Stimulation of blood mononuclear cells with bacterial virulence factors leads to the release of pro-coagulant and pro-inflammatory microparticles
Summary
Severe infectious diseases remain a major and life-threatening health problem. In serious cases a systemic activation of the coagulation cascade and hypovolemic shock are critical complications that are associated with high mortality rates. Here we report that blood mononuclear cells, stimulated with M1 protein of Streptococcus pyogenes or other bacterial virulence factors, produce not only pro-coagulant, but also pro-inflammatory microparticles (MPs). Our results also show that activation of the contact system on MPs contributes to these two effects. Phosphatidylserine (PS) plays an important role in these processes as its upregulation on MPs allows an assembly and activation of the contact system. This in turn results in stabilization of the tissue factor-induced clot and a processing of high-molecular-weight kininogen by plasma kallikrein followed by the release of bradykinin, a potent vascular mediator. Pro-coagulant monocyte-derived MPs were identified in plasma samples from septic patients and further analysis of MPs from these patients revealed that their pro-coagulant activity is dependent on the tissue factor- and contact system-driven pathway.
Introduction
The Gram-positive bacterium Streptococcus pyogenes is a major human pathogen that mainly causes superficial and self-limiting skin and throat infections. However, infections can occasionally develop into serious and life-threatening conditions of which streptococcal toxic shock syndrome (STSS) and necrotizing fasciitis are associated with high morbidity and mortality (Cunningham, 2000). Previous studies have shown that systemic contact activation plays an important role in severe S. pyogenes infections (Sriskandan et al., 2000). The contact system – also known as the intrinsic pathway of coagulation or kallikrein/kinin system – and its activation have been extensively studied on negatively charged artificial and biological surfaces in vitro (Espana and Ratnoff, 1983; Tans and Griffin, 1983; Hojima et al., 1984; Kannemeier et al., 2007; Oehmcke et al., 2009a). It consists of three plasma proteins: factor XII (FXII), plasma kallikrein (PK) and high-molecular-weight kininogen (HK). Upon activation, the contact system is involved in at least three distinct (patho)-physiologic processes: (i) activation of the intrinsic pathway of coagulation, (ii) release of bradykinin (BK) from the HK precursor by the action of PK, and (iii) generation of antimicrobial peptides (for a review see Oehmcke and Herwald, 2009).
The name ‘contact system’ is related to its mode of action, as binding (contact) and assembling of the contact factors to a negatively charged surface cause an activation of the system. Negatively charged phospholipids, such as phosphatidylserine (PS), are integral constituents of all eukaryotic cell membranes. Their composition varies not only among cellular organelles, but also their relative distribution within the inner and outer leaflet of a lipid bilayer can differ considerably (for a review see Leventis and Grinstein, 2010). In quiescent cells, PS is mainly found on the cytosolic side of the plasma membrane. However, upon cell activation, PS can be flipped to the outer plasma membrane, where it drives the cell to a pro-apoptotic and pro-coagulative state. Recently, the role of PS on microparticles (MPs) has attracted considerable attention.
Microparticles are sphere-shaped intact vesicles released from cell membranes of almost all activated orapoptotic cell types. They are less than 1 µm in diameter and limited by their lipid bilayer membrane. Depending on the degree of cell activation, MPs differ in their composition and function. Notably, PS is permanently exposed on the outer membrane of the majority of MPs derived from stimulated cells. This is not observed on the cells of origin, where PS is rapidly, but transiently, externalized to the outer membrane upon a stimulatory signal (Piccin et al., 2007).
Phosphatidylserine plays an important role in many biological processes, for instance in apoptosis, where it acts as one major ‘eat me’ signal and ensures efficient recognition and uptake of apoptotic cells by phagocytes (Chaurio et al., 2009). A translocation of PS is particularly seen on the surface of platelets and MPs. It has been shown that PS is not only critical for the assembly of pro-coagulant complexes, but PS also amplifies the activity of tissue factor (TF), a transmembrane protein essential for the activation of the extrinsic pathway of coagulation (Butenas et al., 2009). Although the pro-coagulant activity of MPs has mainly been attributed to TF, little is known as to what extent the activation of the contact system is involved in these processes.
In 2006 Påhlman and colleagues reported that M protein, one of the classical virulence determinants of S. pyogenes, activates monocytes via the TLR2 receptor (Påhlman et al., 2006). These findings prompted us to study whether M proteins are able to evoke formation of pro-inflammatory and pro-coagulative MPs from peripheral blood mononuclear cells (PBMCs). Indeed, by using different experimental approaches our in vitro results show that the intrinsic and extrinsic pathways are activated on MPs from M1 protein-stimulated PBMCs. Interestingly, also other bacterial virulence factors stimulate the release of pro-coagulant MPs from PBMCs. Our results therefore point to an important and general role for pro-coagulant MPs in infectious diseases. In addition to these findings, we report that MP-induced contact activation contributes to clot stabilization and evokes inflammatory reactions due to the generation of BK. These findings were further confirmed ex vivo by studying pro-coagulant plasma MPs from septic patients, where the source of infection were caused by different bacterial pathogens including S. pyogenes.
Results
MPs from M1 protein-stimulated PBMCs trigger clotting via the extrinsic and intrinsic pathways of coagulation
Previous work has shown that monocytes stimulated with M proteins of S. pyogenes respond with the secretion of pro-inflammatory cytokines and an up-regulation of TF at their cell surface (Påhlman et al., 2006; 2007). To investigate, whether this treatment also triggers the release of pro-coagulant MPs, PBMCs isolated from healthy volunteers were incubated for 24 h with M1 protein of S. pyogenes or buffer alone (negative control). LPS from Escherichia coli served as a positive control (Satta et al., 1994). Cells were then centrifuged and the supernatants were further processed for MP isolation (see Experimental procedures). Trypan blue exclusion revealed that independent of the stimulus, less than 2% of the cells died within 24 h. The size distribution of the recovered MPs was in the range from 0.1 to 1 µm as determined by scanning electron microscopy (SEM, Fig. 1A). Interestingly, most particles (87%) had a size of about 0.1 µm in diameter, independent of their stimulus (Fig. 1B). Additional FACS analysis of MPs (see Experimental procedures) revealed that the number of MPs released from PBMCs did not increase upon stimulation with M1 protein or LPS when compared with MPs released from non-stimulated cells (data not shown).
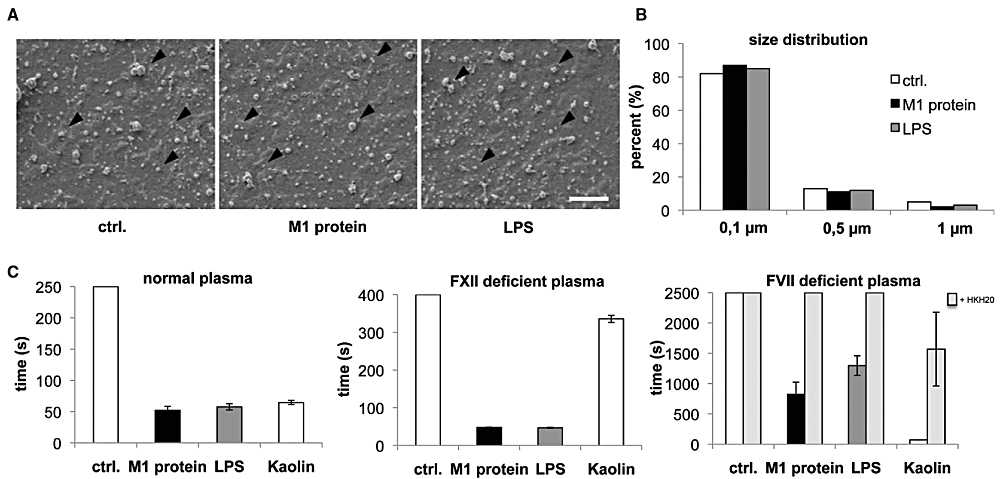
Size distribution and pro-coagulant activity of PBMC MPs. A. Scanning electron microscopy of MPs from non-stimulated (ctrl.), M1 protein- or LPS-stimulated PBMCs (scale bar 2.5 µm). B. Size distribution of MPs from non-stimulated (ctrl.), M1 protein- or LPS-stimulated PBMCs. C. PBMCs were stimulated with M1 protein or LPS for 24 h and MPs were purified from the supernatant. MPs isolated from non-stimulated cells were used as a control (ctrl.). MPs were adjusted to concentration of 150 MPs µl−1. MPs (5 µl) or kaolin (a contact system activator) were then added to re-calcified normal plasma (left panel), FXII-deficient plasma (middle panel) or FVII-deficient plasma (right panel), and the time for clot formation was determined. The contact system inhibitor HKH20 was pre-incubated with microparticles (final concentration of 100 µM) before addition to FVII-deficient plasma, to exclude that clotting is caused by a FVII contamination.
To test the pro-coagulant activity of the MPs, equal numbers of particles were added to normal re-calcified human plasma and clotting times were determined. As shown in Fig. 1C (left panel), MPs from M1 protein- and LPS-stimulated PBMCs, triggered coagulation very efficiently (approximately 1 min), whereas the same number of MPs from non-stimulated cells failed to shorten the clotting time (t > 17 min). Similar results were obtained when FXII-deficient plasma was used (Fig. 1C, middle panel). These results suggest that clotting is dependent on the extrinsic pathway of coagulation. To test whether the intrinsic pathway is also involved, MPs were incubated with FVII-deficient plasma. As seen before, clotting times were reduced when MPs from M1 protein- and LPS-stimulated PBMCs were tested (Fig. 1C, right panel). However, all measuring points were approximately fivefold increased when compared with normal or FXII-deficient plasma. The effect of M1 protein- or LPS-induced MPs in FVII-deficient plasma was inhibited by co-incubation with a contact system inhibitor (HKH20) (Oehmcke et al., 2009b), which proofs that clotting was not triggered by a residual FVII activity in the deficient plasma. Taken together, the results show that M1 protein and LPS are equally potent inducers of pro-coagulant MPs from PBMCs. As both virulence factors signal via Toll like-receptors (Poltorak et al., 1998; Påhlman et al., 2006), we wished to test whether also other bacterial factors can trigger the formation of pro-coagulant MPs. To this end, a panel of previously described virulence determinants from Gram-positive and Gram-negative bacteria was used to generate MPs from PBMCs. Apart from M1 protein, M proteins from serotype M3 as well as the secreted protein SIC (streptococcal inhibitor of complement (Åkesson et al., 1996) from S. pyogenes and lipoteichoic acid (LTA) from Staphylococcus aureus were also found to release pro-coagulant MPs (Fig. S1), whereas MPs derived from protein PAB- (from Finegoldia magna (de Château and Björck, 1994) or protein M5-stimulated cells gave only weak response (Fig. S1). Taken together, the findings implicate that the release of pro-coagulant MPs can be triggered by other bacterial virulence factors as well, and is not restricted to M proteins of S. pyogenes.
Clot structure and endurance against fibrinolysis
Our findings show that pro-coagulant MPs activate both pathways of the coagulation cascade, and suggest that the two arms of the coagulation system have different functions during clot formation. We therefore set up a series of experiments to test whether the extrinsic pathway is important in initiating clotting, while intrinsic pathway contributes to the clot morphology and stability. MPs from M1 protein-stimulated PBMCs were added to re-calcified normal, FVII- or FXII-deficient plasma. The generated clots were fixed immediately (termed as 1 min clot) or 10 min (10 min clot) after clot formation, which should resemble the situation before and after contact activation. Samples were then subjected to morphological analysis by light and scanning electron microscopy. Clots that were processed immediately (1 min clots) appeared as a wide-meshed fibrin network and no differences were observed regardless of whether normal or FXII-deficient plasma was used (data not shown). Note that no clotting was observed in re-calcified FVII-deficient plasma after 1 min. We next analysed 10 min clots that were formed in normal plasma. Microscopic analysis revealed that clots were built up by a dense network, consisting of long and thick fibrin strands that are tightly assembled (Fig. 2A, upper panel). This was not seen with FXII- and FVII-deficient plasma, where the clot architecture was looser with thinner fibrin strands (Fig. 2A, middle panel) or formed by thick and short fibrin strands arranged in a diffuse manner (Fig. 2A, lower panel) respectively.
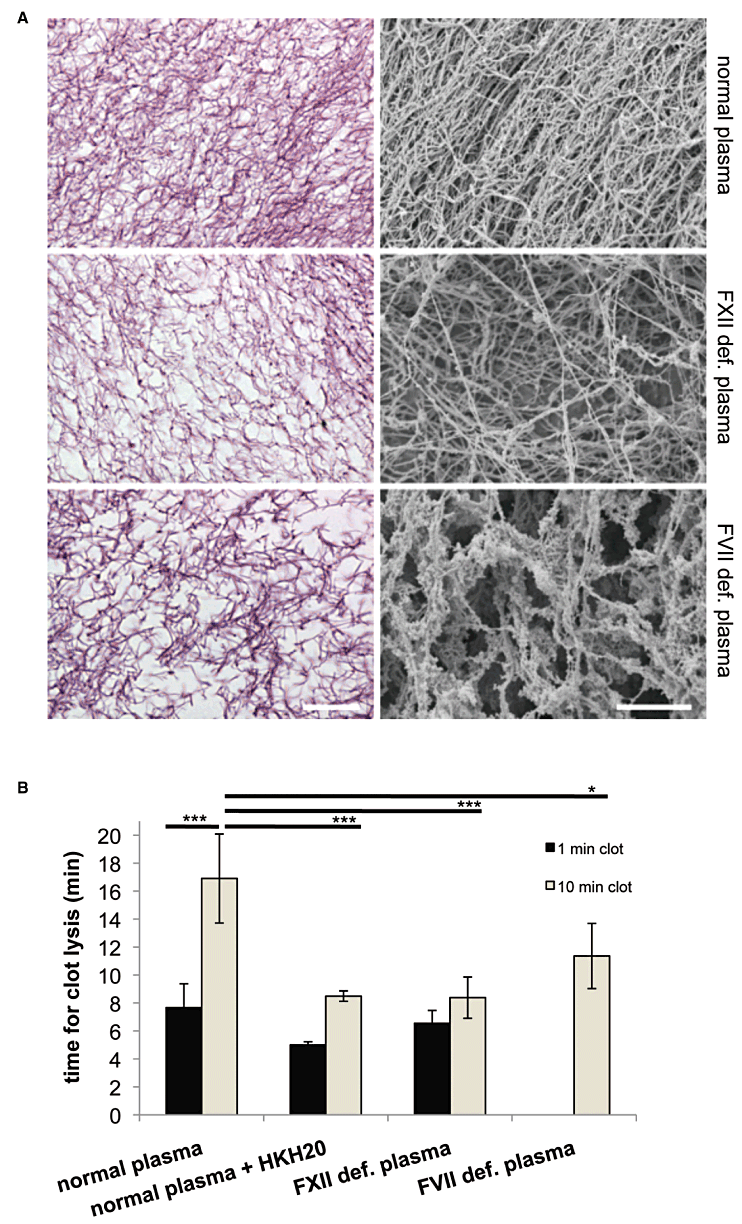
Clot structure and clot lysis. A. Clots were induced by addition of MPs from M1 protein-stimulated PBMCs to re-calcified plasma, incubated for 10 min, fixed and processed for light microscopy (left panel, HE stain, scale bar 10 µm) or scanning electron microscopy (right panel, scale bar 10 µm). B. Clots were generated as described above and tPA were added immediately (1 min clot) or 10 min after (10 min clot) re-calcification. The times for clot lysis were measured in a coagulometer. *P < 0.05, ***P < 0.0001.
The differences in the clot morphology were also reflected in their resistance to breakdown by tissue-type plasminogen activator (tPA). To this end, clots were generated as described above and subjected to fibrinolysis by the addition of tPA. Figure 2B shows that 1 min clots produced from normal plasma were dissolved within 8.6 min, while lysis time for 10 min clots was almost doubled (17 min). To test whether inhibition of the contact system leads to the formation of less stable clots, HKH20 was added to normal plasma. We found that this treatment shortened tPA-induced lysis of the 1 min clots to 5 min and also the lysis time of HKH20 treated 10 min clots was decreased (from 17 to 8.5 min). The latter lysis time was in the same range as the lysis time for the 1 min clots of non-HKH20 treated normal plasma, suggesting that inhibition of the contact system by HKH20 impairs clot stability. Similar findings were also recorded when FXII-deficient plasma was used. Using this approach we observed that the 10 min clots dissolved almost at the same time as the 1 min clots made from normal plasma. Finally, we measured the lysis of clots generated from FVII-deficient plasma. Here, we found that the time for clot lysis was prolonged when compared with normal plasma treated with HKH20 or FXII-deficient plasma (10 min clots). Taken together, the tPA experiments show that an impaired contact system leads to accelerated clot lysis.
TF and PS composition is altered in MPs derived from stimulated PBMCs
In order to activate both coagulation pathways, MPs have altered to a pro-coagulant surface. Exposure of PS on the outer leaflet of the membrane from MPs has been reported to be a critical component in this process (Key, 2010) and, thus, gold-labelled annexin V was employed to determine PS translocation on MPs. Examination and quantification by transmission electron microscopy (TEM) revealed gold-labelled annexin V binds more abundantly to MPs from stimulated PBMCs (Fig. 3A, upper panel) and similar findings were recorded when MPs were analysed by FACS (Fig. 3B). We next studied the distribution of TF on PBMC-derived MPs. Figure 3A depicts that gold-labelled antibodies against TF bound more frequently to MPs from M1 protein or LPS treated cells than to MPs from non-stimulated cells (Fig. 3A, lower panel). The ELISA measurements also showed that TF significantly increased on MPs derived from stimulated PBMCs (Fig. 3C). Collectively, the data show that the pro-coagulant state of MPs from stimulated PBMCs is primed by translocation of PS to the outer membrane in combination with concurrent increase of TF content.
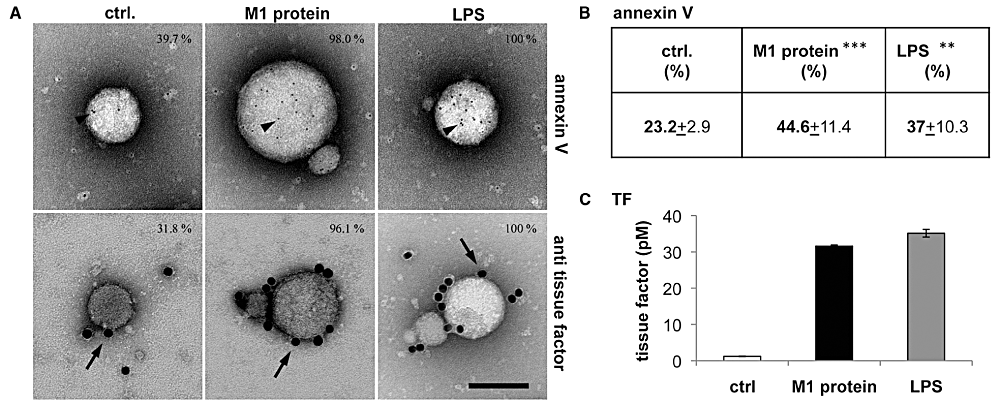
TF and PS exposure on PBMC-derived MPs. A. MPs derived from non-stimulated (ctrl.), M1 protein- or LPS-stimulated PBMCs were incubated with a gold-labelled antibody against TF (15 nm gold, lower panel), or gold-labelled annexin V (5 nm, upper panel), processed by negative staining and analysed in a transmission electron microscope. Arrows point to gold-labelled antibody against TF and arrowheads to gold-labelled annexin V, scale bar 100 nm. B. Measurement of annexin V binding by FACS analysis. MPs were incubated with annexin V and 5000 events were counted and analysed. Positive events are presented as percentage of 5000 events, **P < 0.005, ***P < 0.0001. C. Quantification of TF pro-coagulant activity of MPs (150 MPs µl−1) by ELISA.
MPs bind and activate the contact system
We next studied the effect of pro-coagulant MPs on the contact system. To this end, MPs from stimulated PBMCs were mixed with gold-labelled HK and FXII, while MPs from non-stimulated PBMCs served as control. MPs were also stained with gold-labelled CD14 antibodies, to determine monocytic origin of the particles. Negative staining and analysis by TEM visualized HK (small gold particles) and FXII (middle sized gold particles) that were most frequently attached to MPs from M1 protein- and LPS-stimulated CD14-positive monocytes (big sized gold particles) and to a lesser extent to MPs from non-stimulated monocytes (Fig. 4A). To test whether the binding of contact factors to MPs is followed by an activation of the contact system, we measured PK activity and BK release in plasma treated with equal amounts of MPs from non-stimulated and stimulated PBMCs. Figure 4B depicts that the addition of MPs from non-stimulated PBMCs to plasma did not trigger an increase of PK activity, while MPs from M1 protein- or LPS-stimulated cells induced a significant rise. Addition of HKH20 to the MPs before incubation with plasma reduced PK activity to background levels. Likewise, BK release was also significantly increased, when plasma was incubated with MPs from stimulated, but not from non-stimulated cells (Fig. 4C). As seen before, this effect was blocked, when MPs were co-incubated with HKH20 (Fig. 4C). These results show that MPs from stimulated PBMCs are potent activators of the contact system.
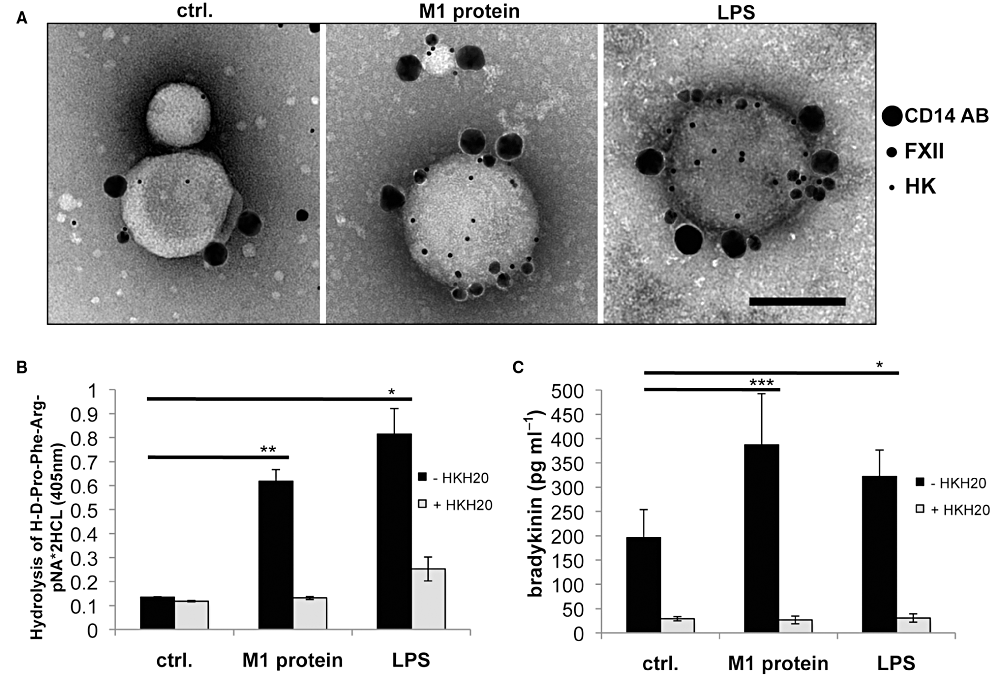
Binding and activation of the contact system on MPs. A. MPs derived from non-stimulated (control), M1 protein- or LPS-stimulated PBMCs were incubated with gold-labelled FXII (15 nm gold), gold-labelled HK (5 nm gold), and gold-labelled anti-CD14 antibody (30 nm gold). CD14-positive MPs were quantified and their FXII and HK content was determined. B. Plasma kallikrein activity was recorded in plasma 30 min after incubation with MPs derived from non-stimulated, M1 protein-stimulated (black column), or LPS-stimulated PBMCs (150 MPs µl−1) in a substrate assay. Contact activation was blocked by the addition of the contact system inhibitor HKH20 (light grey columns), *P < 0.05, **P < 0.005. C. Bradykinin release was measured in plasma 30 min after incubation with MPs derived from non-stimulated, M1 protein-stimulated (black column), or LPS-stimulated PBMCs (100 MPs µl−1) and HKH20 was added to prevent bradykinin formation (light grey columns). The results are representative of at least three experiments performed in triplicates, *P < 0.05, ***P < 0.0001.
PS triggers the release of bradykinin
Earlier studies demonstrated that acidic phospholipids such as PS promote binding and activation of the contact system in plasma (Schousboe, 1988). To further prove whether this activation triggers BK release, we generated vesicles consisting of pure PS, while vesicles made of the non-charged phosphatidylcholin (PC) served as negative control. The vesicular nature of both phospholipids in solution was confirmed by negative staining and TEM. Subsequent electron microscopic analysis revealed that gold-labelled FXII and HK avidly bind to PS, but not to PC vesicles (Fig. 5A, upper panel). Similar findings were also recorded when gold-labelled PS – binding annexin V was used as a positive control (Fig. 5A, lower panel). Based on these findings, we tested whether PS is able to trigger an activation of the contact system. PS and PC vesicles were incubated with normal human plasma and the induction of PK activity was measured. Figure 5B shows that the addition of PS vesicles to plasma leads to a time-dependent rise in plasma kallikrein activity, which was not seen with PC vesicles. Moreover, a significant BK release was measured, when PS, but not PC, vesicles were incubated with plasma (Fig. 5C). These data suggest that an increase in the PS content on MPs promotes the assembly and activation of the contact system and is followed by the release of BK.
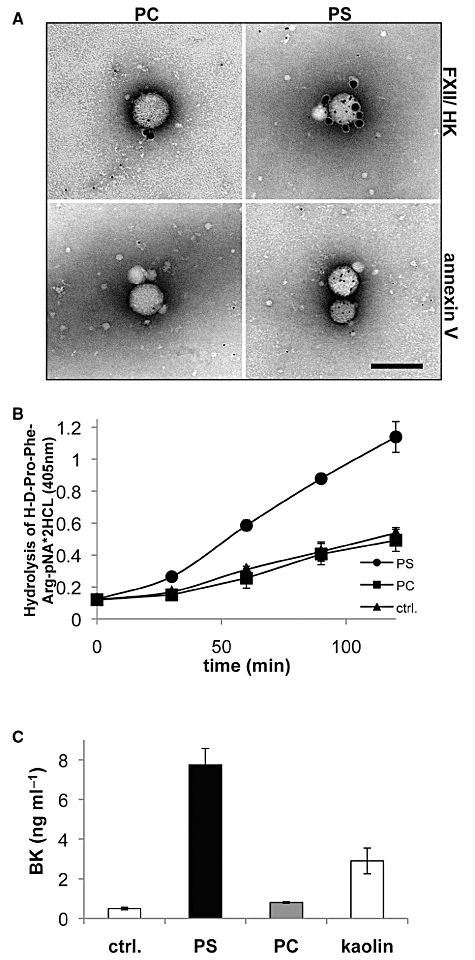
Binding and activation of the contact system on PS vesicles. A. PS and PC vesicles were incubated with gold-labelled HK and FXII (5 nm gold and 15 nm gold respectively; upper panel) or gold-labelled annexin V (5 nm; lower panel). The vesicles were then processed by negative staining and analysed in a transmission electron microscope. Scale bar 100 nm. B. PS and PC vesicles at a final concentration of 100 nM or buffer were incubated with normal human plasma for 2 min before a substrate, specific for plasma kallikrein activity, was added. After indicated time points the absorbance in the reaction mixture was measured at 405 nm. C. PS, PC, kaolin (positive control) or buffer (ctrl.) were incubated with normal human plasma for 30 min followed by measurements of the BK content by ELISA.
The concentration of PS-positive MPs is increased in plasma samples from septic patients
To address whether contact activation can be monitored on plasma MPs from septic patients, we analysed plasma samples from 14 septic patients (including 4 samples from patients suffering from a streptococcal sepsis). The activated partial thromboplastin time (aPTT), as a marker for the intrinsic pathway of coagulation, and the prothrombin time (PT), as a marker for the extrinsic pathway of coagulation, were significantly prolonged in all septic patients, when compared with healthy volunteers (Fig. 6A). On average, the total numbers of plasma MPs were higher in septic patients; however, the difference was not statistically significant (Fig. 6A, right panel).
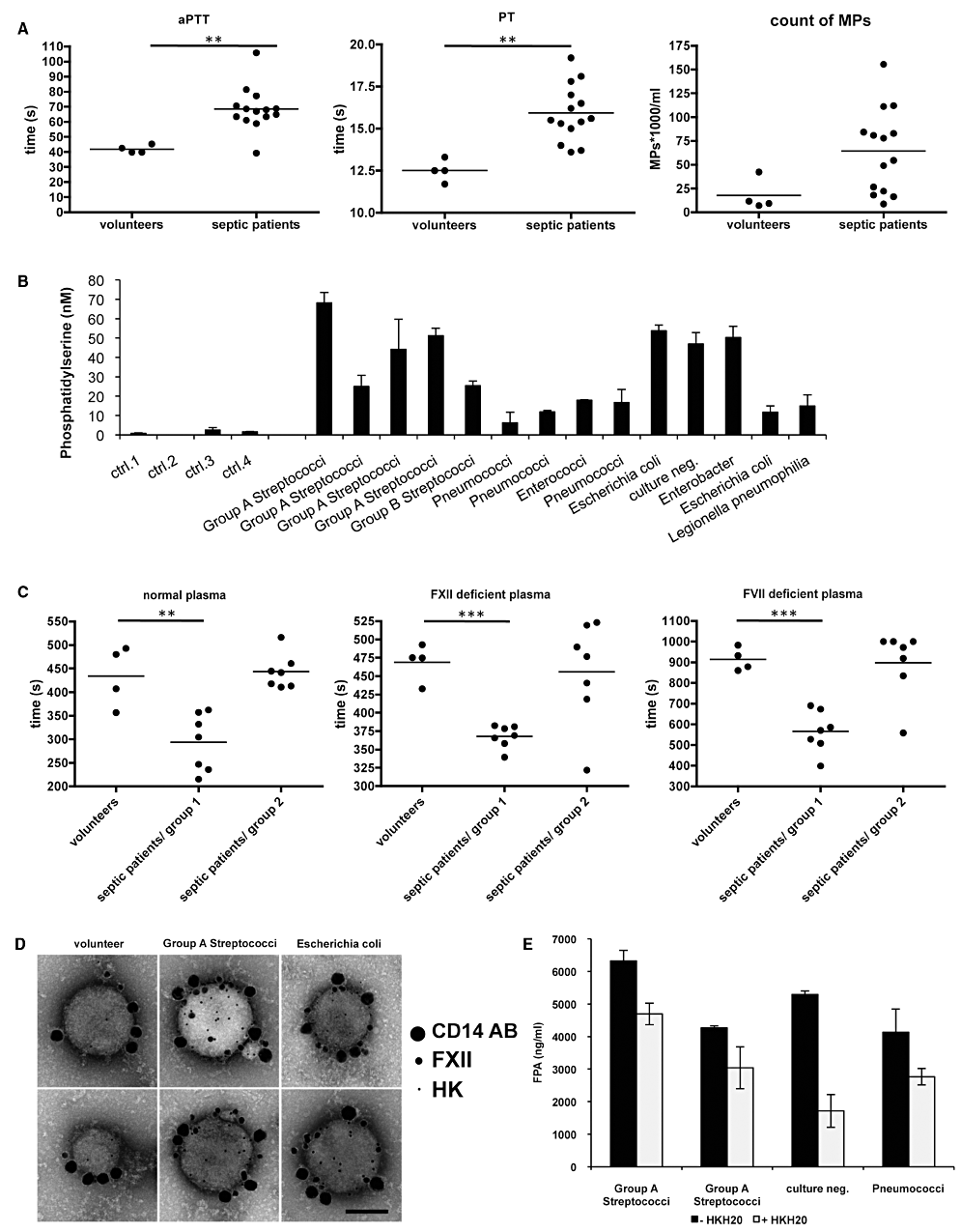
Analysis of plasma and plasma-derived MPs from septic patients. A. Measurement of clotting times and MP content in plasma samples from healthy volunteers and septic patients, **P < 0.005. B. Measurement of pro-coagulant activity of annexin V positive MPs in plasma. Results were expressed as PS equivalent (nM). C. MPs isolated from plasma of healthy persons and septic patients were added to fresh re-calcified normal plasma, FXII- or FVII-deficient plasma and the clotting times were determined, **P < 0.005, ***P < 0.0001. D. Plasma MPs derived from two healthy volunteers and four septic patients were incubated with gold-labelled FXII (15 nm gold), gold-labelled HK (5 nm gold), and gold-labelled anti-CD14 antibody (30 nm gold). Representative pictures from one volunteer and two septic patients are shown. E. Measurement of fibrinopeptide A (FPA) in normal human plasma, 20 min after addition of MPs derived from plasma of septic patients. Samples were also treated with HKH20 (100 µM) to prevent an activation of the contact system.
When measuring the content of PS-positive plasma MPs from sepsis patients (Fig. 6B), we found that PS levels were in the range from 6 to 68 nM (a PS concentration above 10 nM is considered as pathologic), whereas the PS levels in all control samples were below 3 nM. Additional statistical analysis of the MP and PS content in these samples revealed a significant positive correlation (Fig. S2A, r = 0.77). Thus, the data show that the content of PS-positive MPs reaches pathological levels in the plasma of septic patients. This in turn equips the MPs with a pro-coagulant surface and may further promote activation of the contact system and generation of bradykinin.
Plasma MPs from septic patients activate both pathways of coagulation
To dissect the influence of the extrinsic and intrinsic pathway of MPs from septic patients, a series of clotting assays was performed. MPs purified from 200 µl plasma were used undiluted in clotting assays (see Experimental procedures). When the MPs were added to normal human plasma, we observed that MPs from seven septic patients (three from S. pyogenes infected patients) caused significantly shorter clotting times as compared with those from healthy volunteers (Fig. 6C, septic patient group 1). Importantly, MPs from these patients also decreased the clotting times in FXII- or FVII-deficient reconstituted plasma (Fig. 6C), suggesting that both pathways contribute to the pro-coagulative activity. However, MPs from sepsis patients that had no effect in human plasma (group 2) were in general also not able to affect the clotting times in FXII- or FVII-deficient plasma (Fig. 6C). Statistical analysis showed a significant negative correlation between the increase in the PS content and the clotting time, when MPs from healthy volunteers and septic patients were analysed (Fig. S2B). This correlation was even more significant when MPs were incubated with FVII deficient plasma (Fig. S2C), suggesting that activation of the contact system is dependent on the PS content. By contrast, the reconstitution of MPs with FXII deficient plasma was not significantly dependent on the PS content (Fig. S2D). These findings point to an important role of PS in activating the contact system on MPs, but not for the extrinsic pathway of coagulation.
To test whether monocyte-derived MPs can be detected in plasma samples from septic patients, MPs from four patients with high pro-coagulant MPs (three patients with a S. pyogenes sepsis) were incubated with CD14 antibodies for analysis by FACS and TEM. In all four samples 5–6% CD14-positive MPs were found, while only less than 1% of MPs from healthy volunteers were of monocyte origin (data not shown). Additional triple immunogold staining and TEM revealed that CD14-positive MPs from all septic patients were able to bind gold-labelled FXII and HK on their surface (Fig. 6D).
Fibrinopeptide A (FPA) is a 16-amino-acid peptide released from the amino terminus of fibrinogen Aα chains by thrombin cleavage. Elevated levels of FPA indicate an increase in fibrin network formation and, thus, measurements of FPA present a sensitive method to determine fibrinogen conversion to fibrin and subsequent clot formation. When MPs isolated from two healthy volunteers were added to normal re-calcified plasma, the FPA levels in the samples was below the detection level (not shown). This was in contrast to MPs from the four septic patients (see above) where a massive release of FPA was seen when subjected to the same experimental protocol (Fig. 6E). When the experiments were performed in the presence of HKH20 a drop in the FPA content in all four septic samples was measured. These results imply that fibrinogen conversion to fibrin and subsequent fibrin network formation are amplified upon contact activation, which in turn may affect clot stability (Fig. 6E). Taken together, the data show that pro-coagulant monocyte-derived MPs are found in the plasma from septic patients. In addition, our results suggest that pro-coagulant MPs have the ability to activate both arms of the coagulation system, which in a concerted manner can create a stable clot.
Discussion
Microvascular thrombosis is a hallmark in infectious diseases (van Gorp et al., 1999), and it can, under systemic conditions, contribute to serious and life-threatening complications. When activated locally, however, microvascular thrombosis can be also part of the immune response for instance by preventing bacterial spreading (Dixon, 2004). Both scenarios (systemic versus local) depend on a coordinated thrombus formation and in vivo studies have demonstrated an important role for MPs in these processes (Furie and Furie, 2005). When looking at the molecular mechanisms involved, it was found that pro-coagulant MPs can be incorporated into a growing thrombus in a P-selectin-dependent manner. This will then trigger thrombin generation and fibrin clot propagation via TF pathway (Falati et al., 2003). These findings suggest that MPs exposing TF play a central role in the initiation of coagulation under pathological conditions (Nomura et al., 2008).
Streptococcus pyogenes is one of the most important pathogens encountered worldwide (Carapetis et al., 2005) and it was estimated by the WHO that severe streptococcal infections cause the death of at least 517 000 patients, annually (Carapetis et al., 2005). The pathogenesis of invasive infections with this microorganism is often combined with a systemic activation of the coagulation system and an overwhelming inflammatory response. Either of the two host responses is considered to contribute to deleterious conditions during the course of disease. Among the different streptococcal virulence factors studied, M proteins have attracted considerable attention, since they can evoke pathologic pro-coagulative and pro-inflammatory reactions by targeting different cell types such as monocytes, neutrophils, and T-cells (Oehmcke et al., 2010). The aim of this study was to examine whether M protein can in addition evoke systemic responses by the generation of MPs, a highly mobile carrier of pro-coagulative and pro-inflammatory activity. A special focus was set on MPs derived from monocytes, since monocytes are thought to be the major source of TF positive MPs (Owens and Mackman, 2011).
Our results show that purified blood mononuclear cells, stimulated with M1 protein, release pro-coagulant TF positive MPs, and also virulence factors from other bacterial pathogens were found to have similar activities. These findings point to a general patho-physiological role of pro-coagulant MPs in infectious diseases. Our data also show that the pro-coagulant activity of MPs is triggered by the extrinsic pathway in concert with activation of the intrinsic pathway. Notably, other bacterial virulence factors apart from M1 protein are also able to release pro-coagulant MPs from PBMCs, suggesting that the mechanisms described here are of general nature and not restricted to infections caused by S. pyogenes.
Our results raise the question about the pathophysiological relevance of MPs in their ability to activate the extrinsic and the intrinsic pathways of coagulation. It is noteworthy that less than a decade ago it was generally accepted that the contact system only plays a secondary role in hemostasis, because patients with deficiencies in FXII, PK or HK normally do not suffer from bleeding disorders. The point of view has, however, changed when Renné and colleagues reported in 2005 that the contact system contributes to the formation and stabilization of three-dimensional thrombi (Rennéet al., 2005). Taking these findings in consideration it seems plausible that pro-coagulant MPs induce thrombus formation via the TF pathway, followed by an activation of the intrinsic pathway that helps to stabilize the formed clot. As a low concentration of contact factors correlates with a fatal outcome of the disease (Pixley and Colman, 1997), it is tempting to speculate that patients with an increased number of pro-coagulative MPs and low contact factors levels will form non-stable clots that can end-up as microthrombi in the circulation. This condition, also referred as disseminated intravascular coagulation, is often seen in patients with systemic coagulation dysfunction and is associated with high mortality rates.
Having a closer look at the molecular mechanisms that lead to an activation of the contact system on MPs, we established an important role for PS as a docking and activation site for contact factors. The importance of PS as activator of the contact system has been controversially discussed in the literature. For instance Schousboe (1988) found amidolytic activity of contact factors when PS was added to plasma, while Rojkjaer and Schousboe (1997) reported that FXII binds to, but does not become activated on PS vesicles. Our study is in line with the first report and we show a critical role of PS as activator of the contact system, leading to an increased PK activity and the release of BK.
BK release by contact system activation is another example showing how tightly hemostasis and inflammation are linked together. Here we report for the first time that BK is released from MPs. Kinins are potent inducers of vascular leakage, hypovolemic hypotension and oedema formation, which are critical features in severe bacterial infections.
Taken together, the data presented here show an important role for the contact system on pro-coagulant MPs. Our findings also support the notion that this mechanism contributes to clot stabilization and the induction of inflammatory reactions. As hemostasis and inflammation are tightly linked processes, a better understanding of the bacterial virulence mechanisms that target both cascades may lead to novel therapeutic concepts in infectious diseases.
Experimental procedures
M1 protein, M3 protein, M5 protein, peptostreptococcal albumin-binding protein (PAB) and protein SIC were purified described before (Åkesson et al., 1996; Collin and Olsén, 2000; Påhlman et al., 2006). LPS and LTA were obtained from Sigma-Aldrich (USA). Deficient plasmas were from George King Bio-Medical (Overland Park, KS, USA). Plasmas from septic patients were from the University Hospital, Lund and Malmö, Sweden. The project protocol was approved by the ethics committee of Lund University Hospital, and informed consent was obtained from all patients or their close relatives. Blood was transferred to plastic tubes and centrifuged (2000 g for 10 min). To obtain platelet free plasma (PFP) samples were centrifuged (10 000 g for 5 min) and supernatants were frozen at −80°C. HKH20 (HKHGHGHGKHKNKGKKNGKH) was produced as described (Oehmcke et al., 2009b).
Preparation of MPs from PBMCs or plasma
Peripheral blood mononuclear cells were isolated from human citrated blood from healthy volunteers as described (Påhlman et al., 2007). The PBM cell layer was collected; cells were washed twice in PBS and resuspended in RPMI medium (Invitrogen). PBMCs (2 × 106 cells ml−1) were treated with different M proteins (1 µg ml−1), LPS (100 ng ml−1), LTA (1µg ml−1) or medium alone (final volume of 1000 µl). After an overnight incubation on rotation at 37°C, cells were centrifuged (400 g for 20 min). Supernatants were kept frozen (−80°C) until use. Samples (900 µl supernatant or 200 µl plasma) were thawed at 37°C and centrifuged (20 817 g for 30 min) at room temperature to pellet the MPs. Supernatants were removed and 900 µl of a buffer containing 15 mM Hepes (ICN Biomedicals, Aurora, OH, USA), and 135 mM NaCl (Hepes buffer) were added. MPs were pelleted by centrifugation, 800 µl of the supernatant were removed, and the MPs were resuspended in the remaining 100 µl buffer.
Flow cytometric analysis of plasma samples and MPs
To identify the cellular origin of MPs, 10 µl purified MPs were mixed with 30 µl Hepes buffer containing 2.5 mmol CaCl2 and incubated with 5 µl PE-labelled and 5 µl FITC-labelled monoclonal antibody (mAb) for 20 min. The following antibodies and proteins were used: 100 µg ml−1 IgG1-FITC isotype control mouse (Antibodies-online GmbH, Aachen, Germany), IgG1-PE isotype control mouse (Antibodies-online GmbH, 100 µg ml−1), 60 µg ml−1 anti-CD14-PE (Dako, Glostrup, Denmark), 100 µg ml−1 anti-TF-FITC (American Diagnostica, Stamford, CT, USA), and 5 µl annexin V-PE (Invitrogen, Carlsbad, CA, USA). The incubation was terminated by adding 500 µl Hepes buffer containing 2.5 mmol l−1 CaCl2. Samples were analysed in a FACSCalibur flow cytometer with CellQuest software (Becton Dickinson, San Jose, CA, USA). Both forward scatter and sideward scatter were set at a logarithmic gain. MPs were identified on forward scatter, sideward scatter and by binding of annexin V and cell-specific mAbs. Non-specific binding and auto-fluorescence were corrected by subtracting the fluorescence signal of the control IgG antibodies. For all experiments the number of MPs were determined by using a fluorescent bead count based assay (Orozco and Lewis, 2010). Ten microlitres of PFP or purified MPs was mixed with 10 µl Rainbow Calibration Particles (BD Biosciences, 6–6.4 µm) and 550 µl Hepes buffer. Due to their size, Rainbow Calibration Particles can be easy differentiated from MPs when analysed by light scatter. The Rainbow Calibration Particles (200 µl−1) were counted (1000 events) on a separate gate and the number of MPs was calculated.
Clotting experiments
Clotting times were measured using an Amelung coagulometer as described before (Oehmcke et al., 2009b). To test the pro-coagulant effect of MPs, 50 µl of human citrated plasma was reconstituted with 50 µl 30 mM CaCl2 and 5 µl washed MP suspensions was added (concentration 50–150 MPs µl−1). The time for clot formation was determined. Alternatively, FXII- or FVII-deficient plasma was used.
Clot lysis
Clots were generated with MPs from M1 protein-stimulated PBMCs as described above. After 1 and 10 min, 25 µl Actilyse (1 mg ml−1, Boehringer Ingelheim GmbH, Germany) were added to the clot and resuspended. The time to clot lyses was measured in the Amelung coagulometer.
Phospholipid preparation
PC and PS (Sigma-Aldrich, USA) liposomes were prepared by adding the phospholipids (100 µM) to Eppendorf tubes. Samples were centrifuged in a SpeedVac to evaporate chloroform. Hepes buffer was added, the mixture was vortexed for 5 min, and subjected to 10 cycles of ultrasonication (Hielscher Ultrasound Technology, Teltow, Germany).
Chromogenic substrate assay
Microparticles were resuspended (150 MPs µl−1) in Hepes buffer. Alternatively phospholipids were prepared as described above and diluted to a concentration of 100 µM. Ten microlitres of citrated plasma was incubated with 10 µl MPs, or 1 µl phospholipids (PS or PC), or 10 µl Hepes buffer (negative control) for 2 min and added to 100 µl Hepes containing 500 µM ZnCl2 and 1 mM chromogenic substrate S-2302 (Chromogenix, Milano, Italy). After 15, 30 and 60 min of incubation at 37°C, the absorbance was measured at 405 nm in an ELISA reader.
Bradykinin assay
BK contents were determined by ELISA as described (Mattsson et al., 2001). MPs and phospholipids were prepared and diluted as described (chromogenic substrate assay).
TF activity ELISA
The Actichrome TF activity assay kit (American Diagnostica) was used to quantify the TF pro-coagulant activity in the MP samples.
Measurement of MP pro-coagulant activity
The ACTICHROME Microparticle Activity kit (American Diagnostica) was used according to the instructions of the manufactory, to measure the pro-coagulant activity of MPs. Results were expressed as PS equivalent (nM) by employing a standard curve with liposomes of known PS concentrations from the kit. The system does not allow the capture of lipoproteins, and the eventual presence of TF on captured MP does not alter values corresponding to PS content, as it is based on a prothrombinase assay (Jy et al., 2004).
Measurement of fibrinopeptide A (FPA) release
To investigate the release of FPA in human plasma treated with MPs, the IMUCLONE FPA ELISA (American Diagnostica) was used. Ten microlitres of plasma was re-calcified with 10 µl CaCl2 (30 mM) and 2–5 µl of plasma MPs were added. Controls were made with buffer instead of MPs. In some samples HKH20 (100 µM; final concentration) were added. After 20 min of incubation samples were treated twice with 10 µl Bentonite to remove any cross-reactive fibrin(ogen). Samples were kept frozen (−20°C) until ELISA processing. Before usage samples were diluted 100 times in sample dilution buffer.
Histopathological evaluation of clots
Clots were generated as described above and fixed at room temperature for 24 h in buffered 4% formalin (pH 7.4; Kebo). Processing for histopathology occurred as described (Oehmcke et al., 2009b).
Scanning electron microscopy
Clot samples were fixed as previously described (Oehmcke et al., 2009b). Samples were processed and sputtered with palladium/gold as described (Oehmcke et al., 2009a) and examined in a Jeol JSM-350 scanning electron microscope.
Negative staining and transmission electron microscopy
Human proteins (HK, FXII, anti-CD14 AB, and anti-TF AB) were labelled with colloidal gold (30, 15 and 5 nm in diameter, BBI International) as described earlier (Bengtson et al., 2006). MPs or phospholipids were mixed with gold-labelled 20 nM proteins for 20 min at room temperature and processed for negative staining (Bober et al., 2010).
Statistical analysis
Statistical analysis was performed using GraphPadPrism 4.00. The P-value was determined by using the unpaired t-test. All samples were analysed in triplicate and experiments were performed at least three times. The bars in the figures indicate standard deviation.
Acknowledgements
We wish to thank Monica Heidenholm and Maria Baumgarten for excellent technical assistance, and Rita Wallén and Eric Hallberg, Cell and Organism Biology, Lund University, and Lina Gefors, The Jubileum Institute, Lund University for support with electron microscopy. This work was supported in part by the European research council (Marie Curie fellowship), the foundations of Alfred Österlund, Crafoord, Greta and Johan Kock, the Medical Faculty, Lund University, and the Swedish Research Council.