Involvement of suppressor of cytokine signalling-1-mediated degradation of MyD88-adaptor-like protein in the suppression of Toll-like receptor 2-mediated signalling by the murine C-type lectin SIGNR1-mediated signalling
Summary
Dendritic cells recognize pathogens through pattern recognition receptors such as Toll-like receptors and phagocytose and digest them by phagocytic receptors for antigen presentation. This study was designed to clarify the cross-talk between recognition and phagocytosis of microbes in dendritic cells. The murine dendritic cell line XS106 cells were stimulated with the murine C-type lectin SIGNR1 ligand lipoarabinomannan and the Toll-like receptor 2 ligand FSL-1. The co-stimulation significantly suppressed FSL-1-mediated activation of NF-κB as well as production of TNF-α, IL-6 and IL-12p40 in a dose-dependent manner. The suppression was significantly but not completely recovered by knock-down of SIGNR1. SIGNR1 was associated with Toll-like receptor 2 in XS106 cells. The co-stimulation upregulated the expression of suppressor of cytokine signalling-1 in XS106 cells, the knock-down of which almost completely recovered the suppression of the FSL-1-mediated cytokine production by lipoarabinomannan. In addition, it was found that the MyD88-adaptor-like protein in XS106 cells was degraded by co-stimulation with FSL-1 and lipoarabinomannan in the absence, but not the presence, of the proteasome inhibitor MG132 and the degradation was inhibited by knock-down of suppressor of cytokine signalling-1. This study suggests that Toll-like receptor 2-mediated signalling is negatively regulated by SIGNR1-mediated signalling in dendritic cells, possibly through suppressor of cytokine signalling-1-mediated degradation of the MyD88-adaptor-like protein.
Introduction
Detection of bacterial invasion and the uptake and killing of bacteria are key functions of the innate immune system. Phagocytes, such as macrophages, neutrophils and dendritic cells (DCs), play important roles in combating bacterial infection. These phagocytes are able to detect bacterial invasion through various pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) before uptake and degradation of bacteria. To date, more than 10 TLRs have been identified and have been shown to be critical for signalling by pathogen-associated molecular patterns (PAMPs) such as LPS, peptidoglycans, lipoteichoic acid and lipoprotein/lipopeptide (LP/LPT) (Takeda et al., 2003). The activation of innate immunity by TLRs also leads to the development of antigen-specific adaptive immunity. Thus, TLRs also play a key role in bridging between innate immunity and adaptive immunity (Takeda et al., 2003). Therefore, it is thought that TLRs not only detect bacterial invasion but also play essential roles in activating signal transduction pathways leading to the killing and clearance of pathogens, since the events after detection of bacterial invasion are uptake and degradation of pathogens for presentation of antigens in the context of MHC to T cells. Doyle et al. (2004) have recently demonstrated that numerous TLR ligands specifically enhance phagocytosis of bacteria by macrophages. Blander and Medzhitov (2004) have also suggested that TLR-mediated signallingregulates bacterial phagocytosis. We have also demonstrated that the TLR2 ligand FSL-1 [mycoplasmal diacylated LPT, S-(2,3-bispalmitoyloxypropyl)CGDPKHPKSF] (Shibata et al., 2000) upregulates scavenger receptors and C-type lectins (CLs), such as MSR1 (macrophage scavenger receptor 1), CD36, DC-SIGN (dendritic cell-specific ICAM3 grabbing non-integrin) and Dectin-1, through TLR2-mediated signalling pathways and that TLR2 by itself does not function as a phagocytic receptor (Mae et al., 2007). Thus, there have been several reports on how TLR signalling affects phagocytosis of bacteria. However, little is known about how phagocytosis signalling affects recognition of microbes by TLRs.
In the immune system, CLs and CL-like receptors (CLRs) have been shown to function both as phagocytic receptors and as pathogen recognition receptors (Cambi and Figdor, 2003). CLs contain the so-called carbohydrate recognition domain (CRD) that binds a carbohydrate structure in a calcium-dependent manner. CLs are produced either as transmembrane proteins or as soluble proteins. Soluble CLs include members of the collectin family. Transmembrane CLs include selectins (Ley and Kansas, 2004), the mannose receptor family (East and Isacke, 2002) and DC-SIGN (Geijtenbeek et al., 2000). DC-SIGN was first identified as a DC-specific CL that regulates the first contact between DCs and resting T cells by binding to ICAM-3 (Geijtenbeek et al., 2000). Human DC-SIGN is a 44 kDa type II transmembrane receptor displaying a CRD that is separated from the transmembrane region and cytoplasmic tail by a neck domain that consists of seven tandem repeats and one incomplete tandem repeat (van Kooyk and Geijtenbeek, 2003). Recently, we have demonstrated that Ca2+ binding sites in the CRD of DC-SIGN were involved in phagocytosis of bacteria as well as multimerization of DC-SIGN and that the neck region played a role in efficiency of binding to microbes as well as multimerization of the protein (Iyori et al., 2008). DCs are one of the key players that bridge innate immunity to adaptive immunity (Iwasaki and Medzhitov, 2004; Pulendran, 2004). DCs, which are the most effective antigen-presenting cells capable of inducing robust CD4+ and CD8+ T cell immunity, are one of the key regulators in determination of Th1/Th2 balance (Banchereau and Steinman, 1998; Iwasaki and Medzhitov, 2004; Pulendran, 2004). DCs recognize pathogens through PRRs including TLRs and phagocytose them for antigen presentation.
On the basis of these reasons, we have been interested in the cross-talk between recognition of microbes by TLRs and CL-mediated phagocytosis of microbes in DCs. This study was therefore designed to investigate how the murine DC-SIGN homologue SIGNR1 (Koppel et al., 2005)-mediated signals affect the TLR-mediated signals in a murine DC cell line, XS106 (Mohan et al., 2005).
This study demonstrated that SIGNR1 ligation by ManLAM (lipoarabinomannan) suppresses TLR2-mediated production of pro-inflammatory cytokines such as TNF-α, IL-6 and IL-12p40 through degradation of Mal (MyD88-adaptor-like protein; also known as TIRAP), an adaptor molecule downstream of TLR2, possibly mediated by SOCS-1 (suppressor of cytokine signalling-1).
Results
Effects of SIGNR1 ligation on the TLR2-mediated signalling pathway in murine DCs
To begin to examine effects of DC-SIGN ligation on the recognition of diacylated LPT FSL-1 by TLR2, we confirmed whether the murine DC XS106 cells express both the murine DC-SIGN homologue SIGNR1 (Park et al., 2001) and TLR2. It was found that TLR2 was highly expressed on the surface of XS106 cells (Fig. 1A) and that stimulation with the TLR2 ligand FSL-1 induced production of TNF-α by the cells in a dose-dependent manner (Fig. 1B), suggesting that XS106 cells express functional TLR2 on the cell surface. In addition, XS106 cells expressed SIGNR1 at both transcript and protein levels as confirmed by real-time RT-PCR (Fig. 1C) and immunoblotting (Fig. 1D) respectively. An experiment therefore was carried out to determine the effects of SIGNR1 ligation with Man9 (Mannonanose-di-N-acetyl-d-glucosamine) (Fig. 2A) or ManLAM (Fig. 2B) on FSL-1-induced production of TNF-α, IL-6 and IL-12p40 by XS106 cells. FSL-1 stimulation by itself induced production of these cytokines by the cells in a dose-dependent manner, whereas stimulation of the cells with either Man9 or ManLAM did not induce production of these cytokines (Fig. 2A and B). Co-stimulation with Man9 or ManLAM significantly suppressed the FSL-1-induced production of TNF-α, IL-6 and IL-12p40 in a dose-dependent manner (Fig. 2A and B). Co-stimulation with ManLAM also suppressed the production of IL-12p40 by XS106 cells induced by stimulation with other TLR2 ligands, PGN (peptidoglycan) (Fig. 2C) or Pam3CSK4[S-(2,3-bispalmitoyloxypropyl)-N-palmitoyl-CKKKK] (Fig. 2D).
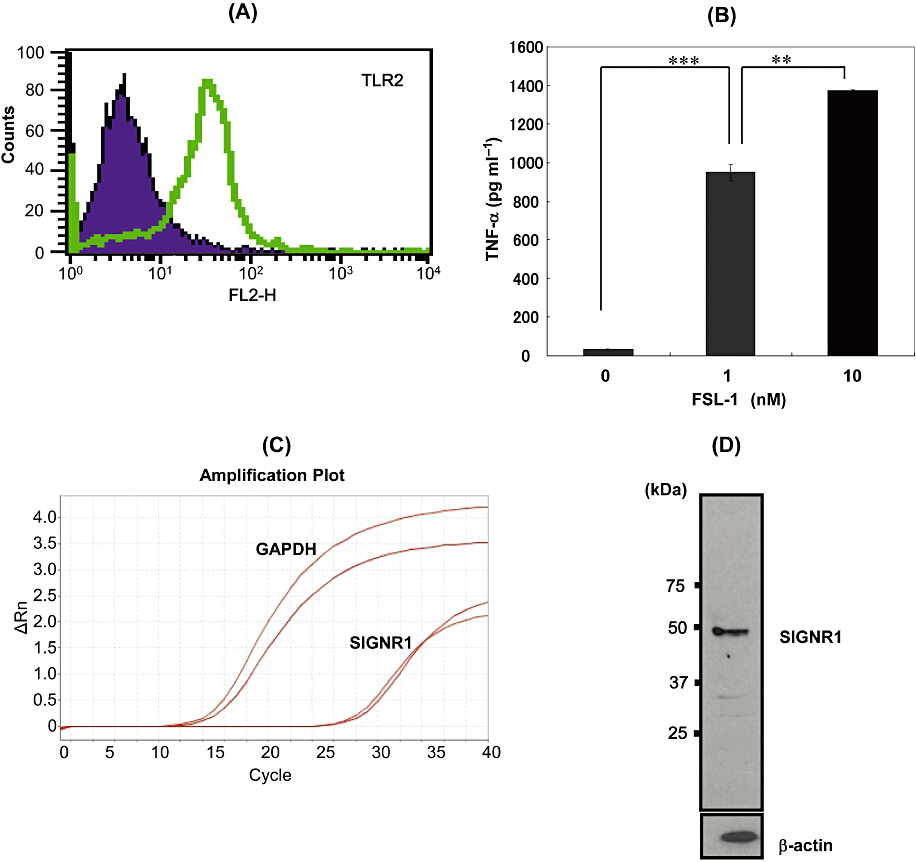
Expression of functional TLR2 and SIGNR1 in XS106 cells. A. XS106 cells were incubated on ice with PE-conjugated anti-mouse TLR2 mAb (green) or PE-conjugated rat IgG2b, κ (purple) as an isotype control. The cells were fixed in PBS containing formaldehyde and then analysed by a flow cytometer. Data for 30 000 cells falling within appropriate forward and side light scatter gates were collected from each sample. Data were analysed using CellQuest software. B. XS106 cells were stimulated at 37°C for 6 h with 0, 1 and 10 nM FSL-1, and the supernatant was collected. The amount of TNF-α produced in the supernatant was determined by ELISA. Results are expressed as means ± SD of values from three separate experiments. Student's t-test (**, 0.001 < P < 0.01; and ***, P < 0.001). C. Total RNA was extracted from XS106 cells and cDNA was synthesized. For real-time PCR analysis, PCR amplification was performed in the presence of a TaqMan probe specific for SIGNR1 by using A StepOne Real-Time PCR system. D. Cell proteins of XS106 cells were separated by SDS-PAGE, transferred to a Nitrocellulose Transfer Membrane, and incubated at room temperature for 1 h with an anti-SIGNR1 Ab or anti-β-actin mAb. Immunoblots were developed by HRP-conjugated anti-mouse IgG Ab. Immunoreactive proteins were detected by using ECL™ detection reagents.
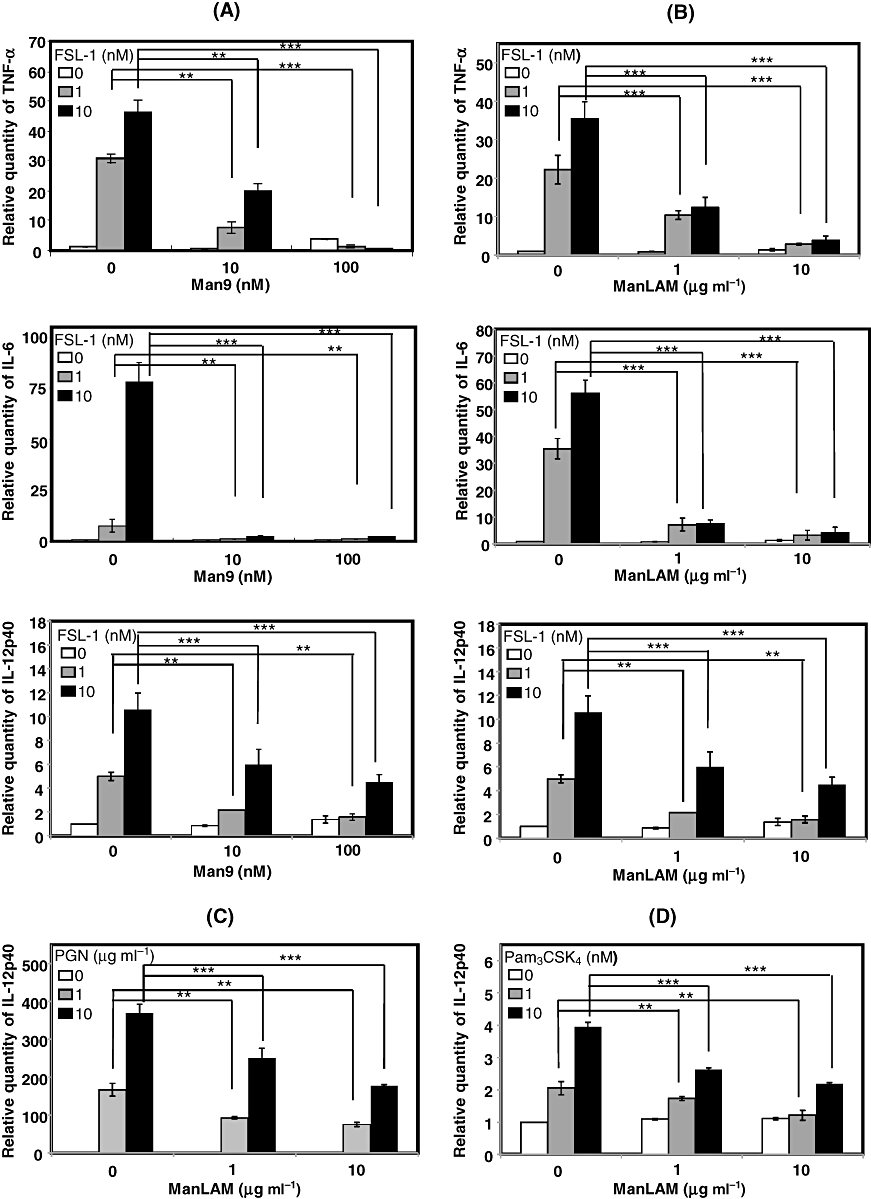
Effects of SIGNR1 ligation on the TLR2-mediated signalling pathway in XS106 cells. XS106 cells were incubated at 37°C for 6 h with 0, 1, 10 nM FSL-1 in the absence or presence of Man9 (10, 100 nM) (A) or ManLAM (1, 10 µg ml−1) (B). Total RNA was extracted from XS106 cells and cDNA was synthesized. For real-time PCR analysis, PCR amplification was performed in the presence of TaqMan probes specific for TNF-α, IL-6 and IL-12p40 by using A StepOne Real-Time PCR system. XS106 cells were stimulated with PGN (C) and Pam3CSK4 (D) as TLR2 ligands and the amount of IL-12p40 was determined. Results are expressed as means ± SD of values from three separate experiments. Student's t-test: *, 0.01 < P < 0.05; **, 0.001 < P < 0.01; ***, P < 0.001.
It is well known that the TLR2-mediated signal leads to the activation of NF-κB (Takeda et al., 2003). Therefore, XS106 cells were transfected with NF-κB luciferase reporter genes and then stimulated with FSL-1 and/or Man9. Expectedly, we found that co-stimulation with Man9 significantly suppressed the FSL-1-mediated activation of NF-κB (Fig. 3).
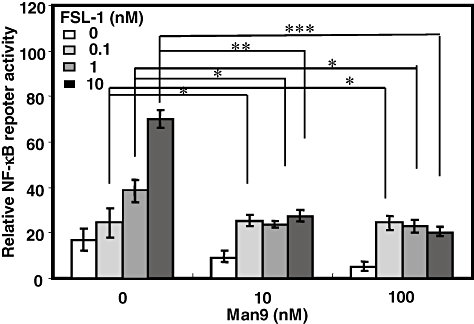
FSL-1-induced NF-κB activation in XS106 cells transiently transfected with an NF-κB reporter plasmid and Renilla luciferase control reporter plasmid. XS106 cells were transiently transfected with an NF-κB reporter plasmid and a construct directing expression of Renilla luciferase under the control of a constitutively active thymidine kinase promote. After 24 h incubation, the cells were stimulated at 37°C for 6 h with various concentrations (0.1, 1, 10 nM) of FSL-1 in the absence or presence of Man9, and luciferase activity was measured using a Dual-Luciferase reporter assay system according to the manufacturer's instructions. Results are expressed as means ± SD of values from three separate experiments. Student's t-test (*, 0.01 < P < 0.05; **, 0.001 < P < 0.01 and ***, P < 0.001).
Thus, these results demonstrated that only SIGNR1 ligation did not induce any cytokine production by murine DCs, whereas co-stimulation with SIGNR1 and TLR2 suppressed the TLR2-mediated production of pro-inflammatory cytokines, possibly through regulation of NF-κB activation.
Since ManLAM is a ligand for macrophage mannose receptor (MMR) (Bernardo et al., 1998), there is a possibility that the suppression of TLR2 signal was induced by MMR-mediated signal. Therefore, in order to further confirm whether the SIGNR1-mediated signal is able to suppress TLR2-mediated NF-κB activation, SIGNR1 was silenced by transiently transfecting XS106 cells with siRNA targeting the SIGNR1 gene. First, we confirmed that the transcription of SIGNR1 was downregulated by transfection with the siRNA (Fig. 4A). An experiment was therefore carried out to determine whether the SIGNR1-induced suppression of TLR2-mediated cytokine production was recovered by silencing of SIGNR1. As a result, it was found that the suppression of FSL-1-induced production of TNF-α, IL-6 or IL-12p40 by ManLAM was significantly but not completely recovered by SIGNR1-specific siRNA (Fig. 4B) but not by non-specific siRNA (Fig. 4C).
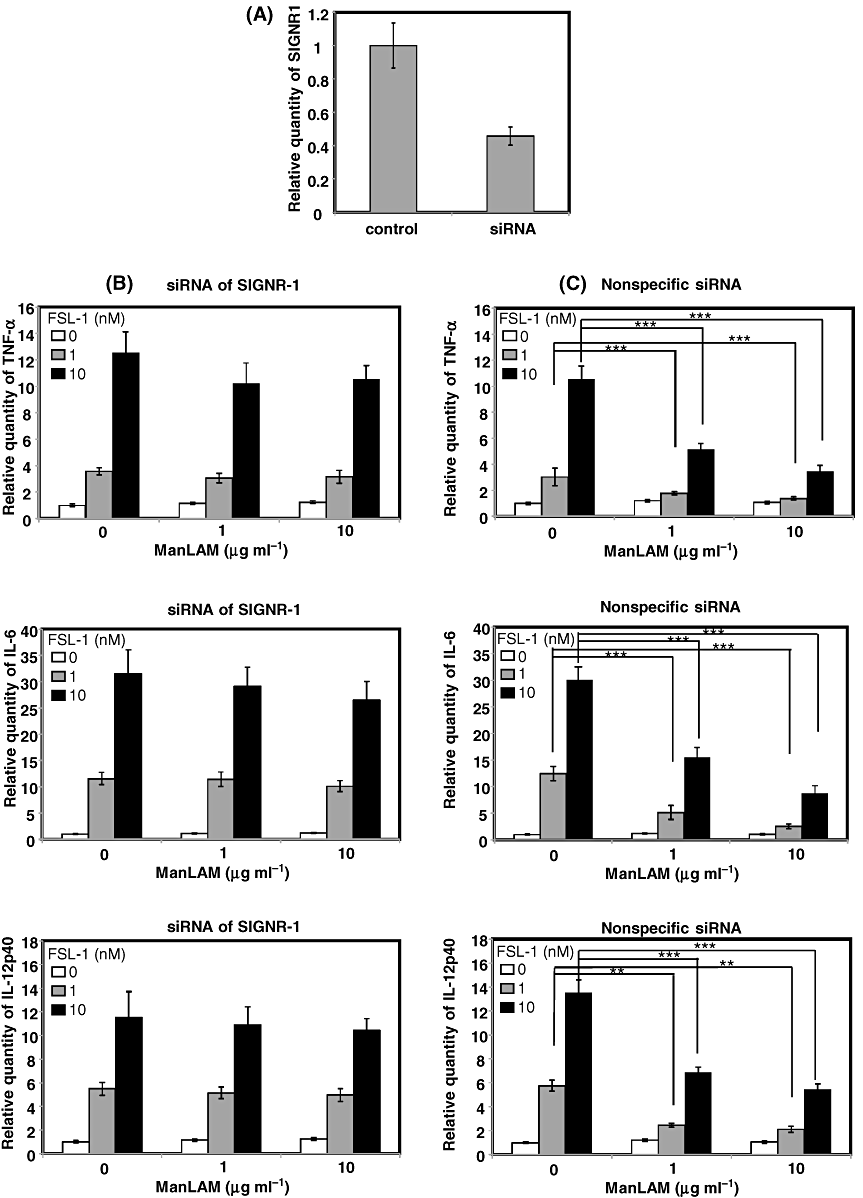
Effects of knock-down of SIGNR1 on the suppression of FSL-1-mediated expression of mRNAs of TNF-α, IL-6 and IL-12p40 by SIGNR1 ligation with ManLAM. XS106 cells were transiently transfected with SIGNR1-specific siRNA (B) or non-specific siRNA (C) by electroporation. Knock-down of SIGNR1 mRNA was confirmed by real-time PCR (A). XS106 cells were incubated with or without 10 nM FSL-1 in the absence or the presence of ManLAM (10 µg ml−1). Total RNA was extracted from XS106 cells and cDNA was synthesized with the High Capacity cDNA reverse transcription kit. For real-time PCR analysis, PCR amplification was performed in the presence of TaqMan probes specific for TNF-α, IL-6 and IL-12p40. Results are expressed as means ± SD of values from three separate experiments. Student's t-test (**, 0.001 < P < 0.01; and ***, P < 0.001).
These results strongly suggest that the SIGNR1-mediated signal, but not MMR-mediated signal, plays an important role in suppression of the TLR2-mediated production of pro-inflammatory cytokines in murine DCs.
Association of TLR2 with DC-SIGN or SIGNR1
It is well known that TLR2 is localized in cell membranes and recognizes triLPTs or diLPTs in collaboration with TLR1 and TLR6 respectively (Takeuchi et al., 2001; Takeda et al., 2003; Kataoka et al., 2006; Nakata et al., 2006). In addition, we and others have found that the human SIGNR1 homologue DC-SIGN is also localized as a tetramer in cell membranes (van Kooyk and Geijtenbeek, 2003) and that it is involved in the phagocytosis of bacteria (Iyori et al., 2008). Therefore, next experiment was carried out to determine whether DC-SIGN was associated with TLR2 in the membrane. For this purpose, HEK (human embryonic kidney) 293 cells were transiently transfected with genes of a FLAG-tagged TLR2 and DC-SIGN and examined for the association of TLR2 and DC-SIGN by immunoprecipitation. As a result, we found that the monomer and oligomer (possibly tetramer) of DC-SIGN were immunoprecipitated with TLR2 in the absence or presence of a BS3[bis(sulfo-succimidyl) suberate] cross-linker, respectively, suggesting that TLR2 is associated with DC-SIGN oligomer (Fig. 5A). In addition, it was found that FSL-1 stimulation enhanced the association of DC-SIGN and TLR2 (Fig. 5A). Thus, DC-SIGN was associated with TLR2 in the membrane of the transfectants. Therefore, next experiment was carried out to investigate whether endogenous SIGNR1 was associated with TLR2 in XS106 cells. We found that monomer and dimer of endogenous SIGNR1 were immunoprecipitated by anti-TLR2 antibody (Ab) but not by its isotype control in XS106 cells (Fig. 5B). FSL-1 but not ManLAM stimulation seems to make the association tight, although SIGNR1 is associated with TLR2 independent of its ligand (Fig. 5B).
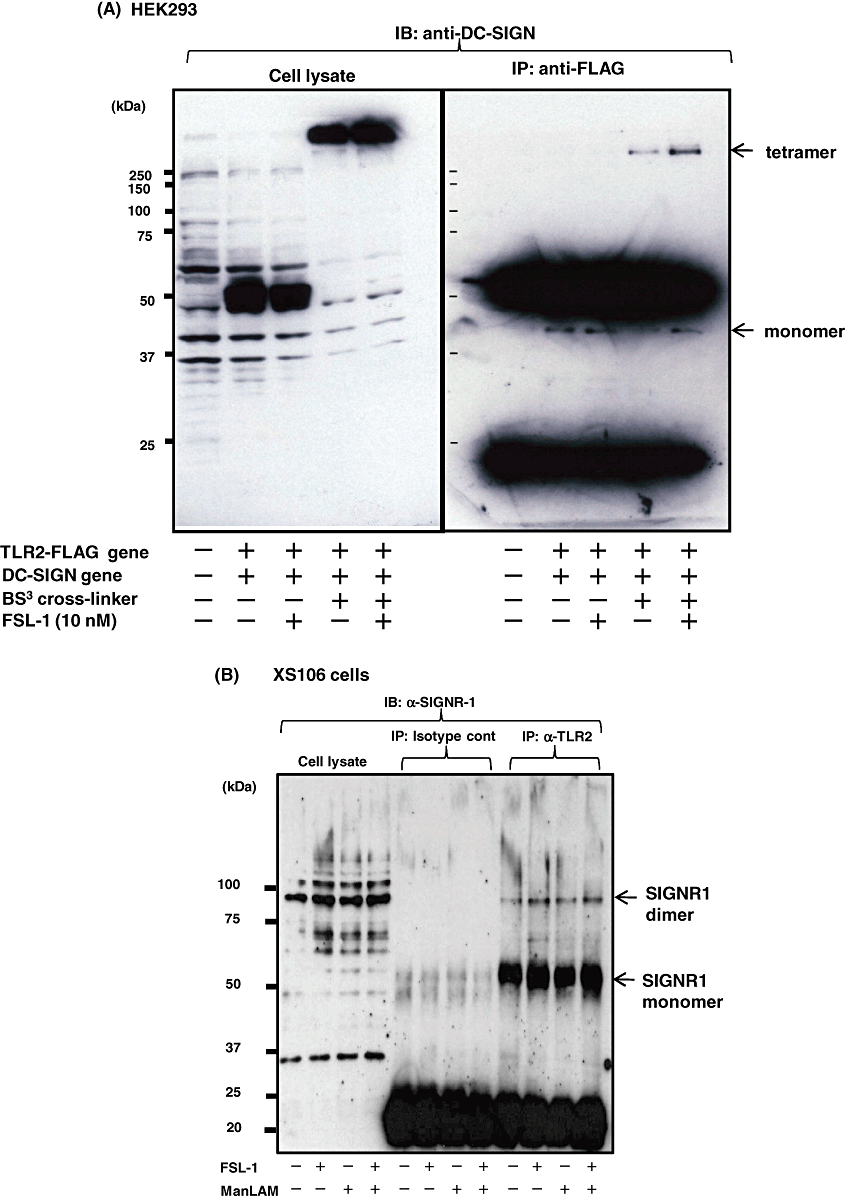
Association of DC-SIGN or SIGNR1 with TLR2 in HEK293/TLR2/DC-SIGN or XS106 cells. HEK293/TLR2/DC-SIGN or XS106 cells were plated at 1 × 107 cells in a 10 cm dish in their suitable media and incubated at 37°C for 16 h. After the cells had been washed three times with base medium, they were stimulated for various periods of time with FSL-1 (10 nM) or ManLAM (10 µg ml−1). Cell stimulation was terminated by a lysis buffer for total-cell lysates and for immunoprecipitation. All control immunoprecipitates (IPs) were performed with rabbit or mouse IgG depending on Abs used. Proteins were separated by SDS-PAGE, transferred to a Nitrocellulose Transfer Membrane, and blotted with the appropriate Abs. For cross-linking experiments, HEK293/TLR2/DC-SIGN cells were treated with a bis[sulfosuccinimidyl] suberate (BS3) before being lysed according to the manufacturer's instructions. See text for details.
These results strongly suggest that SIGNR1 and its human homologue DC-SIGN, possibly as monomer and oligomer, are associated with TLR2 in the membrane of DCs.
Involvement of SOCS-1 in the suppression of TLR2 signalling by SIGNR1 signalling
It has recently been reported that SOCS-1 is associated with DC-SIGN and negatively regulates TLR2 signals during Mycobacterium tuberculosis infection (Srivastava et al., 2009). Therefore, we first examined whether FSL-1 and/or ManLAM affected the expression of SOCS-1 in the murine DC XS106 cells at the protein level. It was found that stimulation of XS106 cells with both ManLAM and FSL-1 upregulated the expression of SOCS-1 (Fig. 6A), which is consistent with the recent finding (Srivastava et al., 2009). Next experiment was therefore carried out to determine whether endogenous SIGNR1 was associated with SOCS-1 in the cells. It was found that monomer of SIGNR1 was associated with SOCS-1 in the presence of a DSS (disuccnimidyl suberate) cross-linker (Fig. 6B). Judging from the finding that SIGNR1 is associated with TLR2, SOCS-1 is thought to be associated with cytoplasmic domains of the SIGNR1 and TLR2 complex in XS106 cells, because SOCS-1 is a cytoplasmic protein.
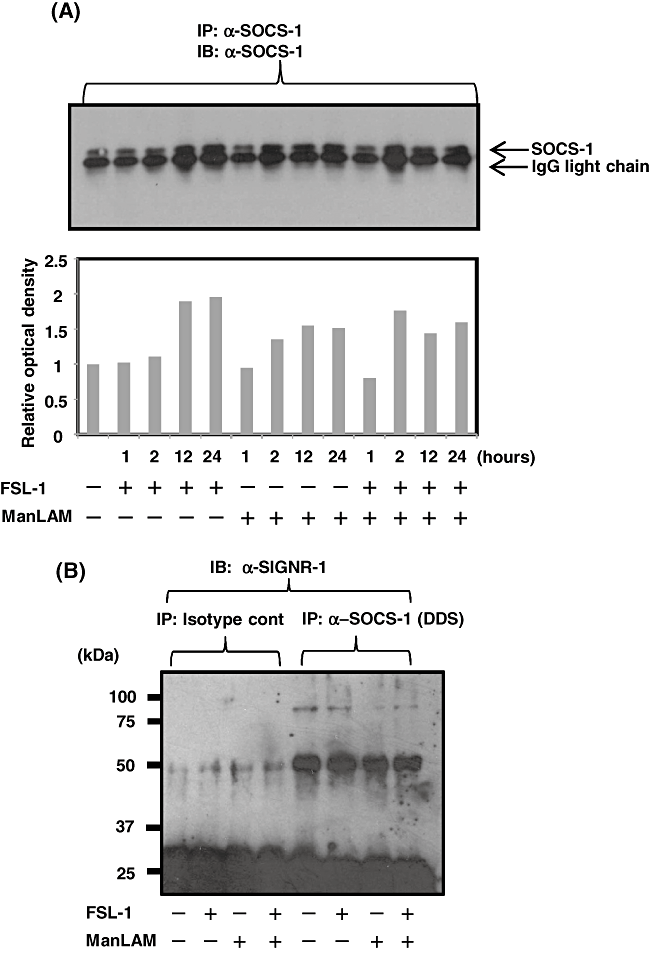
Induction of SOCS-1 in XS106 cells by ManLAM and FSL-1 and association of SOCS-1 with SIGNR1. A. XS106 cells were incubated for various periods of times with 10 nM FSL-1 in the absence or presence of ManLAM (10 µg ml−1). The cells were lysed in a lysis buffer and immunoprecipitated with anti-SOCS-1 Ab. Proteins were separated by SDS-PAGE, transferred to a Nitrocellulose Transfer Membrane and blotted with anti-SOCS-1 Ab. Immunoblots were developed by HRP-conjugated anti-mouse IgG Ab. Density of each SOCS-1 band was analysed by the software image J and are shown in lower figure. B. XS106 cells were incubated with 10 nM FSL-1 in the absence or presence of ManLAM (10 µg ml−1) and then treated with DDS cross-linker. The cells were lysed and treated as described above. Proteins were separated by SDS-PAGE, transferred to a Nitrocellulose Transfer Membrane, and reacted with anti-SIGNR1 mAb. See text for details.
In order to further confirm the involvement of SOCS-1 in the suppression of TLR2 signal by SIGNR1 signal, we examined effects of silencing of SOCS-1 by transiently transfecting XS106 cells with siRNA targeting the SOCS-1 gene on the suppression. First, we confirmed that the transcription of SIGNR1 was downregulated by transfection with the siRNA (Fig. 7A). Next experiment was therefore carried out to determine whether the SIGNR1-induced suppression of TLR2-mediated cytokine production was recovered by silencing of SOCS-1. As a result, it was found that the suppression of FSL-1-induced production of TNF-α, IL-6 and IL-12p40 by ManLAM was almost completely recovered by SOCS-1-specific siRNA (Fig. 7B) but not by non-specific siRNA (Fig. 7C).
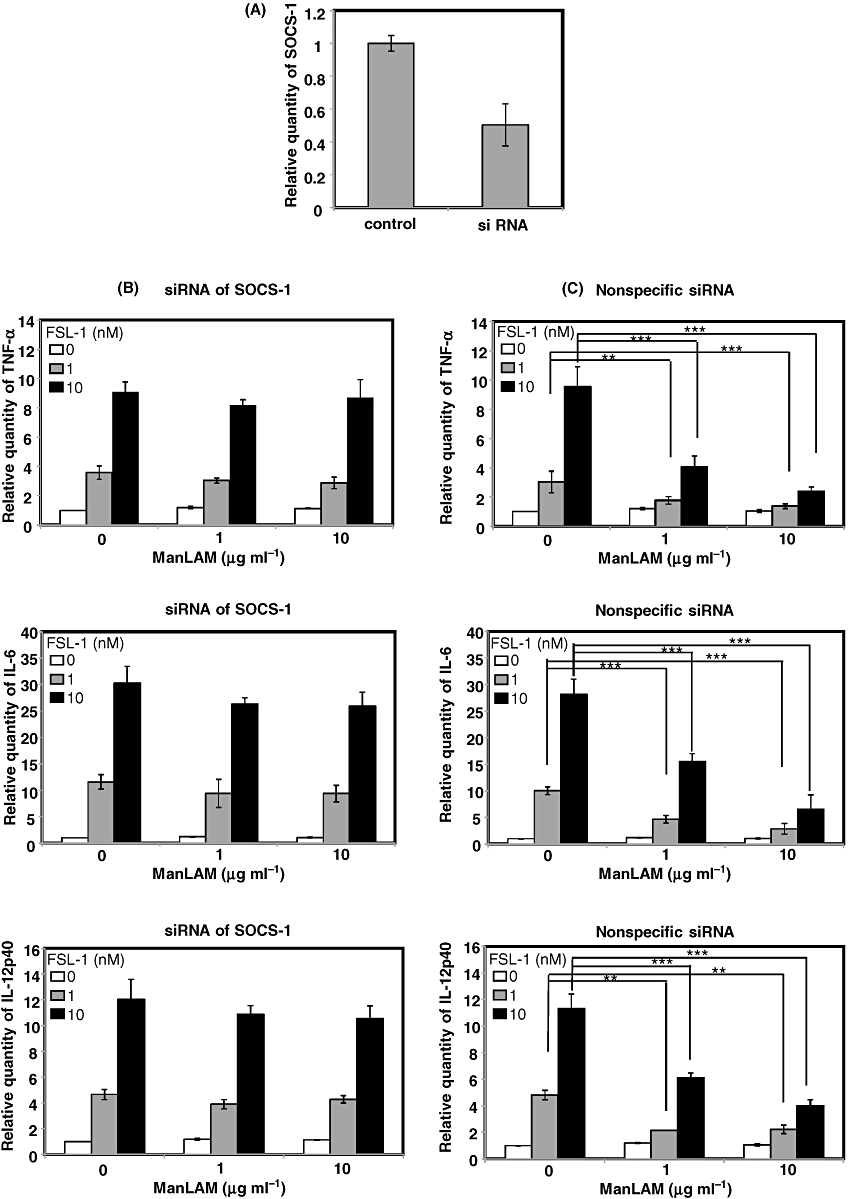
Effects of knock-down of SOCS-1 on the suppression of FSL-1-mediated expression of mRNAs of TNF-α, IL-6 and IL-12p40 by SIGNR1 ligation with ManLAM. XS106 cells were transiently transfected with siRNAs specific or non-specific for SOCS-1 by electroporation. Knock-down SOCS-1 mRNA was confirmed by real-time RT-PCR (A). XS106 cells transfected with siRNAs specific (B) or non-specific (C) for SOCS-1 were incubated at 37°C for 6 h with FSL-1 (0, 1, 10 nM) in the absence or presence of ManLAM (0, 1, 10 µg ml−1). Total RNA was extracted from XS106 cells and cDNA was synthesized with the High Capacity cDNA reverse transcription kit. For real-time RT-PCR analysis, PCR amplification was performed in the presence of TaqMan probes specific for TNF-α, IL-6 and IL-12p40. Results are expressed as means ± SD of values from three separate experiments. Student's t-test (**, 0.001 < P < 0.01; and ***, P < 0.001). See text for details.
Thus, these results strongly suggest that SOCS-1 plays a key role in the suppression of TLR2 signalling by SIGNR1 signalling, although SOCS-1 has already been known as a negative regulator of TLR4 signalling (Kinjyo et al., 2002; Nakagawa et al., 2002).
Ubiquitination and subsequent degradation of Mal
Recently, it has been reported that SOCS-1 binds to tyrosine phosphorylated Mal, an adaptor protein downstream of TLR2, and induces ubiquitination and degradation of Mal in the proteasome, thereby leading to the suppression of activation of NF-κB (Mansell et al., 2006). Therefore, we examined whether stimulation of XS106 cells with both FSL-1 and ManLAM induced degradation of Mal in the absence or the presence of MG132, a proteasome inhibitor. As a result, it was found that the amount of Mal in XS106 cells stimulated with both FSL-1 and ManLAM was significantly smaller than that in cells stimulated with either FSL-1 or ManLAM in the absence of MG132, but not in the presence of MG132 (Fig. 8), suggesting that stimulation of both FSL-1 and ManLAM induces the degradation of endogenous Mal in the proteasome. In addition, we found that the degradation of Mal was also inhibited by silencing of SOCS-1 by specific siRNA (Fig. 8), suggesting that SOCS-1 plays a key role in the degradation of Mal. Next experiment was carried out to determine whether degradation of Mal was observed even in HEK293 transfectants. For this purpose, HEK293 cells (HEK293TLR2/DC-SIGN/Mal–GFP) stably expressing TLR2, DC-SIGN and Mal–GFP fusion protein were established. The transfectant expressed TLR2 and DC-SIGN on the cell surface (Fig. 9A) and Mal–GFP in the cytosol (Fig. 9B). HEK293/TLR2/DC-SIGN/Mal–GFP transfectants mainly consisted of two populations expressing high and low Mal–GFP (Fig. 9B). They were stimulated for 30 min with or without 10 nM FSL-1 and/or 10 µg ml−1 ManLAM. The stimulation induced the reduction in the number of these two populations expressing Mal–GFP and in MFI from 165.8 to 147.7 in the transfectant (Fig. 9C).
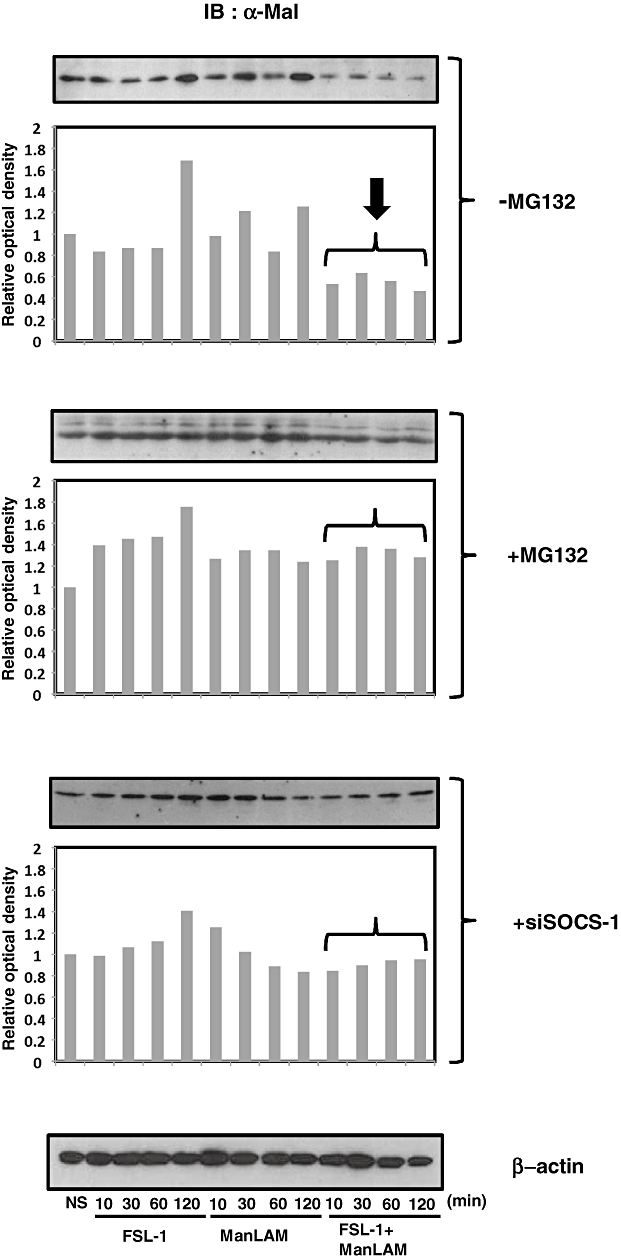
Degradation of Mal in XS106 cells co-stimulated with ManLAM and FSL-1 by Immunoblotting. XS106 cells were incubated for various periods of times with 10 nM FSL-1 and/or ManLAM (10 µg ml−1) in the absence (−MG132) or the presence (+MG132) of the proteasome inhibitor MG132 or under the condition of knock-down of SOCS-1 (+siSOCS-1). Proteins were separated by SDS-PAGE, transferred to a Nitrocellulose Transfer Membrane and reacted with anti-Mal Ab. Immunoblots were developed by HRP-conjugated anti-mouse IgG Ab. Density of each protein band was analysed by the software image J and shown in lower figures. See text for details.
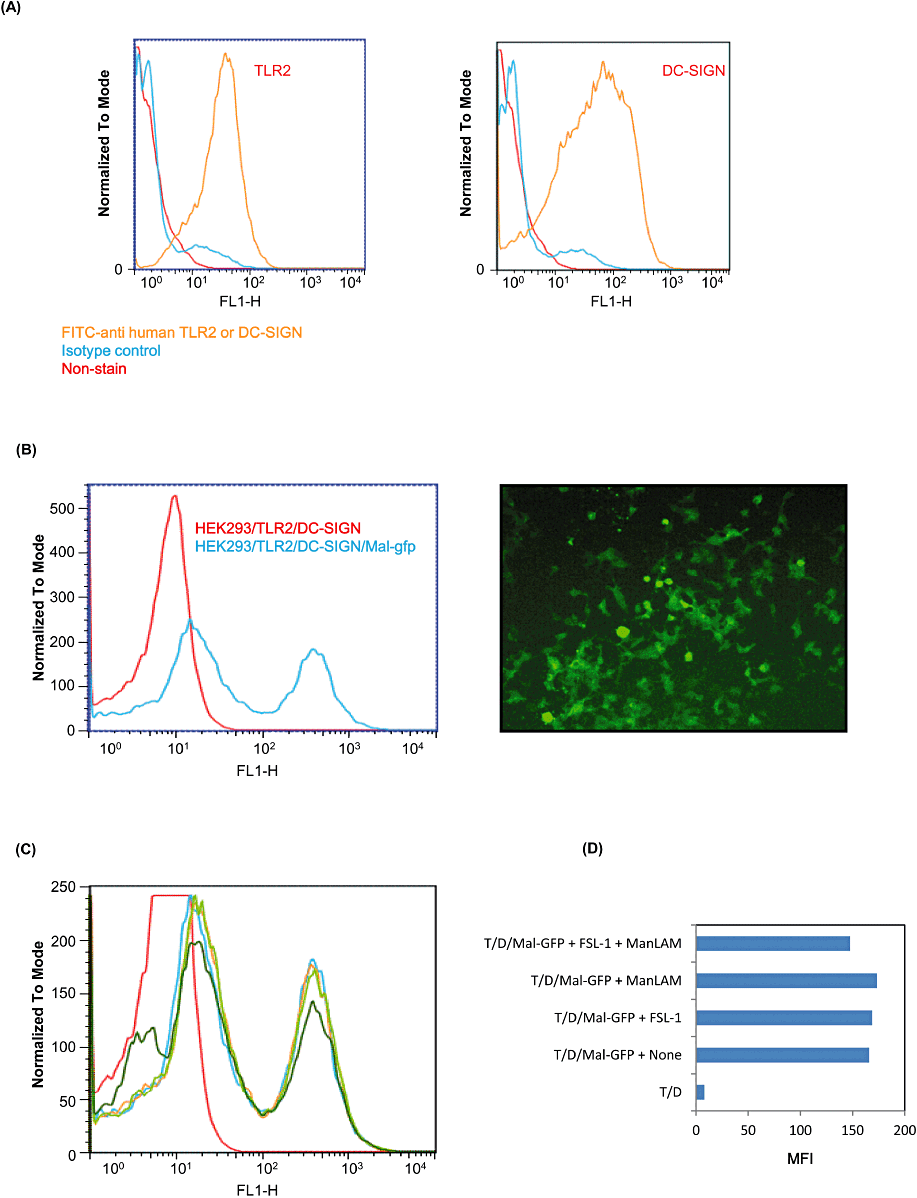
Degradation of Mal in XS106 cells co-stimulated with ManLAM and FSL-1 by flow cytometry. HEK293/TLR2/DC-SIGN cells expressed TLR2 and DC-SIGN the cell surface (A). HEK293/TLR2/DC-SIGN/Mal–GFP cells expressed Mal–GFP by flow cytometry and fluorospectrometer (B). HEK293/TLR2/DC-SIGN/Mal–GFP cells were stimulated with 10 nM FSl-1 and/or 10 µg ml−1 ManLAM. Histogram (C) and Mean Fluorescent Intensity (MFI) (D) are shown. In histogram, red line shows HEK293/TLR2/DC-SIGN; blue line, HEK293/TLR2/DC-SIGN/Mal–GFP + None; yellow line, HEK293/TLR2/DC-SIGN/Mal–GFP + FSL-1; light green line, HEK293/TLR2/DC-SIGN/Mal–GFP + ManLAM; and dark green line, HEK293/TLR2/DC-SIGN/Mal–GFP + FSl-1 + ManLAM. In MFI figure, T/D shows HEK293/TLR2/DC-SIGN cells and T/D/Mal–GFP shows HEK293/TLR2/DC-SIGN/Mal–GFP cells.
Next experiment was therefore performed to investigate whether Mal was ubiquitinated. We found that Mal immunoprecipitated by anti-Mal Ab was ubiquitinated at Lys48 after 30 and 60 min stimulation with both FSL-1 and ManLAM in the presence of MG132, but not in the absence of MG132, and the ubiquitination level increased in a time-dependent manner (Fig. 10A). In addition, we found that the protein immunoprecipitated by anti-Ubiquitin Lys48-specific Ab (anti-Ub K48 Ab) after the stimulation was Mal, the amount of which was higher in the presence of MG132 than in the absence of MG132 and increased along with the incubation time (Fig. 10B).
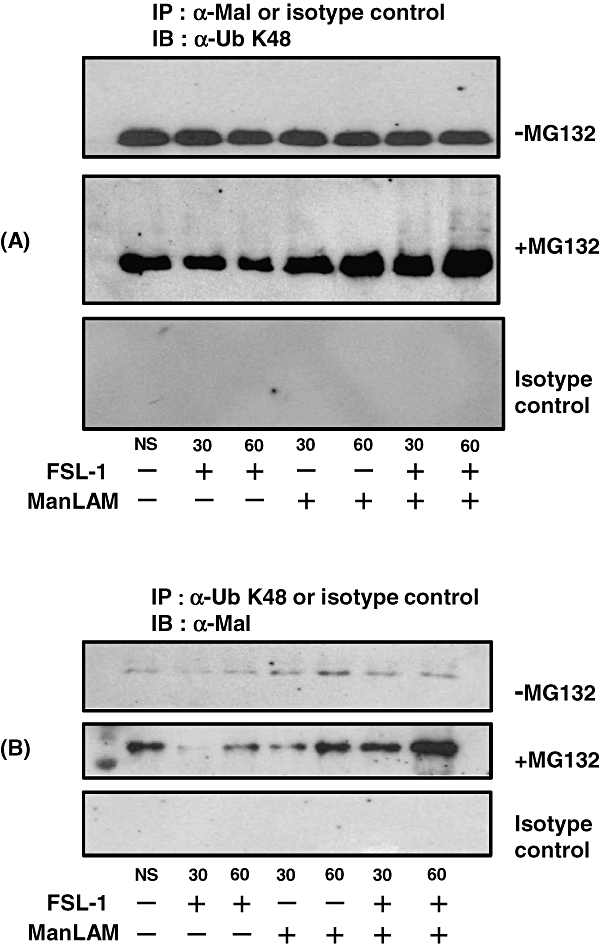
Ubiquitination of Mal. XS106 cells were stimulated with 10 nM FSL-1 and/or 10 µg ml−1 ManLAM in the absence (−MG132) or the presence (+MG132) of MG132. After lysis and then centrifugation, proteins were immunoprecipitated with anti-Mal Ab (α-Mal) or anti-UbK48 Ab (α-UbK48), or their isotype controls. IPs were immobilized by protein G-coupled agarose, washed six times in a lysis buffer and resuspended in SDS sample buffer. Proteins were separated by SDS-PAGE, transferred to a Nitrocellulose Transfer Membrane, and blotted with α-UbK48 for immunoprecipitate by α-Mal (A) or α-Mal for immunoprecipitate by α-UbK48 (B).
Thus, these results strongly suggest that the TLR2 adaptor Mal is ubiquitinated and degraded in the proteasome in XS106 cells in response to the stimulation with both FSL-1 and ManLAM and SOCS-1 plays a key role in the ubiquitination and degradation (Fig. 11).
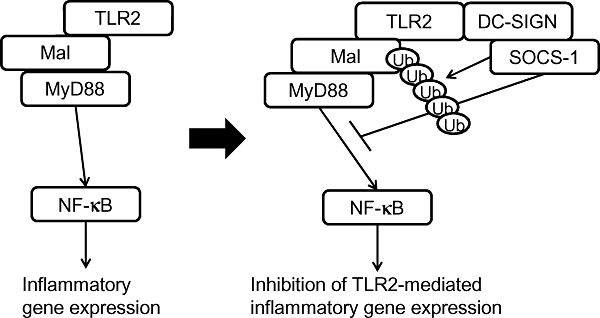
Schematic of suppression of TLR2-mediated signalling by DC-SIGN-mediated signalling via SOCS-1-mediated ubiquitination and subsequent degradation of Mal.
Discussion
Recognition and phagocytosis of microbes are very important events in the innate immune system. Therefore, we have been interested in how recognition of microbes by PRRs such as TLRs affects phagocytosis. This study was designed to investigate how the phagocytic receptor-mediated signalling affects TLR-mediated signalling. First, we investigated how the mouse DC-SIGN homologue SIGNR1 signalling affects TLR2-mediated cytokine production. Several reports on cross-talk between DC-SIGN and TLRs have been reported so far. Gringhuis et al. (2007) have demonstrated that mycobacteria trigger DC-SIGN on human DCs to activate the serine/threonine protein kinase Raf-1, which subsequently leads to induction of the phosphorylation of the NF-κB subunit p65 at Ser276, but only after TLR-induced activation of NF-κB (Gringhuis et al., 2007). Geijtenbeek et al. (2003) have demonstrated that mycobacteria specifically target DC-SIGN through ManLAM to impair DC maturation and induce production of the anti-inflammatory cytokine IL-10. Hovius et al. (2008) have demonstrated that the salivary protein Salp15 of Ixodes ticks, a major vector for Borrelia burgdoferi, the causative agent of Lyme disease, interacts with DC-SIGN on DCs, resulting in activation of the serine/threonine kinase Raf-1, which leads to MEK-dependent decrease of IL-6 and TNF-α mRNA stability and impairment of nucleosome remodelling at the IL-12p35 promoter (Hovius et al., 2008). Caparros et al. (2006) have reported that DC-SIGN ligation on MDDCs by specific Abs does not promote DC maturation but induces ERK1/2 and Akt phosphorylation without p38 activation and that DC-SIGN ligation synergizes with TNF-α or LPS for enhancement of IL-10 production. Thus, co-stimulation of DC-SIGN and TLRs induces immunosuppressive responses. In this study, we also found that co-stimulation of SIGNR1 and TLR2 suppressed the production of pro-inflammatory cytokines in murine DCs (2, 3, 6) and that knock-down of SIGNR1 recovered the suppression (Fig. 4). In addition, we found that DC-SIGN and SIGNR1, possibly as a tetramer, were associated with TLR2 in human HEK293 transfectants with TLR2 and DC-SIGN, and murine DCs respectively (Fig. 5A and B). This is the first study showing the association of DC-SIGN or SIGNR1 with TLR2, although DC-SIGN has already been known to be located with lipid rafts of the membrane (Caparros et al., 2006). Therefore, we tried to clarify the molecular mechanism by which SIGNR1 signalling suppressed TLR2 signalling. More recently, Srivastava et al. (2009) have demonstrated that co-stimulation of TLR2 and DC-SIGNR1 (called SIGNR1 in this study) differentially induces expression of SOCS-1 protein, that DC-SIGNR1 is associated with SOCS-1 and that the induction of SOCS-1 by DC-SIGNR1 is mediated by Raf-1 and Syk (Srivastava et al., 2009). The SOCS protein family consist of eight members (SOCS1–SOCS8), each of which is characterized by an N-terminal kinase inhibitory domain, a central SH2 domain, and a C-terminal SOCS box domain (Yoshimura et al., 2007). SOCS protein was discovered as a cytokine-inducible intracellular negative regulator that inhibits cytokine signalling by interaction with the JAK-STAT signalling cascade (Endo et al., 1997; Naka et al., 1997; Starr et al., 1997). Most SOCS proteins are also induced by various stimuli other than cytokines, such as TLR ligands, isoproterenol, statins and cAMP (Kinjyo et al., 2002; Nakagawa et al., 2002; Baetz et al., 2004; Yoshimura et al., 2007). It has been reported that SOCS-1 is a negative regulator of LPS-induced macrophage activation (Kinjyo et al., 2002; Nakagawa et al., 2002). Several mechanisms by which SOCS-1 negatively regulates TLR-mediated signalling pathways have been clarified. SOCS-1 binds to the p65 subunit of NF-κB and facilitates ubiquitination and degradation of p65 (Ryo et al., 2003). Mansell et al. (2006) have demonstrated that SOCS-1 induced by TLR2 or TLR4 ligands binds to Mal with a tyrosine residue phosphorylated by Btk (Bruton's tyrosine kinase) through the SH2 domain and induces ubiquitination and degradation of Mal, leading to the suppression of transactivation of NF-κB (Mansell et al., 2006). In addition to the TLR-NF-κB pathway, it has been reported that SOCS-1 might regulate the MAPKs JNK and p38 by binding to their upstream activator Ask-1 (He et al., 2006). SOCS proteins induced by TLR stimulation limit the extent of TLR signalling by inhibiting type I IFN signalling but not the main NF-κB pathway (Baetz et al., 2004). It has been reported that various molecules negatively regulate the TLR pathway (Kobayashi et al., 2002; Janssens et al., 2003; Wald et al., 2003; Chuang and Ulevitch, 2004; Divanovic et al., 2005; Liew et al., 2005; Naiki et al., 2005). The TLR4 homologue RP105 inhibits the TLR4 signalling complex by being bound to the microbial ligand (Divanovic et al., 2005). Triad3A induces ubiquitination and degradation of some TLRs (Chuang and Ulevitch, 2004), and ST2 and SIGGIR also function as negative regulators by inhibiting MyD88 via homotypic interaction (Wald et al., 2003). IRAK-M has been described as a negative regulator for TLR signalling (Kobayashi et al., 2002).
The findings by Mansell et al. (2006) gave us a clue to clarify the molecular mechanism by which co-stimulation of DCs with ManLAM and FSL-1 suppressed the FSL-1-induced production of pro-inflammatory cytokines. First of all, we investigated whether stimulation with FSL-1 and/or ManLAM induced expression of SOCS-1, and we found that both stimuli upregulated SOCS-1 at a protein level (Fig. 6) and that co-stimulation with SIGNR1 and FSL-1 induced degradation of Mal but did not in the proteasome inhibitor MG132 and knock-down of SOCS-1 inhibited the degradation of Mal (8, 9). In addition, we found that SOCS-1 was associated with DC-SIGN, which was also associated with TLR2 (Fig. 6), suggesting that a trimer complex consisting of TLR2, DC-SIGN and SOCS-1 is formed in DCs. Knock-down of SOCS-1 recovered the suppression of FSL-1-mediated production of TNF-α, IL-6 and IL-12p40 by SIGNR1 signal (Fig. 7). This study also demonstrated that Mal was ubiquitinated in the presence of the proteasome inhibitor MG132, but not in the absence of the inhibitor (Fig. 10).
These results suggest that both SIGNR1 and TLR2 induced the expression of SOCS-1, which subsequently induces ubiquitination and degradation of Mal. Thus, our findings strongly support the findings of Srivastava et al. (2009) that SOCS-1 plays an important role in the suppression of the TLR2-mediated cytokine production by DC-SIGNR1. However, they have not demonstrated how SOCS-1 regulates cytokine production by stimulation with TLR2 and DC-SIGNR1 through ubiquitination and degradation of Mal, although they discuss the possibilities that SOCS-1 directly binds to the p65 subunit of NF-κB, Mal or Ask-1 and then induces ubiquitination and degradation of them on the basis of previous findings (Ryo et al., 2003; He et al., 2006; Mansell et al., 2006). We also investigated whether co-stimulation of SIGNR1 and TLR2 induced SOCS-1-mediated degradation of Ask-1 and p65 of NF-κB, but we did not find degradation of Ask-1 and p65 of NF-κB (data not shown).
Recognition of microbes and removal of microbes by phagocytosis are two major events in the innate immune system. This study implies an immunologically important truth that the recognition of microbes by TLRs is downregulated after phagocytosis of microbes starts.
Experimental procedures
Reagents and Abs
The glycolipid ManLAM from M. tuberculosis Aoyama-B was obtained from NAKALAI TESQUE (Kyoto, Japan). Man9 was obtained from Sigma-Aldrich (St Louis, MO). The diacylated lipopeptide FSL-1 derived from Mycoplasma salivarium was synthesized as described previously (Shibata et al., 2000). The triacylated lipopeptide Pam3CSK4 was purchased from InvivoGen (San Diego, CA, USA). PGN from Staphylococcus aureus was purchased from Sigma-Aldrich. A goat Ab against SIGNR1 (Park et al., 2001) was purchased from R&D Systems (Minneapolis, MN). A mouse monoclonal Ab (mAb) against β-actin (AC-15) was purchased from Abcam (Cambridge, UK). A mAb against human TLR2 (IMG-319) and its isotype control (mouse IgG1) were purchased from Biocarta (San Diego, CA) and from BD Biosciences (San Jose, CA) respectively. PE-conjugated anti-mouse TLR2 mAb (clone 6C2) was purchased from eBioscience (San Diego, CA); PE-conjugated rat IgG2b, κ as its isotype control from BD Pharmingnen (San Diego, CA); a mouse mAb against human DC-SIGN (MAB161) from R&D Systems; a rabbit polyclonal Ab (H-93) against SOCS-1 from Santa Cruz Biotechnology (Santa Cruz, CA); and a rabbit polyclonal Ab (Pearl-1) against Mal from Enzo Life Sciences International (Plymouth Meeting, PA). Anti-Ub K48 Ab (clone Apu2) and rabbit IgG as its isotype control were purchased from Millipore (Billerica, MA) and BD Pharmingnen respectively. An HRP-conjugated Ab against mouse IgG and FITC-conjugated anti-mouse IgG Ab were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Anti-FLAG Ab were purchased from Sigma-Aldrich. Protein G-Sepharose was from Amersham Bioscience (Uppsala, Sweden). BS3 and DSS cross-linkers were purchased from PIERCE Chemical Corp. (Rockford, IL). MG132, a proteasome inhibitor, was purchased from Calbiochem (Darmstadt, Germany).
All other reagents were purchased from commercial sources and were of analytical or reagent grade.
Genes
Human TLR2 cDNA (19–784th amino acid; containing preprotrypsin leader sequence and FLAG sequence at the N-terminus), which was obtained by RT-PCR of RNA isolated from human monocytic cell line THP-1 cells, was cloned into pcDNA3 vector (Invitrogen, Carlsbad, CA). The construct containing human DC-SIGN (pUNO-human DC-SIGN) was purchased from InvivoGen. The plasmid (pEGFP-Mal) that expresses human Mal/TIRAP as a C-terminal enhanced GFP (EGFP) fusion protein was constructed in pEGFP-N1 (BD Biosciences Clontech). The open reading frame for Mal was amplified from cDNA of THP-1 cells by PCR. Site-directed mutagenesis was performed using the QuickChange XL Site-Directed Mutagenesis Kit (Stratagene, Santa Clara, CA) to delete the 3′ sequence including the stop codon of TIRAP and confirmed by sequence analysis.
HEK293 transfectants
HEK293 cells were transiently transfected with genes of TLR2 and DC-SIGN. The resulting transfectants were used to investigate the association of TLR2 and DC-SIGN, and were also selected by blasticidin to establish HEK293 cells (HEK293/TLR2/DC-SIGN) stably expressing TLR2 and DC-SIGN. HEK293/TLR2/DC-SIGN cells were transfected with pEGFP-Mal and were selected by G418 to establish HEK293/TLR2/DC-SIGN/Mal–GFP cells.
Cell cultures
XS106 cells, a murine DC cell line, kindly provided by Professor Akira Takashima (University of Texas South-Western, TX), is a long-established DC line derived from the epidermis of newborn mice (Mohan et al., 2005). This cell line was cultured in RPMI-1640 medium (XS medium) containing 10% (v/v) FBS (Invitrogen), 2 mM l-glutamine, 1 mM sodium pyruvate, 10 mM HEPES buffer, 100 µg ml−1 each of penicillin and streptomycin (Sigma), 50 µM 2-ME (Sigma), 1% (w/v) non-essential amino acids (Sigma), 0.5 ng ml−1 murine recombinant GM-CSF (PeproTech. Rocky Hill, NJ) and 5% (v/v) culture supernatant derived from the NS47 fibroblast cell line.
HEK293 cells (CRL-1573; ATCC) were maintained in DMEM medium (Sigma) supplemented with 10% (v/v) heat-inactivated FBS and 100 µg ml−1 each of penicillin and streptomycin.
Flow cytometry
XS106 cells were incubated on ice [106 cells in 200 µl of PBS containing 1% (w/v) BSA (PBS/BSA)] with PE-conjugated anti-mouse TLR2 mAb (clone 6C2) (eBioscience) or PE-conjugated rat IgG2b, κ (BD Pharmingen). After 30 min incubation, they were washed and incubated in 200 µl of PBS/BSA and further incubated with FITC-conjugated anti-mouse IgG Ab. The cells were fixed in PBS containing 0.5% (w/v) formaldehyde and then analysed by a FACS Calibur flow cytometer (BD Bioscience). Data for 30 000 cells falling within appropriate forward and side light scatter gates were collected from each sample. Data were analysed using CellQuest software (BD Bioscience).
Luciferase reporter assay
XS106 cells were plated at 1 × 105 cells per well in a 24-well plate pre-coated with poly-l-lysine on the day before transfection. The cells were transiently transfected by METAFECTENE™ Transfection Reagent (Biontex Laboratories GmbH, Martinsried, Germany) with 50 ng of an NF-κB reporter plasmid (pNF-κB-Luc from Stratagene) and 5 ng of a construct directing expression of Renilla luciferase under the control of a constitutively active thymidine kinase promoter (pRL-TK, Promega, Madison, WI) together with 445 ng of pcDNA3 empty vector (Invitrogen). After 24 h incubation, the cells were stimulated at 37°C for 6 h with various concentrations of FSL-1 or Man9 in DMEM base medium, and luciferase activity was measured using a Dual-Luciferase reporter assay system (Promega) according to the manufacturer's instructions.
Quantitative real-time RT-PCR
XS106 cells were plated at 5 × 105 cells per well of a 24-well plate in XS medium or RPMI 1640 complete medium and incubated at 37°C for 16 h. The cells were washed three times with RPMI 1640 base medium and treated at 37°C for 6 h with various concentrations (0.1, 1, 10 nM) of FSL-1 and/or ManLAM (1, 10 µg ml−1) or Man9 (10, 100 nM). The cell culture was centrifuged at 400 g for 10 min. The cell pellets were washed three times with PBS and used for RNA extraction as described below. Total RNA was extracted from the washed cells by the TRI Reagent (Sigma) and cDNA was synthesized with the High Capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA). For real-time PCR analysis, PCR amplification was performed in the presence of a TaqMan probe by using A StepOne Real-Time PCR system (Applied Biosystems). Specific primers for TNF-α, IL-6, IL-12p40, SIGNR1, SOCS-1 and GAPDH were purchased from Applied Biosystems. The cycling threshold (CT) value is defined as the number of PCR cycles in which the fluorescence signal exceeds the detection threshold value. The normalized amount of target mRNA (Nt) was calculated from the CT values obtained for both target and GAPDH mRNA with the equation Nt = 2Ct(GAPDH)−Ct(target). Relative mRNA expression was obtained by setting Nt in non-stimulated samples as 1 in each experiment.
RNA interference
Non-targeting siRNA as a control and siRNAs specific for SIGNR1 (Ambion, Austin, TX) and SOCS-1 (Santa Cruz Biotechnology), final concentrations of which were 10 µM, were transfected into XS106 cells (2 × 106 cells/0.1 ml of Opti-MEM) by electroporation using two-step electroporator (CUY21 Pro-Vitro, NEPA GENE, Tokyo, Japan) at 175 V and 2 ms. The cells were cultured in XS medium at 37°C for approximately 36 h to reach 100% confluence in a 10 cm dish. The cells collected from the dish were then inoculated to a 24-well plate at a concentration of 2 × 105 cells per well. After overnight incubation, the cells were stimulated at 37°C for 6 h with various concentrations of FSL-1 and/or ManLAM. Expression of mRNAs of TNF-α, IL-6 and IL-12p40 was quantified by a real-time PCR system as described above.
Induction of SOCS-1 in XS106 cells by ManLAM and/or FSL-1
XS106 cells were incubated for various periods of times (1, 2, 12, 24 h) with 10 nM FSL-1 in the absence or presence of ManLAM (10 µg ml−1). The cells were lysed in a lysis buffer and immunoprecipitated with anti-SOCS-1 Ab. Immunoprecipitate (IPs) were immobilized by protein G-coupled agarose, washed six times in lysis buffer, and resuspended in SDS sample buffer. Proteins were separated by SDS-PAGE, transferred to a Nitrocellulose Transfer Membrane (GE Healthcare, Piscataway, NJ), and blotted with appropriate Abs. Immunoblots were developed by HRP-conjugated anti-mouse IgG Ab (Jackson ImmunoResearch Laboratories). Immunoreactive proteins were detected by using ECL™ detection reagents (GE Healthcare). Proteins were separated by SDS-PAGE, transferred to a Nitrocellulose Transfer Membrane, and blotted with anti-SOCS-1 Ab. Immunoblots were developed by HRP-conjugated anti-mouse IgG Ab. Density of each SOCS-1 band was analysed by the software image J and are shown in lower figure.
Association of TLR2, SIGNR1 or DC-SIGN and SOCS-1
HEK293 cells (HEK293/TLR2/DC-SIGN cells) transfected with genes of a FLAG-tagged TLR2 and DC-SIGN and XS106 cells were plated at 1 × 107 cells in a 10 cm dish in DMEM and XS medium, respectively, and cultured at 37°C for 16 h. After the cells had been washed three times with the base medium, they were stimulated for various periods of time with FSL-1 or ManLAM. Cell stimulation was terminated by a lysis buffer containing 20 mM Tris-HCl (pH 7.5), 1% (w/v) sodium deoxycholate, 1% (v/v) Triton X-100, 150 mM NaCl, 1 mM each of Na3VO4, β-glycerophosphate, EDTA and sodium pyrophosphate, and 1 tablet/10 ml of complete mini (Roche, Basel, Switzerland) for total-cell lysates and for immunoprecipitation. All control immunoprecipitates (IPs) were performed with rabbit or mouse IgG depending on Abs used. After lysis and then centrifugation at 8000 g for 15 min, proteins were immunoprecipitated (3 µg antibody) with various Abs indicated. IPs were immobilized by protein G-coupled agarose, washed six times in lysis buffer, and resuspended in SDS sample buffer. Proteins were separated by SDS-PAGE, transferred to a Nitrocellulose Transfer Membrane (GE Healthcare), and blotted with appropriate Abs. Immunoblots were developed by HRP-conjugated anti-mouse IgG Ab (Jackson ImmunoResearch Laboratories). Immunoreactive proteins were detected by using ECL™ detection reagents (GE Healthcare). For cross-linking experiments, HEK293/TLR2/DC-SIGN cells were treated with a bis[sulfosuccinimidyl] suberate (BS3) for a cell-surface protein and for association of SIGNR1 and endogenous SOCS-1 XS106 cells were treated with disuccinimidyl suberate (DSS) at a final concentration of 5 mM before being lysed according to the manufacturer's instructions.
Ubiquitination and subsequent degradation of Mal
XS106 cells were plated at 1 × 107 cells in a 10 cm dish in XS medium and cultured at 37°C for 16 h. After the cells had been washed three times with the base medium, they were stimulated for 10, 30, 60 and 120 min with 10 nM FSL-1 and/or 10 µg ml−1 ManLAM in the absence or the presence of 10 µM MG132 (Sigma-Aldrich) or under the condition of knock-down of SOCS-1 by its specific siRNA described above. Cell stimulation was terminated by a lysis buffer described above. After lysis and then centrifugation at 8000 g for 15 min, proteins were immunoprecipitated (3 µg antibody) with anti-Mal or anti-UbK48 Ab, or their isotype controls. IPs were immobilized by protein G-coupled agarose, washed six times in a lysis buffer and resuspended in SDS sample buffer. Proteins were separated by SDS-PAGE, transferred to a Nitrocellulose Transfer Membrane (GE Healthcare), and blotted with anti-Ub K48 for immunoprecipitate by anti-Mal Ab or anti-Mal for immunoprecipitate by anti-U K48 Ab. Immunoblots were developed by HRP-conjugated anti-mouse IgG Ab. Immunoreactive proteins were detected by using ECL™ detection reagents.
Density of each protein band was analysed by the software image J.
Statistical analysis
Student's t-test for multiple observations was used for statistical analyses. Statistical significance was set at a P-value of less than 0.05.
Acknowledgements
We would like to express our hearty thanks to Professor Akira Takashima (University of Texas South-Western, TX) for providing the murine DC cell line XS106 and to the persons concerned who are working at Hokkaido Red Cross Blood Center (Sapporo, Japan) for providing human heparinized whole blood. This work was supported by a Grant-in-Aid for Science Research B 22390352 provided by the Japan Society for the Promotion of Science.