Rho1 has distinct functions in morphogenesis, cell wall biosynthesis and virulence of Fusarium oxysporum
Summary
Rho-type GTPases regulate polarized growth in yeast by reorganization of the actin cytoskeleton and through signalling pathways that control the expression of cell wall biosynthetic genes. We report the cloning and functional analysis of rho1 from Fusarium oxysporum, a soilborne fungal pathogen causing vascular wilt on plants and opportunistic infections in humans. F. oxysporum strains carrying either a Δrho1 loss-of-function mutation or a rho1G14V gain-of-function allele were viable, but displayed a severely restricted colony phenotype which was partially relieved by the osmotic stabilizer sorbitol, indicating structural alterations in the cell wall. Consistent with this hypothesis, Δrho1 strains showed increased resistance to cell wall-degrading enzymes and staining with Calcofluor white, as well as changes in chitin and glucan synthase gene expression and enzymatic activity. Re-introduction of a functional rho1 allele into the Δrho1 mutant fully restored the wild-type phenotype. The Δrho1 strain had dramatically reduced virulence on tomato plants, but was as virulent as the wild type on immunodepressed mice. Thus, Rho1 plays a key role during fungal infection of plants, but not of mammalian hosts.
Introduction
Establishment of cell polarity and maintenance of unipolar growth at the hyphal tip is a characteristic feature of filamentous fungi (Momany, 2002). Rho-type GTPases regulate polarized cell growth through the reorganization of the actin cytoskeleton as well as through signalling pathways that control the expression of cell wall biosynthesis genes (Levin, 2005). GTPases are molecular modulators which cycle between an inactive GDP-bound and an active GTP-bound form. Switching between the two states is controlled by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) (Tanaka and Takai, 1998).
Saccharomyces cerevisiae has six Rho-type GTPases, named Rho1 to Rho5 and Cdc42, which reside at the plasma membrane and serve related but distinct roles in cell polarity establishment and maintenance (Schmidt and Hall, 1998). Rho1, the founding member of the family, is an essential protein which acts as a master regulator of cell wall integrity signalling by serving a variety of functions. First, it activates the Pkc1-Slt2 cell integrity MAP kinase cascade in response to cell wall stress (Bickle et al., 1998). Second, as a part of the glucan synthase complex, it stimulates β-1,3-glucan synthase activity which catalyses the biosynthesis of the major structural component of the yeast cell wall (Cabib et al., 1998). Additional roles of Rho1p in S. cerevisiae include organization of the actin cytoskeleton via binding to Bni1p (Imamura et al., 1997), polarized secretion (Guo et al., 2001) and endocytosis (Eitzen et al., 2001).
Rho1 orthologues in other yeasts seem to carry out similar functions as in S. cerevisiae. In fission yeast, which has six Rho-type GTPases (Garcia et al., 2006), the functional Rho1 homologue is an essential regulatory subunit of the β-1,3-glucan synthase complex and localizes at the growing tips, where it controls cell wall biosynthesis and actin cytoskeleton organisation (Arellano et al., 1996; 1997). The rho1 gene from the human pathogen Candida albicans can functionally complement a S. cerevisiae rho1 deletion strain and its product interacts directly with the β-1,3-glucan synthase catalytic subunit (Kondoh et al., 1997). The YlRHO1 gene from Yarrowia lipolytica was also able complement rho1 lethality in S. cerevisiae but, unlike S. cerevisiae, a Δylrho1 disruptant strain of Y. lipolytica is viable (Leon et al., 2003).
Only limited knowledge exists on the role of Rho1 orthologues in filamentous fungi. Antibodies against human RhoA protein detected the presence of a Rho-homologue during spore germination of the zygomycete Phycomyces blakesleeanus, suggesting a possible role during the shift from isotropic to polarized growth (Ramirez-Ramirez et al., 1999). The RHO1 orthologue of Cryptococcus neoformans, CnRHO1, was cloned by functional complementation of a temperature-sensitive rho1–104ts mutant of S. cerevisiae. Overexpression and site-specific mutagenesis of CnRHO1 suggested that it has distinct features compared with yeast RHO1 (Chang and Penoyer, 2000). An orthologue of Rho1 in Aspergillus fumigatus was found to be part of the glucan synthase complex, together with the catalytic glucan synthase Fks1p (Beauvais et al., 2001). A. nidulans strains carrying ectopic copies of a constitutively active rhoAG14V allele showed restricted growth and an abnormal branching pattern, whereas a strain carrying an ectopic dominant rhoAE40I allele exhibited cell lysis and hypersensitivity to calcofluor and caspofungin, suggesting a role of RhoA in morphogenesis and cell wall biogenesis (Guest et al., 2004). So far, targeted deletion of rho1 has only been performed in one filamentous species, Ashbya gossypii. Agrho1 null mutants exhibited severely restricted growth which gave rise to tiny colonies showing cell lysis and colony death after a few days (Wendland and Philippsen, 2001). Collectively, these results indicate that Rho1 is required for the establishment and maintenance of cell polarity, as well as for proper composition and integrity of the fungal cell wall.
Polarized hyphal growth is a key element in fungal pathogenicity (Madhani and Fink, 1998). During invasion of their hosts, pathogenic fungi differentiate infection hyphae that penetrate and colonize the underlying tissue, thereby causing disease. This process is likely to require both polarized growth to breach the host surface and maintenance of cell wall integrity to withstand the host defences. At present, the role of Rho1 during fungal infection of plant or animal hosts remains unknown.
In this work, we have functionally characterized the rho1 orthologue from the vascular wilt fungus Fusarium oxysporum. This ubiquitous soilborne pathogen attacks more than a hundred different plant species and has been reported as an opportunistic human pathogen (Di Pietro et al., 2003). We found that a F. oxysporum mutant lacking rho1 was viable, but severely impaired in growth on solid media, and exhibited alterations in cell wall structure and in expression of cell wall biogenesis genes. The Δrho1 strain showed a dramatic decrease in virulence on tomato plants, thus providing the first evidence for an essential role of Rho1 in fungal pathogenesis on plants.
Results
Characterization of the F. oxysporum rho1 gene encoding a putative Rho-like GTPase
The rho1 gene encoding a putative Rho-like GTPase of F. oxysporum f.sp. lycopersici was previously cloned from a λEMBL3 genomic library, using as a probe a PCR fragment amplified from genomic DNA with degenerate primers derived from conserved regions of fungal Rho genes (Caracuel et al., 2005). The sequence of F. oxysporum rho1 has been deposited in GenBank under accession number AY884607. Rho1 spans an open reading frame of 585 bp encoding a putative polypeptide of 195 amino acids with a predicted molecular mass of 22 kDa and a pI of 6.2. Sequence alignment of a cDNA clone obtained by RT-PCR with gene-specific primers with the genomic sequence revealed the presence of four introns of 318, 133, 77 and 99 bp respectively (Fig. S2). Southern analysis of total genomic DNA of F. oxysporum treated with different restriction enzymes produced a banding pattern consistent with the presence of a single copy of rho1 in the genome (data not shown). However, the presence of additional bands hybridizing at lower intensity indicated that F. oxysporum contains at least one other gene structurally related to rho1. In agreement with this, BLASTN and BLASTP searches of the F. oxysporum genome database (http://www.broad.mit.edu/annotation/genome/fusarium_group/MultiHome.html) detected one predicted copy of rho1 (FOXG_13835) which was correctly annotated, as well as a second gene (FOXG_03575) which shared 50 bp of complete identity with the rho1 probe and corresponded to an orthologue of the Rac GTPase cflB (Boyce et al., 2003).
Alignment of the amino acid sequence of the predicted F. oxysporum Rho1 protein with sequences in the databases revealed the presence of a single orthologue in yeasts and filamentous fungi. Sequence identity between RHO1 orthologues ranged between 70% and 96% and was correlated with phylogenetic distance (Fig. S1). F. oxysporum Rho1 contained the domains previously shown to be required for S. cerevisiae Rho1p function (Saka et al., 2001), including the GDGACGKT region involved in transition of the GTP/GDP molecule and the YVPTVFENY domain which binds the regulatory GAP. Based on sequence homology, we conclude that F. oxysporum rho1 encodes a structural orthologue of ScRho1p.
Generation of Δrho1 loss-of-function and rho1G14V gain-of-function alleles
To investigate the biological role of rho1 in F. oxysporum, we generated loss-of-function and gain-of-function alleles of the gene. Loss-of-function mutants were produced using targeted gene replacement with a Δrho1 allele disrupted in a highly conserved domain within the predicted protein (Fig. S2B; see Experimental procedures for details). Among 68 hygromycin-resistant transformants analysed by PCR with different combinations of gene-specific primers, one transformant produced PCR amplification products indicative of homologous integration-mediated gene replacement (data not shown). Southern blot analysis confirmed the replacement, in this transformant, of a 9 kb EcoRI fragment corresponding to the wild-type rho1 allele, by two fragments of 2.5 and 6.5 kb (Fig. S2D). By contrast, other hygromycin-resistant transformants still contained the wild-type 9 kb fragment together with additional hybridizing fragments, indicating ectopic insertion of the replacement vector (data not shown). We conclude that the Δrho1 strain carries a disrupted copy of the rho1 gene.
To confirm that the phenotypes of the Δrho1 mutant were caused by loss of rho1, a 2.2 kb DNA fragment encompassing the complete F. oxysporum rho1 gene was introduced into the Δrho1 strain by cotransformation. Phleomycin-resistant transformants were analysed for the presence of a functional rho1 allele by PCR with gene-specific primers RHO19 and RHO7 (Fig. S2A). A 2.2 kb amplification product identical to that obtained from the wild-type strain was detected in multiple phleomycin-resistant transformants, but not in the Δrho1 mutant (results not shown). Southern blot analysis of one of these transformants revealed an additional 11 kb EcoRI hybridizing fragment, confirming the presence of the newly introduced functional rho1 allele (Fig. S2D, lane 3). We conclude that the Δrho1 + rho1 strains carry an intact ectopic copy of the wild-type allele. In subsequent experiments, several complemented transformants showed similar phenotypes.
To further investigate the role of Rho1 in F. oxysporum, we used site-directed mutagenesis to generate a rho1G14V gain-of-function allele, in which the conserved G14 residue was changed to V (Fig. S2C). This mutation mimics that of the S. cerevisiae RHO1G14V allele which prevents dissociation of GTP, resulting in a constitutively active form of Rho1 (Saka et al., 2001). A PCR fragment of the rho1G14V allele was introduced into the Δrho1 background by cotransformation with the phleomycin resistance marker. Phleomycin-resistant transformants were analysed for the presence of the rho1G14V allele by PCR with gene-specific primers RHO19 and RHO7. A 2.2 kb amplification product of identical size to that obtained from the wild type was detected in multiple transformants (Fig. S2E, # 2, 4, 6), but not in the Δrho1 mutant. Southern blot analysis of transformants # 2 and 6 revealed an additional 13 kb EcoRI hybridizing fragment, confirming the presence of the newly introduced rho1G14V allele (data not shown). We conclude that these transformants, named Δrho1 + rho1G14V, carry a rho1G14V allele in their genomes.
Expression of rho1 during vegetative growth of F. oxysporum in submerged culture was determined by northern hybridization analysis. High transcript levels were present in the wild-type strain, which were further increased in the presence of the osmotic stabilizer sorbitol (Fig. 1A). By contrast, an extremely faint signal was detected in the Δrho1 loss-of-function mutant (Fig. 1A and Fig. 5, upper panel). As mentioned in the previous section, this faint signal was probably caused by cross-hybridization of the probe with the transcript of the cflB gene. A moderate hybridization signal was observed in the Δrho1 + rho1G14V strain (Fig. 5, upper panel).
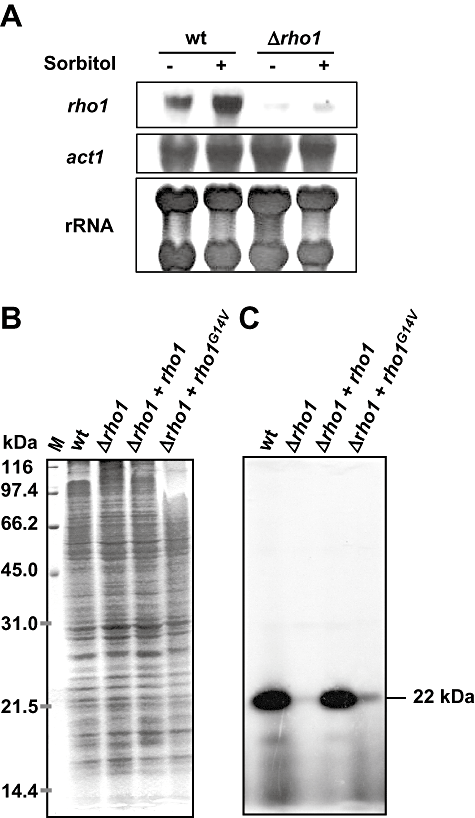
A. Northern analysis of rho1 transcript accumulation in the wild-type and the Δrho1 strain grown on synthetic medium in the presence or absence of 1.2 M sorbitol. Total RNA was fractionated in agarose gels, blotted onto nylon membranes and hybridized to the rho1 probe (see Fig. S2A). As loading controls, equal amounts of RNA were hybridized to the act1 gene, and RNA was stained directly on the filter with 0.02% methylene blue (rRNA).B and C. Detection of Rho-type proteins by ADP-ribosylation with exoenzyme C3 from Clostridium botulinum. Mixed membrane fractions of the indicated strains obtained by ultracentrifugation were incubated with C. botulinum exoenzyme C3 in the presence of [32P]-NAD, followed by separation of the reactions by SDS-PAGE. Gels were stained with Coomassie blue (B) and subsequently subjected to autoradiography (C). The relative molecular masses and positions of the size markers in B are indicated to the left. The deduced molecular mass of the radioactive protein band in C is indicated to the right.
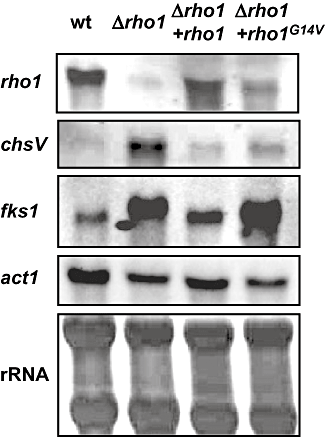
Mutation of rho1 affects expression of genes encoding cell wall biosynthetic enzymes. Total RNA was extracted from mycelia of the indicated strains grown for 12 h on liquid potato dextrose broth medium, fractionated in agarose gels, blotted onto nylon membranes, and hybridized to the probes indicated to the left. As a loading control, RNA was hybridized to the actin gene probe (act1), or stained directly on the filter with 0.02% methylene blue (rRNA).
The presence of Rho-type proteins in the different F. oxysporum strains was explored by ADP-ribosylation with exoenzyme C3 from Clostridium botulinum (Aktories et al., 1988). Mixed membrane fractions of fungal mycelia were obtained by ultracentrifugation and incubated with C. botulinum exoenzyme C3 in the presence of radioactively labelled [32P]-NAD, followed by separation of the reactions by SDS-PAGE. Proteins were stained with Coomassie blue (Fig. 1B), and radioactively labelled Rho-type proteins were detected by autoradiography (Fig. 1C). A robust signal corresponding to a protein of 22 kDa, the predicted size of Rho1, was observed in the wild type and the Δrho1 + rho1 strains. This major band was absent in the Δrho1 mutant, suggesting lack of Rho1 protein in this strain. An extremely faint signal was still detectable, again indicative of the presence of additional Rho-type proteins of similar molecular mass that are susceptible to ADP-ribosylation. The Δrho1 + rho1G14V strain showed a moderate signal, suggesting the presence of relatively low levels of Rho1G14V protein.
Mutations in rho1 specifically affect hyphal growth on solid substrates
The role of rho1 in hyphal growth and development of F. oxysporum was examined by comparing the growth phenotypes of the different mutants with that of the wild-type strain. No significant differences in hyphal morphology and growth rate (determined as mycelial dry weight) were found between mycelia of the strains grown in liquid culture, either on potato dextrose broth or minimal medium with or without 1.2 M sorbitol (Fig. S2A). By contrast, the Δrho1 mutant exhibited a dramatically restricted colony phenotype on solid media (Fig. 2, Fig. S2B). Stereomicroscopical examination of the Δrho1 colonies revealed burying of the colony margin into the agar, although no clear differences in hyphal morphology were observed (results not shown). The restricted phenotype was partially remediated by addition of 1.2 M sorbitol to the medium (Fig. 2). Cell wall stresses such as Congo red or incubation at 37°C strongly inhibited growth of the Δrho1 strain compared with the wild type. Moreover, colonies of the Δrho1 mutant grown in the presence of 5-Br-4-Cl-3-indoyl phosphate (BCIP) showed blue staining, indicative of hyphal lysis at the centre of the colony, whereas no such staining was observed in wild-type colonies. The phenotypes of the Δrho1 mutant were largely restored to wild type in the complemented Δrho1 + rho1 strains, but not in strains carrying the rho1G14V gain-of-function allele, except for a partial recovery in growth at 37°C (Fig. 2). We conclude that mutations in the rho1 gene specifically affect hyphal development on solid surfaces and produce a colony phenotype indicative of structural alterations in the cell wall.
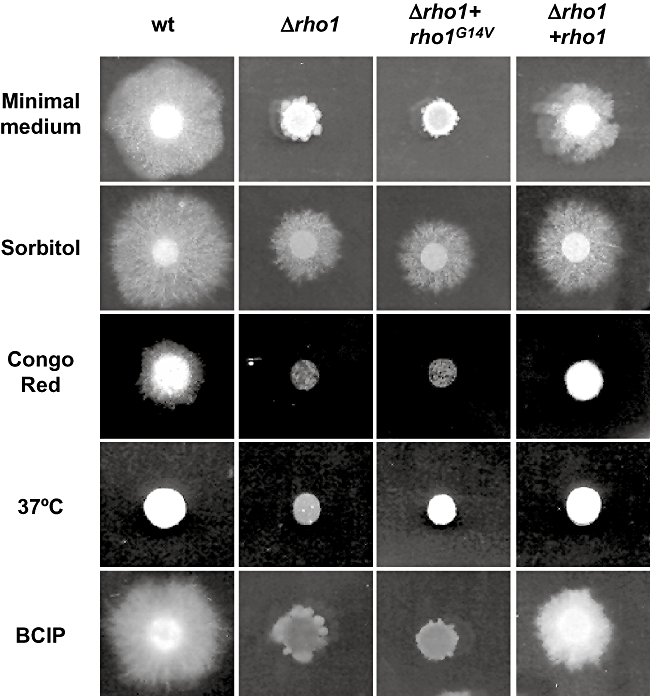
Mutation of rho1 affects hyphal growth on solid surfaces. Colonies of the indicated strains were grown for 3 days at 28°C on minimal medium alone, or supplemented with 1.2 M sorbitol, 0.5 mg l−1 Congo red, 600 mg l−1 5-Br-4-Cl-3-indoyl phosphate (BCIP) or incubated at 37°C.
Loss-of-function and gain-of-function mutations in rho1 differentially affect cell wall integrity and cell wall biosynthetic enzyme activity
The nature of the colony phenotype of the Δrho1 and the Δrho1 + rho1G14V strains prompted us to examine the relative cell wall integrity in these mutants. Germinated microconidia were exposed to a mixture of cell wall-degrading enzymes and the release of protoplasts was monitored over time. As shown in Fig. 3, the Δrho1 mutant showed dramatically increased resistance to the activity of the protoplasting enzymes, whereas the Δrho1 + rho1G14V strain was somewhat more sensitive than the wild-type strain. Next, the relative amount of exposed chitin was assessed microscopically by staining with the chitin-specific dye Calcofluor white (CFW) in combination with DAPI. The CFW signal was much more intense in hyphae of the Δrho1 strain than in those of the wild-type and the Δrho1 + rho1 strain ( Fig. 4). By contrast, the Δrho1 + rho1G14V mutant showed slightly reduced CFW staining. These results indicate that the amount of chitin exposed to CFW is significantly increased in the Δrho1 mutant and decreased in the Δrho1 + rho1G14V strain.
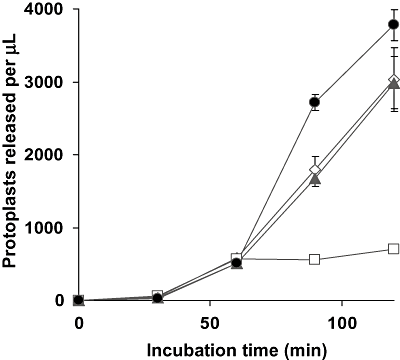
Mutations in rho1 affect sensitivity of F. oxysporum to cell wall-degrading enzymes. Germlings of the wild-type strain (filled triangles), the Δrho1 strain (empty squares), the Δrho1 + rho1 strain (empty diamonds) and the Δrho1 + rho1G14V strain (filled circles) were incubated with protoplasting enzyme mix at 30°C in osmotically stabilized buffer and the number of protoplasts released was determined microscopically at the indicated times. Each data point represents the mean from three independent samples. Bars indicate standard deviations.
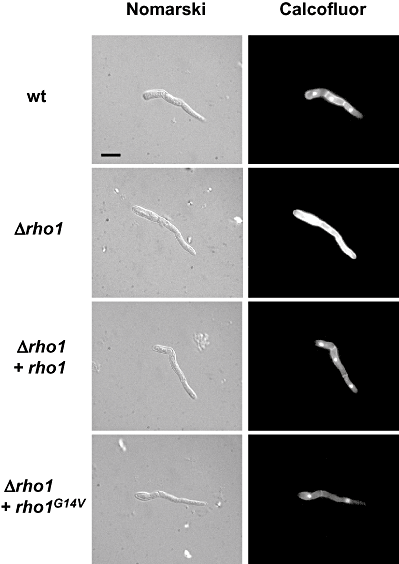
Mutants in rho1 show differential staining with Calcofluor white (CFW). Microconidia of the indicated strains were allowed to germinate for 8 h in potato dextrose broth, stained with a combination of CFW and DAPI and observed in a Leica fluorescence microscope. Bar represents 10 μm.
We next tested whether the observed alterations in cell wall structure of the rho1 mutants correlated with changes in gene expression and activity levels of cell wall biosynthetic enzymes. Northern blot analysis revealed drastically increased transcript levels of the chsV gene, encoding a class V chitin synthase (Madrid et al., 2003), in the Δrho1 mutant compared with the wild-type, the Δrho1 + rho1 and the Δrho1 + rho1G14V strains (Fig. 5). In agreement with this result, specific chitin synthase activity in mixed membrane fractions was approximately twofold enhanced in the Δrho1 mutant compared with the other strains (Fig. 6A).
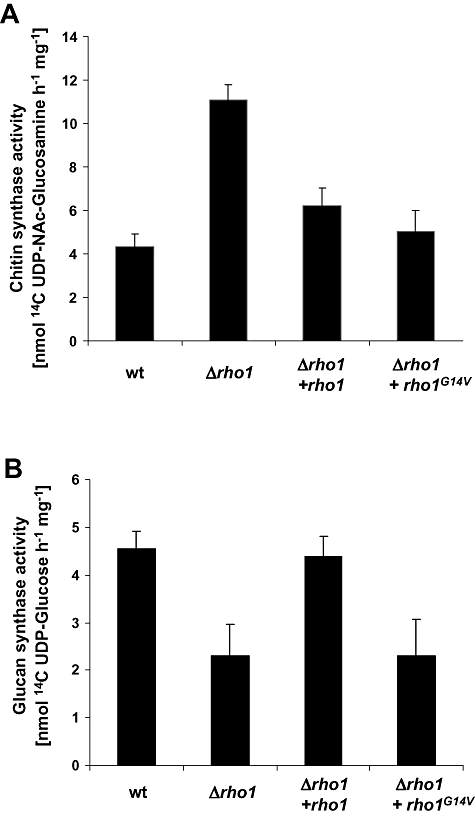
Mutation of rho1 affects cell wall biosynthetic enzyme activity. Specific chitin synthase (A) and glucan synthase activities (B) were determined in mixed membrane fractions of the indicated strains using UDP [14C]-N-acetylglucosamine or UDP [14C]-glucose respectively (see Experimental procedures for details). Specific activity is expressed in (nmol of sugar incorporated) h−1 (mg protein)−1. Each column represents the mean from three independent samples. Bars indicate standard deviations.
To study the effect of rho1 mutation on β-1,3-glucan synthesis, we cloned the F. oxysporum orthologue of the S. cerevisiae FKS1 gene encoding the major β-1,3-glucan synthase catalytic subunit (Douglas et al., 1994) (see Experimental procedures for details). Northern blot analysis detected enhanced transcript levels of fks1 in the Δrho1 and Δrho1 + rho1G14V strains, compared with the wild-type and the complemented strain (Fig. 5). By contrast, specific glucan synthase activity in mixed membrane fractions was significantly reduced in the Δrho1 and Δrho1 + rho1G14V mutants compared with the wild-type and the complemented strain, suggesting that Rho1 may regulate β-1,3-glucan synthase activity at the post-translational level (Fig. 6B). Thus, whereas both Δrho1 and Δrho1 + rho1G14V mutants have reduced glucan synthase activity, only the Δrho1 mutant has enhanced chitin synthase activity and an increased amount of chitin exposed at the cell surface.
F. oxysporum rho1 is required for virulence on plants but not on mammals
Two different bioassays were used to determine the effect of rho1 mutations on virulence of F. oxysporum f.sp. lycopersici on its natural plant host tomato. First, the capacity of the different strains to grow invasively on fruit tissue was assayed by injecting microconidial suspensions directly into tomato fruits. For comparison, the Δfmk1 mutant which is completely impaired in invasive growth (Di Pietro et al., 2001) was included in the experiment. Whereas the wild-type and the complemented Δrho1 + rho1 strains efficiently colonized and macerated the tomato fruit tissue, the Δrho1 and the Δrho1 + rho1G14V mutants showed a moderate reduction in invasive growth (Fig. 7A).
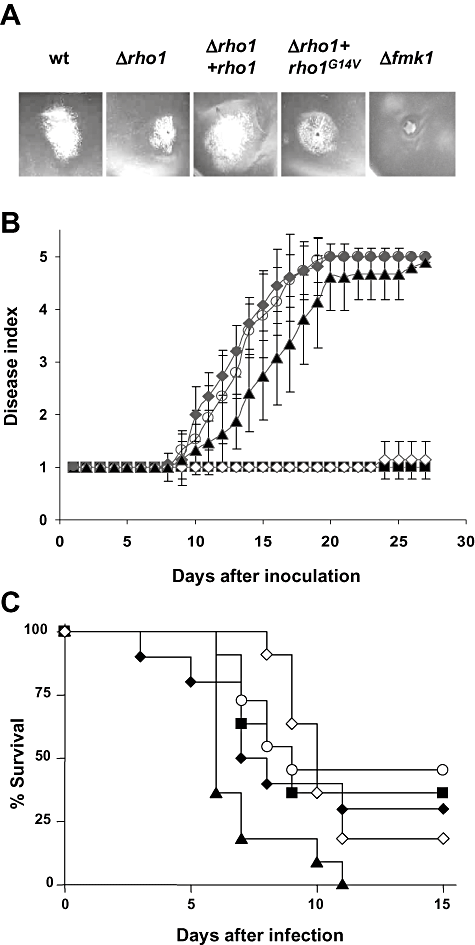
Rho1 is required for infection of tomato plants but not for virulence on immunodepressed mice.A. Invasive growth on tomato fruits. Inoculation was performed by injecting 5 × 105 microconidia of the indicated strains. The photograph was taken after 4 days' incubation at 28°C and 100% relative humidity.B. Incidence of Fusarium wilt after root inoculation of tomato plants (cultivar Monica). Severity of disease symptoms was recorded at the indicated times after inoculation, using an index ranging from 1 (healthy plant) to 5 (dead plant). Symbols refer to plants inoculated with the wild type (open circles), Δrho1 (open diamonds), Δrho1 + rho1 (filled diamonds), Δrho1 + rho1G14V (filled triangles) and the uninoculated control (filled squares). Error bars indicate standard deviations calculated from 10 plants for each treatment.C. Virulence of different strains of F. oxysporum on immunodepressed mice. Groups of 10 mice were infected with 2 × 107 microconidia of each strain and per cent survival was plotted for 15 days. Symbols refer to the same strains as in B, except that the Δgas1 strain was included (filled squares). The data shown are from one representative experiment.
For whole-plant infection assays, roots of tomato plants were immersed for 30 min in a microconidial suspension of the F. oxysporum strains, planted into minipots and maintained in a growth chamber. Severity of wilt symptoms was recorded at different times after inoculation (Fig. 7B). Disease symptoms in plants inoculated with the wild type increased steadily throughout the experiment. Initially, leaf tips turned yellow and curved, then the stalk weakened and decayed, and most of the plants were dead 20 days after inoculation. Plants inoculated with the Δrho1 + rho1 strain showed a similar pattern of disease development. By contrast, plants inoculated with the Δrho1 mutant failed to develop any disease symptoms, similar to the control plants treated with water. Unexpectedly, the Δrho1 + rho1G14V strain was nearly as virulent as the wild-type strain. We conclude that F. oxysporum rho1 is essential for root infection of tomato plants, but not for invasive growth on fruit tissue.
Fusarium oxysporum f.sp. lycopersici strain 4287 was previously reported to infect and kill immunodepressed mice (Ortoneda et al., 2004). To test the role of Rho1 in virulence of F. oxysporum on mammalian hosts, microconidia of the different strains were injected into the lateral tail vein of immunodepressed mice and survival was recorded over time. The Δrho1 and the Δrho1 + rho1 strain caused mortality rates that were not significantly different from those produced by the wild-type strain (Fig. 7C). By contrast, the Δrho1 + rho1G14V strain caused significantly increased mortality rates. These results indicate that Rho1 is dispensable for virulence of F. oxysporum on mammalian hosts.
Discussion
We explored the role of the small GTPase Rho1, a major regulator of cell polarity and cell wall synthesis in yeast, in development and virulence of the fungal pathogen F. oxysporum. In contrast to S. cerevisiae and S. pombe (Arellano et al., 1997; Levin, 2005), a targeted mutant lacking rho1 was viable, demonstrating that rho1 is not an essential gene in F. oxysporum. A similar nonessential role of rho1 has also been reported in Y. lipolytica (Leon et al., 2003). Lack of lethality might be due to the presence of at least one other rho1-like gene in F. oxysporum with partially overlapping function. This idea is supported by the presence of a faint signal observed in northern blots and ADP-ribosylation assays of the Δrho1 mutant (Fig. 1), and by the identification of a predicted gene in the F. oxysporum genome database sharing significant homology with rho1. The exact role of this second gene which encodes a putative orthologue of the Rac GTPase CflB (Boyce et al., 2003), and the extent of its possible functional overlap with rho1 remains to be determined.
A role of Rho1 in hyphal growth and cell wall organization
Even though rho1 is not essential in F. oxysporum, mutations in the gene lead to dramatic phenotypes: F. oxysporum strains carrying either a Δrho1 loss-of-function or a rho1G14V dominant-activating allele showed severely restricted rates of hyphal extension and colony growth on plates, but not in submerged culture. This striking difference suggests that rho1 must have a specific function during hyphal growth of F. oxysporum on solid surfaces. The restricted colony phenotype on solid media is similar to that reported for F. oxysporum mutants lacking Gas1, a β-1,3-glucanosyltransferase (Caracuel et al., 2005). Indeed, Δrho1 and Δgas1 mutants share additional phenotypes such as partial relief from restricted colony growth by sorbitol, increased resistance to cell wall-degrading enzymes or enhanced transcript levels of the chsV gene encoding a class V chitin synthase (Caracuel et al., 2005 and this work). In agreement with the latter result, the Δrho1 strain showed a significant increase in membrane-associated chitin synthase activity and in CFW staining. By contrast, Δrho1 had reduced glucan synthase activity. A likely interpretation of these results is that Rho1 positively regulates glucan synthase activity at the post-translational level, and that interference with its function leads to major changes in cell wall structure, including resistance to lytic enzymes and an increase in surface-exposed chitin.
The phenotypes of F. oxysporum rho1 and gas1 mutants have interesting parallels in S. cerevisiae. Yeast Δgas1 mutants are highly resistant to the protoplasting enzyme zymolyase (Popolo et al., 1993) and have an increased chitin content (Ram et al., 1998), correlating with a surge in expression of Chs3p chitin synthase (Valdivieso et al., 2000; Carotti et al., 2002; Lagorce et al., 2003). Moreover, Δfks1 mutants lacking a catalytic subunit of the β-1,3-glucan synthase complex show reduced β-1,3-glucan synthase activity, a pronounced slow-growth phenotype and a drastically altered cell wall composition, with lower β-1,3- and β-1,6-glucan levels and enhanced chitin and mannan levels (Douglas et al., 1994; Ram et al., 1998). Some of these phenotypes are highly reminiscent of those of the F. oxysporumΔrho1 mutant.
The mechanism of the cell wall reorganization response in F. oxysporum is currently unknown. In S. cerevisiae, Rho1 is one of the key upstream components in the cell integrity pathway (Madaule et al., 1987), which is activated in response to cell wall insults such as those caused by loss of gas1 (Bickle et al., 1998). The observation that expression of rho1 is increased in Δgas1 mutants (Caracuel et al., 2005) suggests that Rho1 may function in a similar cell wall reorganization pathway in F. oxysporum.
Rho1 regulates fungal pathogenicity on plants
A F. oxysporum strain lacking Rho1 is non-pathogenic on tomato plants, but fully virulent in an immunodepressed mouse model. To our knowledge, this is the first report on the role of a Rho1 orthologue in fungal pathogenesis. Previously, other members of the Rho family of GTPases have been implicated in fungal infection on plant or mammalian hosts. Cdc42 was found to control invasive hyphal growth in the human pathogen C. albicans (Bassilana et al., 2003) and virulence in the plant pathogens Claviceps purpurea and Colletotrichum trifolii (Scheffer et al., 2005; Chen et al., 2006). In the maize pathogen Ustilago maydis, deletion of either one of the small GTPases cdc42 or rac1 resulted in loss of virulence without interfering with cell viability (Mahlert et al., 2006).
The specific role of Rho1 during plant infection by F. oxysporum is currently unknown. One possibility is that hyphal invasion of a host plant mimics growth on a solid surface, implying a causal relationship between the growth defect of the Δrho1 strain on solid media and its inability to infect plants. This hypothesis falls short of explaining two key observations. First, rho1 mutants are still able to invade tomato fruit tissue. Second, both Δrho1 and the rho1G14V alleles confer restricted colony growth on plates, but only Δrho1 abolishes virulence on tomato plants, suggesting a lack of correlation between restricted growth on solid surfaces and virulence on plants.
A second hypothesis is that the role of Rho1 in virulence is related to maintenance of cell wall integrity during infection. Proper function of the cell integrity response may be a prerequisite to withstand the arsenal of cell wall-targeting compounds deployed by the plant, which includes chitinases, β-1,3-glucanases and phytoalexins (Dangl and Jones, 2001). Evidence against this hypothesis is provided by two lines of evidence. First, the non-pathogenic Δrho1 strain is highly resistant to protoplasting enzymes which contain a mixture of chitinases and β-1,3-glucanases, suggesting that this mutant has enhanced rather decreased cell wall integrity. By contrast, the Δrho1 + rho1G14V strain which is highly sensitive to cell wall-degrading enzymes is still virulent on tomato plants. Second, both loss-of-function and gain-of-function mutants are similarly sensitive to the cell wall-targeting compound Congo red, yet the two strains differ dramatically in virulence. These results again suggest a lack of correlation between sensitivity to cell wall-targeting molecules or enzymes and virulence of the two classes of rho1 mutants.
We currently favour a third hypothesis, according to which Rho1 plays a specific role in maintaining the fungal cell wall architecture in a way that makes it difficult to detect for the plant host. Plants are known to possess a exquisitely sensitive immune system that detects microbial invaders through common pathogen-associated molecular patterns (PAMPs) (Nurnberger et al., 2004). Binding of PAMPs to distinct pattern-recognition receptors activates convergent signalling pathways triggering broad-spectrum immunity (Jones and Dangl, 2006). Fungal cell wall polysaccharides such as N-acetylglucosamine (chitin) or β-1,3-glucan are among the best-characterized PAMPs (Nurnberger et al., 2004). Recently, a specific chitin receptor (CEBiP) was identified in the plasma membrane of rice cells and shown to play a key role in perception and transduction of the chitin elicitor signal that leads to activation of the defence response (Kaku et al., 2006). Thus, both β-1,3-glucan and chitin from fungal cell walls are major determinants of host–pathogen recognition.
Successful fungal pathogens have evolved strategies to avoid early recognition and activation of the immunity response by the host (He et al., 2007). One of these mechanisms consists of making the elicitor-active chitin and glucan polymers invisible to the host surveillance system. In F. oxysporum, as well as in yeast, the inner cell wall layer of chitin and glucan is separated from the extracellular space by an external electron-dense layer of mannosylated proteins (Schoffelmeer et al., 1999; De Groot et al., 2005). This coat of mannoproteins has multiple functions in shaping the properties of the fungal surface (De Groot et al., 2005). One of these functions could be to shield the structural cell wall polysaccharides from the attack by plant hydrolases, thus avoiding the generation of elicitor-active oligosaccharins. Even though the Δrho1 mutant was more resistant to cell wall-degrading enzymes, probably due to its higher chitin content, the N-acetylglucosamine might be more accessible at the cell surface. Alternatively, the altered cell wall structure of the Δrho1 mutant may result in shedding of chitin, glucan and/or outer cell wall proteins into the surrounding medium, leading to an increased elicitor activity even without exposure to plant cell wall-degrading enzymes. Thus, major cell integrity regulators such as Rho1 would play a crucial role in establishing and maintaining the hyphal architecture in a way that prevents cell matrix components from being detected by the cognate host receptors.
A recent genome-wide screen in S. cerevisiae identified a network of genes required for masking of cell wall glucans from detection by the mammalian immune system (Wheeler and Fink, 2006). A significant fraction of the identified genes fell into the class of regulators of polarized cell wall remodelling (Wheeler and Fink, 2006). Rho1 itself belongs to this class, although it was not detected in the mutant screen, probably because rho1 is an essential gene in S. cerevisiae. However, a number of components functioning upstream or downstream of Rho1 were identified, including the GDP-GTP exchange factor Tus1, the MAPK Slt2 or the formin Bni1, as well as Gas1. Moreover, treatment with caspofungin which specifically targets β-1,3-glucan synthesis, a major downstream function of Rho1, caused unmasking of β-glucan in C. albicans and elicitation of a robust immune response (Wheeler and Fink, 2006).
Downstream signalling components of Rho1 or other regulators of cell wall assembly have previously been associated with fungal virulence on plants. Mutants of the rice blast fungus Magnaporthe grisea lacking Mps1, the orthologue of the yeast cell integrity MAP kinase Slt2, were completely non-pathogenic on rice (Xu et al., 1998). Importantly, the Δmps1 strains were still able to trigger strong defence responses in plant cells, including accumulation of autofluorescent compounds or rearrangement of the actin cytoskeleton. The glucanosyltransferase Gas1, another putative downstream target of Rho1, was required for correct assembly of the cell wall and virulence on tomato plants by F. oxysporum (Caracuel et al., 2005). In the two plant pathogens Colletotrichum lagenarium and M. grisea, deletion of the orthologue of S. cerevisiae SSD1, a key regulator of cell wall assembly, lead to increased sensitivity to CFW and loss of pathogenicity (Tanaka et al., 2007). Interestingly, appressorial penetration of the Δssd1 mutants was restricted by plant cell wall-associated defence responses such as papilla formation or accumulation of reactive oxygen species (Tanaka et al., 2007).
We propose that deletion of rho1 in F. oxysporum leads to significant changes in wall architecture, including an increase in the amount of cell wall polymers such as chitin exposed at the hyphal surface or released to the surrounding medium. These elicitor-active compounds then act as triggers of the plant defence responses, effectively blocking pathogenic development. This hypothesis is supported by the finding that the non-virulent Δrho1 strain, but not the virulent Δrho1 + rho1G14V strain showed enhanced staining with CFW, a dye that specifically detects chitin exposed at the hyphal surface. Second, chitin synthase activity and expression of chsV encoding a class V chitin synthase were significantly upregulated in Δrho1, but not in Δrho1 + rho1G14V strains, suggesting a clear correlation between increased chitin synthase activity, surface-exposed chitin and loss of virulence on tomato plants.
In summary, the results of this study support the emerging view that regulators of cell wall architecture such as Rho1 play crucial roles in fungal infection of plants, by avoiding recognition by the host immune system. A thorough analysis of the cell wall remodelling network in plant pathogenic fungi may thus lead to the discovery of new targets for fungicide development.
Experimental procedures
Strains and growth media
Fusarium oxysporum f.sp. lycopersici strain 4287 (race 2) was originally obtained from J. Tello, Universidad de Almería, Spain, and stored at −80°C with 30% glycerol as a microconidial suspension (Di Pietro and Roncero, 1998). The pathotype of the isolate on tomato was routinely confirmed by plant infection assays. The generation and characterization of the Δfmk1 mutant lacking a MAP kinase was described previously (Di Pietro et al., 2001). For extraction of genomic DNA and for microconidia production, cultures were grown in potato dextrose broth (PDB; Difco, Detroit, MI) or on synthetic medium (SM) containing 1% sucrose as the carbon source, as described previously (Di Pietro and Roncero, 1998). For analysis of gene expression, freshly obtained microconidia were germinated for 14 h in PDB, germlings were washed twice in sterile water and transferred for 2 h to SM with or without 1.2 M sorbitol. For qualitative and quantitative analysis of colony growth, drops containing 2 × 105 freshly obtained microconidia were transferred onto plates of potato dextrose agar (PDA; Difco) or SM containing 1.5% agar, 1% (w/v) sucrose as the carbon source and 0.1% NaNO3 as the nitrogen source. Where indicated, sorbitol was added at a concentration of 1.2 M. Congo red (final concentration 0.5 mg l−1) or BCIP (600 mg l−1) (all from Sigma) were added to the medium after autoclaving. Inoculated plates were incubated for 3 days at 28°C or 37°C. For determination of fungal biomass, freshly obtained microconidia (2.5 × 106 ml−1) were transferred to PDB for 24 h, mycelia were harvested on pre-weighed Whatman filters, dried overnight at 80°C, and fungal dry weight was determined. For determination of sensitivity to cell wall-degrading enzymes, germlings were incubated with protoplasting enzyme (Lallzyme; Lallemand SA, St Simon, France) at 30°C as described (Di Pietro and Roncero, 1998), and protoplast release over time was quantified microscopically. CFW and DAPI staining was performed as described (Martin-Udiroz et al., 2004). Microscopic observations were carried out in a Leica DMR microscope using the Nomarsky technique or epifluorescence. Photographs were recorded with a Leica DC 300F digital camera.
Nucleic acid manipulations and gene cloning
Total RNA and genomic DNA were extracted from F. oxysporum mycelium as previously described (Raeder and Broda, 1985; Chomczynski and Sacchi, 1987). Southern and Northern analyses and probe labelling were carried out as described (Di Pietro and Roncero, 1998) using the non-isotopic digoxigenin labelling kit (Roche Diagnostics SL). Other routine nucleic acid manipulations were performed according to standard protocols (Sambrook and Russell, 2001).
For cloning of the F. oxysporum fks1 gene, genomic DNA of strain 4287 was used for PCR amplification on a Perkin Elmer GeneAmp System 2400 with the degenerate primers FKS1-3 (5′-AAYCARGAYAAYTAYYTNGARGARTG-3′) and FKS1-4 (5′-CCRCAYTGRWARTAYTGRCARTGYTTDAT-3′), derived from highly conserved regions of fungal Fks1 proteins. PCR reactions were routinely performed with the Long Template PCR system (Roche Diagnostics SL). The obtained fragment was cloned into the pGEM-T vector (Promega) and after confirming its identity by sequencing, the fragment was used as a probe to screen a lambda EMBL3 genomic library of F. oxysporum isolate 4287. Sequencing of a hybridizing genomic clone identified an open reading frame of 5358 nucleotides, interrupted by three putative introns. The GenBank accession number of F. oxysporum fks1 is EF694038. The probe for Northern analysis was obtained using primers FKS1-5 (5′-TTTGAGAGATGAGACTGACA-3′) and FKS1-6 (5′-GAGGGCATTCATACCAGCGT-3′). Library screening, subcloning and other routine procedures were performed following standard protocols (Sambrook and Russell, 2001). Sequencing of both DNA strands of the obtained clones was performed at the Servicio de Secuenciación Automática de DNA, SCAI (University of Córdoba, Spain) using the Dyedeoxy Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) on an ABI Prism 377 Genetic Analyzer apparatus (Applied Biosystems). DNA and protein sequence databases were searched using the blast algorithm (Altschul et al., 1990) at the National Center for Biotechnology Information (Bethesda, MD, USA).
Construction of rho1 loss-of-function and gain-of-function alleles and production of fungal mutants
Gene replacement vector pDrho1 was constructed by introducing a BamHI site at position 624 of the rho1 coding region using the overlapping method (Ho et al., 1989) with the primers RHO10B (5′-TGACCAGCAggatccCATAGTGCGAG-3′) and RHO9B (5′-CTCGCACTATGggatccTGCTGGTCA-3′), and the final product was cloned into pGEM-T. This construct was linearized with BamHI and a 1.8 kb BamHI fragment containing the hygromycin B resistance gene under the control of a Cochliobolus heterostrophus promoter (Turgeon et al., 1987) was cloned into it (Fig. S2B). A linear fragment containing the interrupted rho1 allele was obtained by amplifying the construct with primers RHO11 (5′-TCCTCTTTCTCTATCCATCCAT-3′) and RHO7 (5′-CCCAAACAAGAAAAAAGACATCT-3′) and used to transform protoplasts of F. oxysporum strain 4287 to hygromycin resistance, and transformants were purified by monoconidial isolation (Di Pietro and Roncero, 1998). Transformants showing homologous insertion of the construct were detected by Southern analysis of genomic DNA treated with EcoRI and hybridized with a labelled rho1 probe obtained by PCR amplification with primers RHO8 (5′-TGTCGAGGTTGATGGCAAGC-3′) and RHO3 (5′-AACACTTCACGGACACCCTC-3′). For complementation experiments, a 2.2 kb DNA fragment encompassing the entire rho1 gene was amplified by PCR from F. oxysporum genomic DNA using primers RHO19 (5′-TCTCAACAACCCTGCCCAAC-3′) and RHO7, and introduced into protoplasts of the Δrho1 strain by cotransformation with the phleomycin resistance cassette amplified from plasmid pAN8-1 (Mattern et al., 1988). Phleomycin-resistant transformants were selected as described (Di Pietro et al., 2001). The presence of the wild-type rho1 allele in the complemented transformants was detected by PCR on genomic DNA with primers RHO19 and RHO7 and by Southern analysis with the rho1 probe.
The constitutively active rho1G14V allele was constructed using the overlapping method described above, by substituting nucleotide G at position 41 of the rho1 coding region with a T, using the primers RHO15MUT (5′-GTCGGCGATGtTGCTTGTGGT-3′) and RHO16MUT (5′-ACCACAAGCAaCATCGCCGAC-3′) with the complete rho1 gene as the template (Fig. S2C). The amplified fragment was cloned into pGEM-T and entirely sequenced to confirm the mutation. A 2.2 kb DNA fragment encompassing the rho1G14V allele was amplified with primers RHO19 and RHO7, and introduced into protoplasts of the Δrho1 strain by cotransformation with the phleomycin resistance cassette. The presence of the rho1G14V allele in phleomycin-resistant transformants was detected by PCR on genomic DNA with primers RHO19 and RHO7 and by Southern analysis with the rho1 probe.
Isolation of mixed membrane fractions
Freshly obtained microconidia were germinated for 14 h in PDB, washed three times in sterile water, harvested by filtration, immediately frozen in liquid nitrogen and stored at −80°C until use. Frozen samples were re-suspended in lysis buffer [50 mM Tris-HCl pH 7.5, 5 mM EDTA, 5 mM NaF, 10% (v/v) glycerol] supplemented with a protease inhibitor cocktail (25 μg ml−1 soy trypsin inhibitor, 5 μg ml−1 antipain, 5 μg ml−1 leupeptin and 5 μg ml−1 pepstatin A, all from Sigma) and lysed using glass beads (0.45 mm in diameter) in a bead beater. Cell walls were removed by centrifugation at 1500 g during 5 min, and supernatants were subjected to ultracentrifugation at 100 000 g during 1 h at 4°C. The pellet containing the mixed membrane fraction was re-suspended in lysis buffer and stored at −20°C until use.
Glucan and chitin synthase activity assays
Glucan and chitin synthase activities were determined in mixed membrane fractions. For glucan synthase activity assays, samples containing 100 μg protein were incubated in 50 mM Tris-HCl pH 8.0, 25 mM NaF, 1 mM EDTA with 50 μM GTP and 600 μM UDP[14C]-glucose in a final volume of 125 μl. After 1 h at 22°C, the reaction was stopped by adding 125 μl of 20% trichloroacetic acid (TCA). Samples were filtered through Whatman B glass fibre filters (2.4 cm in diameter), washed twice with 5% TCA and twice with 100% ethanol, and dried at 50°C for 12 h. Five millilitres of scintillation fluid [0.2% (v/v) 1,5-diphenyloxazoil and 0.01% 1,4-bis-2–4-methyl-5-phenyloxazoil-toluene in toluene] was added, and samples were counted in a Beckman LS 6500 counter. Specific glucan synthase activity was expressed in (nmol Glc incorporated) h−1 (mg protein)−1.
For chitin synthase activity assays, samples containing 100 μg protein were incubated in 50 mM Tris-HCl pH 7.0, 20 mM MgCl2, 100 μM ATP, 10 μM trypsin, 20 mM N-acetylglucosamine and 15 μM UDP[14C]-N-acetylglucosamine in a final volume of 50 μl. After 1 h at 22°C, the reaction was stopped by adding 25 μl of glacial acetic acid, samples were filtered as described above, washed twice with 1 M acetic acid:95% ethanol (70:30 v/v), dried and counted as described above. Specific chitin synthase activity was expressed in (nmol GlcNAc incorporated) h−1 (mg protein)−1.
ADP ribosylation assays
Mixed membrane fractions (100 μg protein) were incubated in reaction buffer [50 mM Tris-HCl pH 7.4, 1 mM MgCl2, 0.5 mM DTT, 0.5 mM EDTA, 0.2% (w/v) n-octylglucoside, 2 μM NAD, 0.01 μCi μl−1[32P]-NAD], either in the presence or absence of 0.02 μg μl−1Clostridium botulinum exotoxin C3 (Calbiochem) in a total volume of 60 μl. After 1 h at 30°C, sample buffer was added and samples were boiled for 5 min, subjected to SDS-PAGE, stained with Coomassie blue, dried under vacuum and subjected to autoradiography at −80°C for 5–8 days.
Virulence assays on plants and mice
Root inoculation assays were performed as described (Di Pietro and Roncero, 1998). Briefly, 2-week-old tomato seedlings (cultivar Vemar) were inoculated with F. oxysporum strains by immersing the roots in a microconidial suspension, planted in vermiculite and maintained in a growth chamber. At different times after inoculation, severity of disease symptoms was recorded using an index ranging from 1 (healthy plant) to 5 (dead plant). Ten plants were used for each treatment. Assays for invasive growth on tomato fruits (cultivar Daniela) were carried out as described (Di Pietro et al., 2001). Virulence experiments were performed three times with similar results.
Infection assays on immunodepressed mice were performed as described (Ortoneda et al., 2004). Experimental conditions were approved by the Animal Welfare Committee of the Faculty of Medicine of Universitat Rovira i Virgili. Briefly, mice were immunosuppressed with a single intraperitoneal 200 mg kg−1 dose of cyclophosphamide (Laboratorios Funk SA, Barcelona, Spain) and with an intravenous 150 mg kg−1 dose of 5-fluorouracil (Fluoro-uracil, Roche SA, Madrid, Spain) on day 0. Groups of 10 animals were infected by injecting 0.2 ml of an inoculum of 108 conidia ml−1 of sterile saline into a lateral vein of the tail on day 0. Survival was recorded each day for 15 days. Infection experiments with each strain were performed at least twice with similar results. Survival was estimated by the Kaplan–Meier method and compared among groups using the log-rank test.
Acknowledgements
The authors are grateful to Esther Martinez Aguilera, Universidad de Córdoba, for valuable technical assistance. This work was supported by grants BIO-2004-00276 and BIO-2004-01240 from the Spanish Ministerio de Ciencia y Tecnología (MCyT). A.L.M.R. was supported by a PhD fellowship from MCyT. A.D.P. was recipient of a Ramón y Cajal grant from MCyT.




