotein (HDL) cholesterol and triglycerides, as well as higher rates of alcohol abuse, diabetes and lipohypertrophy, were associated significantly with severe HS. In addition, elevated plasma viral load levels were also associated significantly with HS. In contrast, serum HCV-RNA levels correlated inversely with HS. In the multivariate analysis, only a higher BMI, elevated alcohol consumption, lipohypertrophy, lower serum HCV-RNA and (conversely) higher plasma viral load levels remained as independently associated to severe HS.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age (years) | 1 (0.97–1.04) | 0.73 | ||
| BMI (kg/m2) | 1.15 (1.04–1.27) | 0.003 | 1.2 (1.04–1.41) | 0.012 |
| Elevated alcohol consumption (n%) | 3.13 (1.48–6.60) | 0.003 | 7.49 (1.81–30.87) | 0.005 |
| Diabetes (n, %) | 3.9 (1.66–9.13) | 0.002 | ||
| Dyslipidaemia (n, %) | 1.19 (0.70–2.02) | 0.51 | ||
| Lipohypertrophy (n, %) | 2.54 (1.19–5.41) | 0.015 | 5.02 (1.56–16.1) | 0.007 |
| Plasma viral load (HIV-1 RNA copies/mL) | 1.32 (1.01–1.73) | 0.04 | 2.05 (1.19–3.52) | 0.009 |
| CD4 count (cells/μL) | 1 (0.99–1) | 0.78 | ||
| Antiretroviral drug exposure (months) | 1 (0.99–1) | 0.43 | ||
| NRTI exposure (months) | 1 (0.99–1) | 0.23 | ||
| Serum HCV-RNA (copies/mL) | 0.36 (0.20–0.65) | 0.001 | 0.22 (0.08–0.63) | 0.005 |
| Advanced liver fibrosis (Metavir F3–F4) | 2.38 (1.10–5.11) | 0.026 | ||
| Glucose (mg/dL) | 1.02 (1–1.04) | 0.002 | ||
| ALT (IU/mL) | 1 (0.99–1) | 0.97 | ||
| AST (IU/mL) | 1 (0.99–1) | 0.94 | ||
| Alkaline phosphatase (IU/mL) | 1 (1.004–1.007) | 0.029 | ||
| Triglycerides (mg/dL) | 1 (1.002–1.009) | 0.001 | ||
| LDL cholesterol (mg/dL) | 1 (0.99–1) | 0.04 | ||
| HDL cholesterol (mg/dL) | 0.98 (0.98–0.99) | 0.002 | ||
- ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CI, confidence interval; HAART, highly active antiretroviral therapy; HCV, hepatitis C virus; HDL, high-density lipoprotein, LDL, low-density lipoprotein; NRTI, nucleoside reverse transcriptase inhibitor; OR, odds ratio.
Discussion
Fat infiltration of the liver constitutes a relevant pathological condition, given its potential for progression to end-stage liver disease [1] and eventually hepatocellular carcinoma, throughout intermediate steps of steatohepatitis and cirrhosis [39]. In both HIV-negative and HIV-positive individuals, HS is known to be associated with elevated alcohol consumption, chronic HCV infection and metabolic abnormalities such as diabetes, hyperlipidaemia and lipodystrophy [40,41]. ART and HIV itself might also play a role in persons with HIV infection, although this is subject to controversy.
The prevalence of HS depends largely on the diagnostic method. Liver US represents a non-invasive procedure with a fairly good sensitivity and specificity when used to assess moderate-to-severe degrees of fat accumulation in the liver [33,34,42]. However, milder degrees of HS can be missed by US. In our study, only severe HS, which has been correlated by others with histological findings of fat deposition in more than 30% of hepatocytes [34–36], was considered using US. The prevalence of severe HS in our HIV-positive population was 13%, which is below the 40–69% rate reported by others using liver biopsy in patients co-infected with HIV and HCV [16–18]. In the absence of clear justification for a liver biopsy (i.e. chronic HCV), it would be very difficult to assess the prevalence of HS in a broader spectrum of HIV-positive patients, as we did in our study. To improve the diagnostic accuracy of US, we limited our examination to severe HS, and in this regard we obtained novel and unique information. Severe HS was observed in nearly one-in-eight HIV-infected patients who underwent liver US for any reason.
Factors associated with severe HS in our HIV population were those classically recognized as predisposing to HS in HIV-negative individuals, such as being overweight, elevated alcohol consumption, diabetes, elevated LDL cholesterol and triglycerides. Of those variables, only elevated alcohol consumption [odds ratio (OR) 7.49, 95% confidence interval (CI) 1.8–30.9] and high BMI (OR 1.2, 95% CI 1.04–1.41) remained significantly associated with severe HS in HIV-positive patients in the multivariate analysis.
It is noteworthy that in our study patients with severe HS had HCV antibodies and active HCV replication less frequently than controls. Moreover, serum HCV-RNA titres were lower in HCV viraemic patients with severe HS than in controls. These observations are in contrast with prior reports [16,43]. However, it should be remembered that HS in patients with chronic HCV has been linked predominantly to genotype 3, which was present in only 20% of our co-infected population. Moreover, controls were more likely to be intravenous drug users than patients with HS and had significantly lower BMI (perhaps reflecting poor nutrition). Altogether, these circumstances might explain why we did not observe more severe HS in patients with HCV. It remains unclear why an inverse association could exist between serum HCV load and HS in our study. This aspect warrants further research.
The good performance of FibroScan for staging liver fibrosis in patients with non-alcoholic steatohepatitis has been demonstrated recently [44]. Moreover, it is important to note that HS does not influence elastometric results by itself [38]. Our study showed a good relationship between severe HS and higher degrees of liver fibrosis assessed by transient elastography, as reported previously [15,17]. Therefore, HS on its own or in combination with underlying chronic viral hepatitis B or C could accelerate liver fibrosis progression.
Some ARV agents (e.g. stavudine) and a longer exposure to ART have been implicated in the development of HS in HIV-infected individuals [15,17]. However, these results have not been confirmed by others [16,21]. In our study, we did not find a significant association between length of ART (neither as a combination nor considering single drugs separately) and severe HS. Nevertheless, there was a trend in favour of more frequent severe HS in patients with more prolonged duration of ART, and specifically those with longer exposure to NRTI. This observation could indirectly reflect the well-established potential for mitochondrial toxicity of some nucleoside analogues [45], which may induce fat accumulation in the liver.
Lipohypertrophy was associated significantly with severe HS in our study, in accordance with other studies of HIV-infected individuals [46]. In the general population, abdominal obesity also constitutes a pathological condition classically linked to fat accumulation in the liver [47].
The recognition of plasma viral load as an independent predictor of severe HS after adjusting for other variables was another interesting finding. Patients with high HIV viraemia experienced severe HS more frequently than patients with low viraemia. This finding may suggest a direct involvement of HIV replication on fat accumulation. To our knowledge this study is the first to report such an association, and this finding clearly merits further research.
In summary, severe HS in HIV-infected individuals appears to be associated with classical risk factors, such as alcohol abuse and obesity. Interestingly, higher plasma viral load could contribute to fat accumulation in the liver. If proven, this observation might favour the early use of potent ART, at least in the sub-set of patients with underlying hepatic conditions such as chronic hepatitis B/C – in whom the progression of liver fibrosis could be accelerated by steatosis.
Acknowledgements
This work was supported in part by grants from Fundación IES (Investigación & Educación en SIDA), Agencia Laín Entralgo, Red de Investigación en SIDA (RIS, project ISCIII-RETIC RD06/006) and the European Commission VIRGIL and NEAT projects.




