Race and ethnicity impact on the maximum proliferative response in peripheral blood lymphocytes from HIV-seropositive individuals
Summary
The effects of race and ethnicity on immunological function have not been fully studied in patients infected with HIV-1. To study such differences, 54 patients on virally suppressive highly active antiretroviral therapy (HAART) with CD4 counts >200 cells/μL had their peripheral blood lymphocytes (PBL) evaluated for response to recall antigen. Significant differences were found in the maximum responses for PBL from black individuals compared with those from white individuals, and the differences were highly significant when responses for African-Americans were compared with those for white-Hispanics. These findings support work delineating ethnicity and race as significant variables to be taken into account when looking at vaccination strategies and responsiveness to therapeutic pharmacological interventions.
Introduction
The effects of race and ethnicity on immunological responsiveness have not been well studied in HIV-1-infected individuals. Indirect evidence of racial differences can be found in studies on viral load and disease progression when socioeconomic variables are controlled for. A number of studies provide data showing that black individuals naïve to therapy have lower plasma viral burdens than their white counterparts [1–3]. In addition, the rate of CD4 cell decline over time is lower for black patients than for white patients naïve to therapy [3,4]. Most recently, in a paediatric HIV/AIDS population, African-American children infected with HIV were matched with Hispanic HIV-infected children for age, gender and viral burden; the study showed that African-American children had a higher HIV-1 gag response than the Hispanic children [5].
With the increasingly disparate ethnic and racial distribution of HIV-1 infection in the USA, it is important to fully define whether immunological differences exist and persist after highly active antiretroviral therapy (HAART). There are few data on how these differences could affect vaccination strategies, pharmacological interventions, or viral escape and disease progression. To better understand these issues it is important to assess whether immunological differences exist in the virally suppressed individual as a function of race and ethnicity. In the present study, we evaluated the proliferative responses of peripheral blood lymphocytes (PBL) from a cohort of HAART-suppressed HIV-seropositive patients attending an urban out-patient clinic. Data are presented showing differences between black and white infected patients with respect to the maximum lymphocyte proliferative response to Casta recall antigen.
Materials and methods
Human subjects
HIV-1-infected patients followed in the Adult HIV Outpatient clinics at the Jackson Memorial Hospital/University of Miami Medical campus were considered for participation in the study if they had a recent CD4 count >200 cells/μL and a nondetectable viral load (<400 HIV-1 RNA copies/mL). All patients were on a HAART-containing regimen. Sixty-five patients gave informed consent (approved by the Human Institutional Review Board of the University of Miami) to participate in the study. All individuals were asymptomatic at the time of enrolment. Demographic information, CD4 cell nadir determination, and duration of viral load suppression (<400 copies/mL) were abstracted from the medical records.
Materials
Casta antigen (from Candida albicans) was obtained from Greer Laboratories, Inc. (Lenoir, NC). H3-thymidine was obtained from New England Nuclear (Boston, MA). Complete media consisted of RPMI (Roswell Park Memorial Institute) with antibiotics, l-glutamine, minimal essential medium (MEM) nonessential amino acids, MEM sodium pyruvate and 10 mm Hepes plus 10% human AB+ serum (Atlanta Biologicals, Lawrenceville, GA).
Cells
Whole blood was collected in ethylenediaminetetraacetic acid (EDTA)-containing tubes. Following collection, PBL were isolated using density separation following the manufacturer's protocol [lymphocyte separation media (LSM®) from ICN Biomedicals, Inc., Aurora, OH]. The PBL were subsequently frozen at −80°C in 50% complete medium plus 40% foetal bovine serum (FBS) plus 10% dimethyl sulphoxide (DMSO), for later use. Data from the AIDS Clinical Trials Group (ACTG) Immunology Laboratories [6] have shown that proliferation to recall antigen of cryopreserved PBL is preserved when compared with that of freshly isolated PBL.
Antigen proliferation experiments
PBL were plated in quadruplicate for each experimental point in wells (105 cells/well) of a 96-well U-bottomed microtitre tissue culture plate. Casta antigen was added to experimental wells to final concentrations of 3.0, 1.0, 0.3, 0.1 and 0.0 μg/mL. After addition of reagents, the experimental plate was incubated for 6 days in a 5% CO2 incubator at 37°C. After the 6-day incubation, 1 μCi of H3-thymidine was added per well and the plate was incubated overnight. Wells were subsequently harvested and DNA-incorporated radioactivity was measured in counts per minute (CPM). Determinations of specific counts were obtained by subtracting nonspecific counts (no antigen: Pmed) from absolute counts (Pexp). The added reagents were present for the total period of incubation. In general, a proliferative response was considered significant if the stimulation index (SI=Pexp/Pmed) was >5, and a change of >5000 CPM occurred in the specific proliferation (Pspec=Pexp–Pmed). The average spontaneous release was 1092 CPM with a standard deviation of 1186 CPM, demonstrating little skewing in spontaneous release.
Determination of K50 and SImax
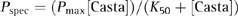 (1)
(1)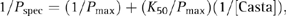 (2)
(2)When 1/Pspec is plotted as a function of 1/[Casta], the resulting data can be fitted with a straight line and then, using Eqn 2, estimates of K50 and Pmax can be obtained. In order to compare the relative maximum obtained between data, the maximum stimulation index (SImax, defined as Pmax/Pmed) is used so that the magnitude of the experimental increase over the control is appropriately modified. Of the 65 PBL samples evaluated with titration curves, 11 could not be analysed either because there were too few points to enable the data to be fitted accurately or because there was too much experimental scatter. Therefore, 54 PBL samples were evaluated.
Statistical methods
Descriptive statistics were used to assess the characteristics of the different variables, their frequency distribution, normality and association patterns. Variables with skewed distributions were transformed using, for example, natural log transformations before they were included in analyses requiring normality. χ2 analyses were used to assess the association between nominal variables; independent t-tests were used to compare two groups with respect to variables with normal or approximately normal distributions, and Mann–Whitney tests were used in the case of variables with skewed distributions. Simple and multiple linear regression models were used to assess different variables as predictors of log-transformed K50 and SImax. Analyses were performed using stata version 8.0 (Stata Corp., College Station, TX) and all significance tests were two-tailed.
Results
Patient demographic and immunological characteristics
Of the 54 patients evaluated, five had a viral load <400 copies/mL, eight had a viral load <200 copies/mL and 41 had a viral load <50 copies/mL at the time the PBL samples were obtained, as determined by the assay sensitivity. Table 1 shows the demographic and immunological characteristics for black and white patients. There were no statistically significant differences between groups with respect to the variables shown in Table 1.
| Characteristic | White* (n=28) | Black** (n=26) |
|---|---|---|
| Nadir | ||
| <200/mm3 [n (%)] | 16 (57.1) | 14 (53.8) |
| >200/mm3 [n (%)] | 12 (42.9) | 12 (46.2) |
| Gender | ||
| Male [n (%)] | 23 (82.1) | 15 (57.7) |
| Female [n (%)] | 5 (17.9) | 11 (42.3) |
| Age | ||
| Mean ± SD | 46.3 ± 9.4 | 47.5 ± 9.1 |
| Median (range) | 46.5 (30, 63) | 51 (30, 63) |
| CD4 Count | ||
| Mean ± SD | 568 ± 255 | 616 ± 340 |
| Median (range) | 521 (238, 1487) | 517 (255, 1711) |
| Time from VL Suppression | ||
| Mean ± SD | 25.8 ± 20.7 | 19 ± 15.5 |
| Median (range) | 25 (1, 92) | 15.5 (2, 61) |
- * Among the 28 white participants 26 were Hispanic.
- ** Among the 26 black participants 20 were African-American and six Haitian/Bahamian/Jamaicans.
SImax is dependent on race
Proliferation response curves were studied for each PBL sample, and SImax and K50 were obtained as described in the Materials and methods section. To evaluate which independent variables predicted SImax, a multiple regression analysis was performed. When lnSImax was used as the dependent variable, and age, time since viral load suppression, percentage CD4, CD4 cell nadir and ethnicity were used as independent variables, only race was found to be a significant independent predictor of lnSImax [β=0.50; 95% confidence interval (CI) 0.03–0.96; P=0.035]. Furthermore, in a subset analysis restricted to African-American and white-Hispanic individuals, after adjusting for the same covariates, race was highly significant (β=0.67; 95% CI 0.19–1.16; P=0.008). This can be seen in Fig. 1, where the SImax is shown for each responder according to ethnicity/race and the median is shown to further demonstrate the difference between populations. These data show that black HIV-infected individuals on suppressive HAART had a greater maximum response to recall antigen than white individuals and, in this study, the significance of the differences was further increased when SImax values for African-American PBL were compared with those for white-Hispanic PBL. In multiple regression analyses, race was not a significant independent predictor of ln-transformed K50 (β=0.12; 95% CI 0.62–0.86; P=0.743).
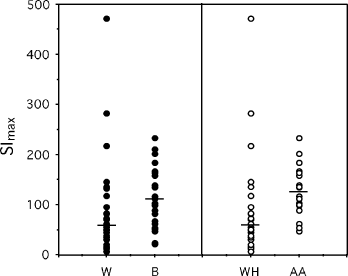
The maximum stimulation index (SImax) as a function of ethnicity/race. The SImax, as determined using Eqn 2 in the Materials and methods section, is shown for white (W) and black (B) HIV-infected individuals in the left panel (closed circles) and for subgroups consisting of white-Hispanic (WH) and African-American (AA) HIV-infected individuals in the right panel (open circles). The median is shown in each case. Significant differences and confidence intervals are given in the text.
Discussion
Evidence for immunological differences between black and white HIV-1-infected individuals has been mostly indirect. Early work evaluated the effect of racial differences on viral load and rate of decay of CD4 count. A number of studies suggested that those individuals who were black (nonwhite) had a slower decline in their CD4 count when compared with their white counterparts [3,4]. Whether clade differences accounted for these differences was unclear [7,8]. In early studies [3,9], a correlation between race and viral load was not found. The results of these studies may have been confounded by the fact that some patients were receiving antiretroviral medication while others were not, and/or by the fact that results were combined for Hispanic and black participants in the analysis. However, more recent work in antiretroviral-naïve individuals suggested that black patients infected with HIV-1 did have lower viral loads than their white counterparts when results were controlled for CD4 count [1–3].
In other fields, the effects of racial differences on immunological function have been studied. The findings are consistent in that black patients have a higher proliferative response to antigen than white patients. In renal transplantation and in asthma studies, suboptimal therapeutic immune suppression has been related to increased PBL responsiveness to antigen in black compared with white patients. This has resulted in poorer outcomes because of disease breakthrough while on usual suppressive therapy [10,11]. Black individuals with hepatitis C were found to have a greater proliferative response to hepatitis C virus antigens than white individuals with hepatitis C [12], although in that study the in vitro response to hepatitis C virus was felt to be dysfunctional secondary to an aberrant co-ordination between proliferation and interferon-γ production leading to an ineffective immune response in situ.
There are few comparative immunological studies of HIV-1-infected individuals by ethnicity or race. One study showed that nonwhite individuals treated with interleukin-2 had a greater increase in their CD4 cell count than white patients infected with HIV [13]. Another study by Rizzardini et al. evaluated immune activation in patients from Uganda and Italy, as well as production of types 1 and 2 cytokines to envelope (env) peptides [14]. They found significant differences in these populations and suggested racial differences may impact on disease progression. More recently, HIV-1 T-cell specific responses in African-American and Hispanic children were studied by Sharp et al. [5]. In that study, they found that when children were matched according to age, viral load and CD4 count, African-American children had a significantly greater gag-specific response than Hispanic children. A study by Frahm et al. evaluated overlapping HIV peptides to screen for immunodominant regions that would demonstrate a cytotoxic T-lymphocyte (CTL) response independent of racial and ethnic background [15]. However, when viewed from the perspective of whether race/ethnicity impacts on CTL response, their data demonstrated that African-American HIV-infected individuals had a greater number of responders to the majority of the immunodominant peptides compared with the number of responders in the Hispanic HIV-infected group.
Our current study confirms that black and, in particular, African-American HIV-infected patients have a significantly greater proliferative response to Casta recall antigen than white (predominantly Hispanic) HIV-infected patients. This is the first study comparing black and white patients with respect to proliferative responses in a chronically HAART-suppressed population after adjusting for percentage CD4, CD4 cell nadir and age.
In our work, specific differences in memory cell subsets were not evaluated. Although possible, it is not likely that such differences affected our findings as stabilization of memory cell populations occurs after 12 months of suppressive HAART. The median length of suppression in the current study was greater than 12 months and not significantly different between groups. This difference in proliferative responses between groups was enhanced when African-Americans were compared with white-Hispanic patients. The use of proliferation–concentration curves to determine the maximum proliferative responses and the K50 allowed delineation of the proliferative differences between these populations. This strategy provided the means to overcome problems in comparing single concentration responses when suboptimal amounts of stimulating antigen are used.
With the increasing incidence of HIV-1 infections among African-American and Hispanic individuals in the USA, it is important to understand differences in immunological responses when viewed from a therapeutic point of view. As we plan interventions and devise strategies for recruiting the immune system into therapeutic participation, differences in race and ethnicity may play a role in the forthcoming discussion.
Acknowledgements
MAK was financially supported, in part, by an independent Medical Study Grant from Pfizer Pharmaceuticals; LJT was supported, in part, by a grant from Humana Innovations Center.




