The Pseudomonas aeruginosa type III secreted toxin ExoT is necessary and sufficient to induce apoptosis in epithelial cells
Summary
Type III secreted (T3SS) effectors are important virulence factors in acute infections caused by Pseudomonas aeruginosa. PA103, a well-studied human lung isolate, encodes and secretes two effectors, ExoU and ExoT. ExoU is a potent cytotoxin that causes necrotic cell death. In addition, PA103 can induce cell death in macrophages in an ExoU-independent but T3SS-dependent manner. We now demonstrate that ExoT is both necessary and sufficient to cause apoptosis in HeLa cells and that it activates the mitochondrial/cytochrome c-dependent apoptotic pathway. We further show that ExoT induction of cell death is primarily dependent on its ADP ribosyltransferase domain activity. Our data also indicate that the T3SS apparatus can cause necrotic cell death, which is effectively blocked by ExoT, suggesting that P. aeruginosa may have evolved strategies to prevent T3SS-induced necrosis.
Introduction
Many microbial pathogens utilize various strategies to induce cell death in their host (Fink and Cookson, 2005). The study of pathogen-induced host cell death has gained significant momentum in recent years with the recognition that this phenomenon is not merely a consequence of infection but rather a regulated process with significant implications for pathogenesis (Gao and Abu Kwaik, 2000). Indeed, pathogens have been key to elucidating new components of cell death pathways and their functions (Moss et al., 1999; Weinrauch and Zychlinsky, 1999; Gao and Abu Kwaik, 2000).
Programmed cell death is a key regulator of development in multicellular eukaryotic organisms (Gulbins et al., 2000). Dysregulation of cell death pathways can have dire consequences that lead to cancer and autoimmune diseases (Lupulescu, 1996a,b; Cohen, 1997; Evans et al., 1998). Programmed cell death can be broadly divided into two categories, apoptosis and necrosis/oncosis. Apoptotic cell death is characterized by distinct morphological features, including membrane blebbing, formation of apoptotic bodies and nuclear condensation (Cohen, 1996; 1997). In contrast, cells undergoing necrotic cell death manifest swelling, sudden cellular collapse and release of cellular content (reviewed in Fink and Cookson, 2005). Apoptosis involves the sequential activation of a cascade of proteases called initiator and effector caspases (Philchenkov, 2004). Necrotic cell death is associated with severe cellular trauma, such as membrane damage, and remains incompletely defined at the mechanistic level.
Pseudomonas aeruginosa is a ubiquitous Gram-negative pathogen that is a leading cause of nosocomial infections (Mandell et al., 2000). P. aeruginosa possesses a type III secretion system (T3SS) that it uses as a conduit to translocate effector proteins directly into its host cytoplasm. Four T3SS effectors have been identified in P. aeruginosa: ExoU, a phospholipase; ExoY, an adenylate cyclase; and ExoT and ExoS, which are bifunctional proteins with N-terminal GTPase-activating protein (GAP) activity and C-terminal ADP ribosyltransferase (ADPRT) activity (Yahr et al., 1998; Barbieri and Sun, 2004; Sato and Frank, 2004). Most strains do not possess all four effectors but virtually all strains encode and express ExoT (Feltman et al., 2001), suggesting a key role for this virulence factor in the context of P. aeruginosa pathogenesis.
Various P. aeruginosa virulence factors have been associated with necrotic or apoptotic cell death. ExoS has been shown to be necessary and sufficient to induce a caspase-3-dependent cell death (Alaoui-El-Azher et al., 2006; Jia et al., 2006), while the phospholipase activity of ExoU results in necrotic cell death (Sato and Frank, 2004). Additionally, the pore-forming activity associated with the insertion of the T3SS needle complex has been shown to lead to cell death in several Gram-negative pathogens, including P. aeruginosa (Viboud and Bliska, 2001; Stockbauer et al., 2003; Lee et al., 2005). In at least some cases, this death is associated with the inflammasome and caspase-1 activation, leading to increased production and secretion of IL1-β and induction of inflammatory response (Mariathasan and Monack, 2007).
Previously, we demonstrated that PA103, an ExoT- and ExoU-expressing lung isolate, induced cell death in macrophages in a T3SS-dependent manner that was, to a large extent, independent of ExoU (Hauser and Engel, 1999). Dying cells exhibited some features of apoptosis including a positive TUNEL assay and release of nucleosome-bound DNA into the cytoplasm. In this communication, we demonstrate that ExoT, the other known T3SS effector produced by PA103 strain, is necessary and sufficient to cause apoptotic cell death in HeLa cells.
Results
PA103ΔU induces cell death by at least two distinct pathways in HeLa cells
We used time-lapse fluorescent and phase videomicroscopy to examine the ExoU-independent cell death. HeLa cells were infected at a multiplicity of infection (moi) of 10 with isogenic mutants of PA103, including PA103ΔU, which carries an in-frame deletion in the exoU gene but produces ExoT; PA103ΔUΔT, which carries deletions in both the exoU and exoT genes but retains a functional T3SS; and PA103pscJ, which carries a transposon insertion in an operon encoding structural compo nents of the T3SS and fails to secrete or translocate the type III secreted effectors (Table 1). Propidium iodide (PI), an impermeant nuclear dye that only stains the nucleus of membrane-compromised cells, was included in the media to identify dead cells. As shown in Fig. 1A and Movie S1, by 10 h post infection, nearly all HeLa cells infected with PA103ΔU took up PI. PA103ΔUΔT also caused PI uptake, albeit with slower kinetics (Fig. 1A and B and Movie S2) but the T3SS mutant, PA103pscJ, failed to cause cell death up to 25 h post infection (Fig. 1A and Movie S3). Z-Val–Ala–Asp(OMe)-CH2F (ZVAD), a broad pan-caspase inhibitor known to block apoptosis (Callus and Vaux, 2007), prevented PI uptake in HeLa cells infected with PA103ΔU but had little effect on PA103ΔUΔT-induced cell death (Fig. 1B and Movies S4 and S5). All strains exhibited similar growth curves, eliminating the possibility that differences in the extent or the kinetics of cell death were due to differences in bacterial load (data not shown).
| Plasmids and strains | Relevant characteristics | Source or reference |
|---|---|---|
| Plasmids | ||
| pIRESKII-EGFP | pIRES-EGFP (Clonetech) with bases 1870–1910 removed | A kind gift of M. Mohrs and R. Locksley |
| pIRESKII-EGFP/ExoT | Coding region of wild-type ExoT cloned into the EcoRI site of pIRESKII | Garrity-Ryan et al. (2004) |
| pIRESKII-EGFP/ExoT(G−A−) | Coding region of ExoT double mutant cloned into the EcoRI site of pIRESKII, as described | Garrity-Ryan et al. (2004) |
| pEGFP | pIRES2-EGFP | BD Biosciences, Clontech |
| pExoT–GFP | Modified pIRES2-EGFP vector, harbouring ExoT directly fused at its C-terminus to EGFP | Shafikhani and Engel (2006) |
| pExoT(G+A−)–GFP | Modified pIRES2-EGFP vector, harbouring ExoT with functional GAP and mutant ADPRT, directly fused at its C-terminus to EGFP, as described | Shafikhani and Engel (2006) |
| pExoT(G+A−)–GFP | Modified pIRES2-EGFP vector, harbouring ExoT with mutant GAP and functional ADPRT, directly fused at its C-terminus to EGFP, as described | Shafikhani and Engel (2006) |
| pExoT(G−A−)–GFP | Modified pIRES2-EGFP vector, harbouring ExoT double mutant directly fused at its C-terminus to EGFP, as described | Shafikhani and Engel (2006) |
| Strains | ||
| PA103pscJ | PA103 harbouring Tn5 Gent insertion in the pscJ gene, defective in T3SS | Kang et al. (1997) |
| PA103ΔU | PA103 harbouring an in-frame deletion in exoU | Garrity-Ryan et al. (2000) |
| PA103ΔUΔT | PA103ΔU with a xylE/aacC1 cassette containing Gmr, replacing amino acids 36–348 of exoT | Garrity-Ryan et al. (2000) |
| PA103ΔU/T(G−A+) | PA103ΔUΔT expressing full-length ExoT, mutated in the GAP domain, at exoT locus | Garrity-Ryan et al. (2000) |
| PA103ΔU/T(G+A−) | PA103ΔUΔT expressing full-length ExoT, mutated in the ADPRT domain, at exoT locus | Garrity-Ryan et al. (2000) |

PA103ΔU induces cell death by at least two distinct pathways in HeLa cells. HeLa cells were infected in the absence (A) or presence (B) of ZVAD-fmk (60 μm) with PA103ΔU, PA103ΔUΔT, PA103pscJ at an moi of ca. 10 for the indicated time. Host cell death was assessed by uptake of PI by simultaneous time-lapse phase and fluorescent videomicroscopy. Note that both PA103ΔU and PA103ΔUΔT cause cell death whereas the T3SS mutant strain does not. ZVAD inhibits cell death induced by the ExoT-producing strain PA103ΔU but fails to prevent cell death induced by PA103ΔUΔT.
The ExoT-dependent apoptotic killing of HeLa cells was further confirmed by flow cytometry. Dead cells are hypodiploid in DNA content and appear as a sub-G1 population in a flow cytometry histogram (Fraker et al., 1995). HeLa cells were infected with the aforementioned isogenic strains for 5 h, bacteria were removed by washing, fresh medium containing antibiotics was added to prevent bacterial growth, and cells were analysed 15 h after bacterial removal. As shown in Fig. 2A and C, infection with PA103ΔU resulted in nearly fourfold increase in cell death (80%, P < 0.001), compared with the uninfected or PA103pscJ infected cells, and this cell death was inhibited by ZVAD (Fig. 2B and C). Infection with PA103ΔUΔT also led to increased cell death, albeit to a lesser degree than PA103ΔU (∼41%) (Fig. 2B and C). This effector-independent cell death was reduced to 31% in the presence of ZVAD (P < 0.01), indicating that caspase-dependent apoptotic cell death only accounted for 20% of cell death in response to PA103ΔUΔT infection. Time-lapse videomicroscopy studies further revealed that HeLa cells infected with PA103ΔU exhibited membrane blebbing and apoptotic body formation, hallmarks of apoptosis (Kothakota et al., 1997), which was not seen in cells infected with PA103ΔUΔT (Fig. 2D and data not shown). Collectively, these data indicate that in the presence of ExoT, HeLa cell death is primarily apoptotic in nature and that the T3SS can cause cell death that is mostly necrotic in nature and is effectively blocked by ExoT.
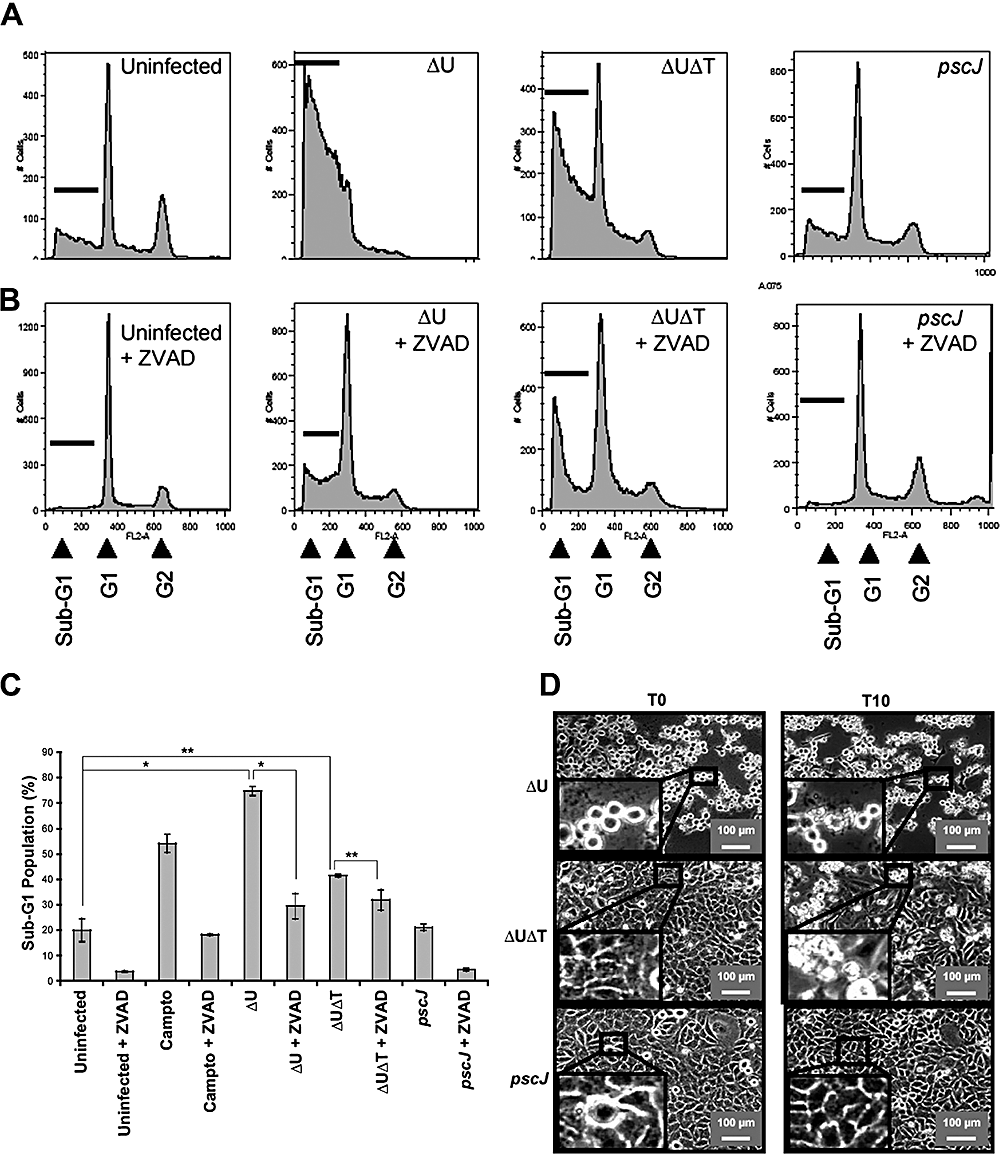
ExoT-expressing bacteria induce apoptosis in HeLa cells. HeLa cells were infected with the indicated strains for ∼5 h at an moi of ca. 10 in the absence (A) or presence (B) of ZVAD. Bacteria were then removed and fresh medium containing antibiotics was added to kill remaining bacteria. Cell death was analysed by flow cytometry (A–C) or time-lapse videomicroscopy (D). In (A)– (C), cells were collected 20 h post infection and analysed for DNA content by flow cytometry. Each experiment was repeated in triplicate and the average ± SEM (*P < 0.001 and **P < 0.01 is graphed in (C). In (D) cell morphology was analysed by time-lapse videomicroscopy after removal of bacteria (T = 0). Note that ExoT-producing PA103ΔU strain increases the fraction of sub-G1 cells (A–C) in a ZVAD-sensitive manner and causes membrane blebbing and apoptotic body formation (D).
ExoT activates the mitochondrial apoptotic pathway
Apoptosis proceeding through the main intrinsic or extrinsic pathways leads to the release of cytochrome c and the subsequent activation of the effector caspase-3 (Cory and Adams, 2002; Philchenkov, 2004). We used two criteria, loss of mitochondrial membrane potential (MMP) and activation of caspase-3, to examine the involvement of the mitochondrial pathway in ExoT-induced cell death. Loss of MMP was assessed by immunofluorescence (IF) microscopy, using MitoCaptureTM. This cationic dye accumulates and aggregates in intact mitochondria, resulting in punctate staining but remains in the cytoplasm in its monomeric form upon disruption of the mitochondrial membrane potential, exhibiting diffuse staining (see Experimental procedures). HeLa cells were infected for 5 h, stained with the dye for 15 min, and live cells were visualized by IF without fixation. As a positive control, uninfected HeLa cells were treated with camptothecin, a known inducer of the intrinsic pathway, for 10 h prior to staining. As shown in Fig. 3A and B, 60% of uninfected cells exposed to camptothecin and greater than 80% of HeLa cells infected with PA103ΔU exhibited loss of MMP as manifested by diffuse cytoplasmic staining. In contrast, untreated cells or cells infected with PA103ΔUΔT or PA103pscJ showed no evidence of MMP loss and exhibited punctate mitochondrial staining.
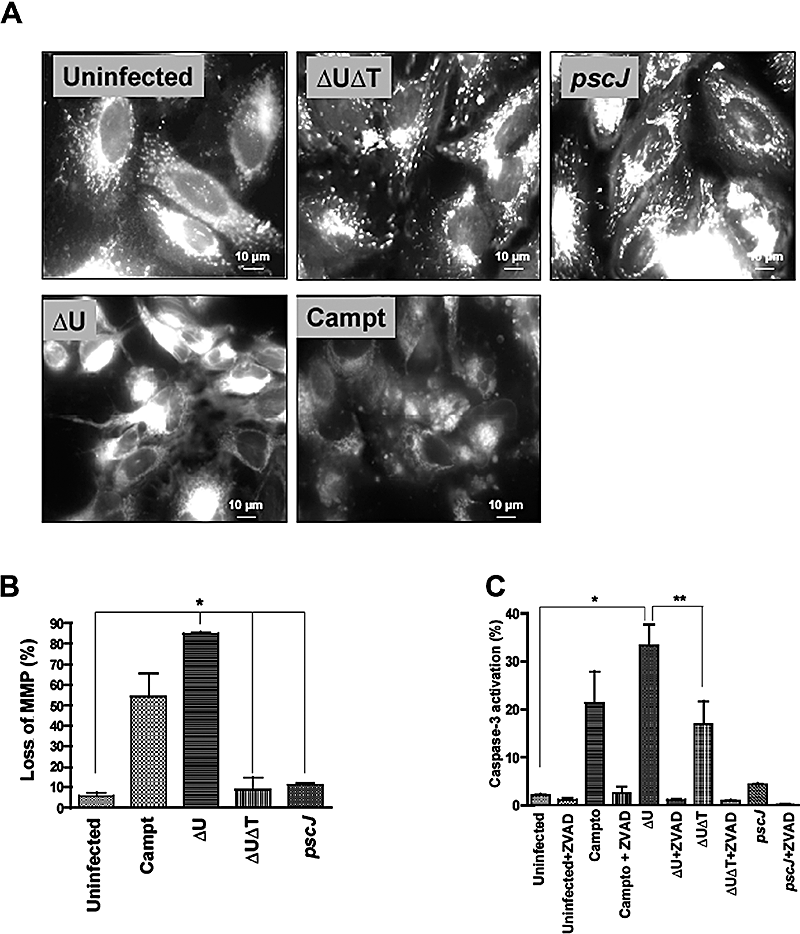
ExoT activates the mitochondrial/cytochrome c-dependent apoptosis.A. HeLa cells were infected with the indicated strains at an moi of ca. 10 for 5 h, bacteria were removed and medium containing MitoCaptureTM stain was added to stain cells. Cells were imaged by IF microscopy without fixation. Punctate mitochondrial staining is apparent in uninfected, PA103pscJ-infected and PA103ΔUΔT-infected cells. In contrast, PA103ΔU-infected or camptothecin-treated cells exhibit diffuse cytoplasmic staining, consistent with disruption of the mitochondrial membrane potential.B. The fraction of cells with diffuse staining from six random fields for each sample is tabulated and shown as the mean ± SEM (*P < 0.001).C. HeLa cells were infected with the indicated strains in the presence or absence of ZVAD as described in Fig. 2. Bacteria were removed 5 h post infection, cells were harvested at 20 h post infection, and triplicate samples were analysed for activated caspase-3 by flow cytometry using APO ACTIVE 3TM.The mean ± SEM is shown (*P < 0.001).
Using an antibody that specifically recognizes the activated form of caspase-3, we then examined the effect of ExoT on caspase-3 activation. Treatment with camptothecin or infection with PA103ΔU resulted in greater than fivefold activation of caspase-3 (Fig. 3C), compared with uninfected or PA103pscJ-infected cells (P < 0.001). Infection with PA103ΔUΔT also led to caspase-3 activation, although to a lesser extent (∼50% of PA103ΔU). ZVAD effectively blocked the caspase-3 activation in all cases (Fig. 3C). Together with 1, 2, these data suggest that PA103ΔUΔT can induce two types of cell death, a minor branch that is ZVAD-sensitive and apoptotic in nature and a major branch that is ZVAD-insensitive and necrotic. Our results further indicate that ExoT effectively blocks the necrotic branch of T3SS-induced cell death (Fig. 1B, top, and Fig. 2A–C).
ExoT is sufficient to induce apoptotic cell death
To determine whether ExoT was sufficient to cause apoptosis in the absence of other bacterial factors, HeLa cells were transiently transfected in the absence or presence of ZVAD with a mammalian expression vector expressing wild-type ExoT or the enzymatically inactive form of ExoT, in which both domains are mutated [ExoT(G−A−)], and fused to GFP at the C-terminus (Table 1). We then utilized time-lapse videomicroscopy of live cells in the presence of PI as well as IF microscopy of fixed cells: (i) to determine the kinetics of cell death, (ii) to examine the morphology of dying cells and (iii) to assess the activation of caspase-3.
We used time-lapse videomicroscopy in the presence of PI to establish the mean time to death (MTD) in cells transfected with ExoT–GFP. The time to death was defined as the time between the appearance of GFP (green), an indicator of transfected gene expression, and the time at which the cell membrane integrity was compromised resulting in the uptake of PI (red). We only included cells in our subsequent analysis that could be followed for at least one MTD plus one standard deviation from the mean.
Nearly 100% of cells transfected with wild-type ExoT exhibited PI uptake (n = 143, MTD = 8.5 ± 1.3 h), compared with only 33% (n = 98) of cells transfected with either the ExoT(G−A−) double mutant, or 22% (n = 63) of cells transfected with the vector alone (P < 0.001, Fig. 4A and B, Movies S6 and S7, and data not shown). ZVAD significantly blocked PI uptake (Fig. 4B and C, and Movie S8, P < 0.01). HeLa cells expressing ExoT first appeared green (defined as T = 0) and rounded up, consistent with the known ability of ExoT to disrupt the actin cytoskeleton (Garrity-Ryan et al., 2000). They then turned yellow (T = 8 h), indicating that PI had gained access to the nucleus. At later time points (T = 15 h), only PI staining was apparent, a result that is in agreement with our previous report demonstrating that ExoT is unstable and is degraded in HeLa cells (Balachandran et al., 2007). Identical results were obtained when ExoT and GFP were expressed from an IRES-containing vector in which they were co-transcribed but translated and produced as separate proteins (Table 1), indicating that GFP fusion did not alter ExoT activity in a way that would lead to cell death (data not shown).
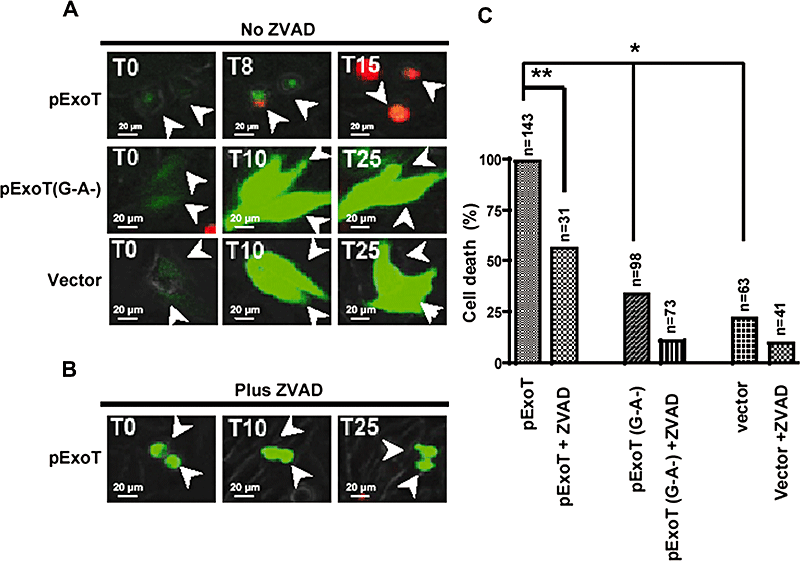
ExoT is sufficient to induce cell death in HeLa cells.A and B. HeLa cells were transiently transfected in the absence (A) or presence (B) of ZVAD with a mammalian expression vector expressing either wild-type ExoT or ExoT(G−A−) fused to GFP at the C-termini. Cell death was analysed by time-lapse videomicroscopy in the presence of PI. Video images were captured every 15 min. The arrows show representative transfected cells.C. The tabulated results, collected from multiple movies, are shown. Expression of ExoT induces nearly 100% cell death whereas the ExoT double mutant or vector alone results in much less cell death. ZVAD significantly inhibits ExoT-induced cell death. The numbers above each column indicates the total number of transfected cells that were scored (*P < 0.001; **P < 0.01, χ2 test).
Consistent with our data that bacterially translocated ExoT induces apoptosis in HeLa cells (1-3), 80% of the cells (n = 270) transfected with pExoT exhibited nuclear condensation and fragmentation, morphological hallmarks of apoptosis, and ZVAD reduced this to less than 30% (n = 126, Fig. 5B and C). In contrast, only 12% of HeLa cells transfected with the pExoT(G−A−) double mutant exhibited nuclear condensation (n = 237; Fig. 5A and C, P < 0.001). It is worth noting that although the nuclei of pExoT-transfected cells appeared smaller than the nuclei of the untransfected cells or cells transfected with pExoT(G−A−), they were not condensed or fragmented in the presence of ZVAD (Fig. 5B and C).
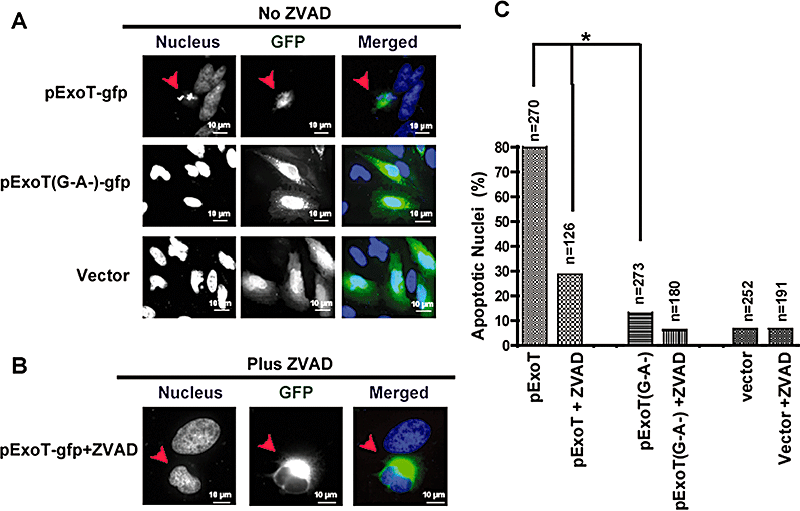
Transient transfection of ExoT is sufficient to induce apoptosis in HeLa cells.A and B. HeLa cells were transiently transfected in the absence (A) or presence (B) of ZVAD with a mammalian expression vector expressing wild-type ExoT or ExoT(G−A−) fused to GFP at the C-termini, or control vector. At 15–20 h post transfection, cells were fixed and stained with DAPI (blue). Transfected cells (green) were assessed for nuclear condensation and nuclear fragmentation, indicators of apoptosis. The arrow points to a representative ExoT transfected cell, showing apoptotic nuclear condensation.C. The tabulated results, collected from multiple transfection events are shown. The numbers above each column indicates the total number of transfected cells that were scored (*P < 0.001; χ2 test).
Immunofluorescence imaging was then used to assess the activation status of caspase-3. As shown in Fig. 6, transfection with wild-type ExoT but not ExoT(G−A−) or the control vector was sufficient to activate caspase-3, further confirming that ExoT-induced cell death is apoptotic in nature.
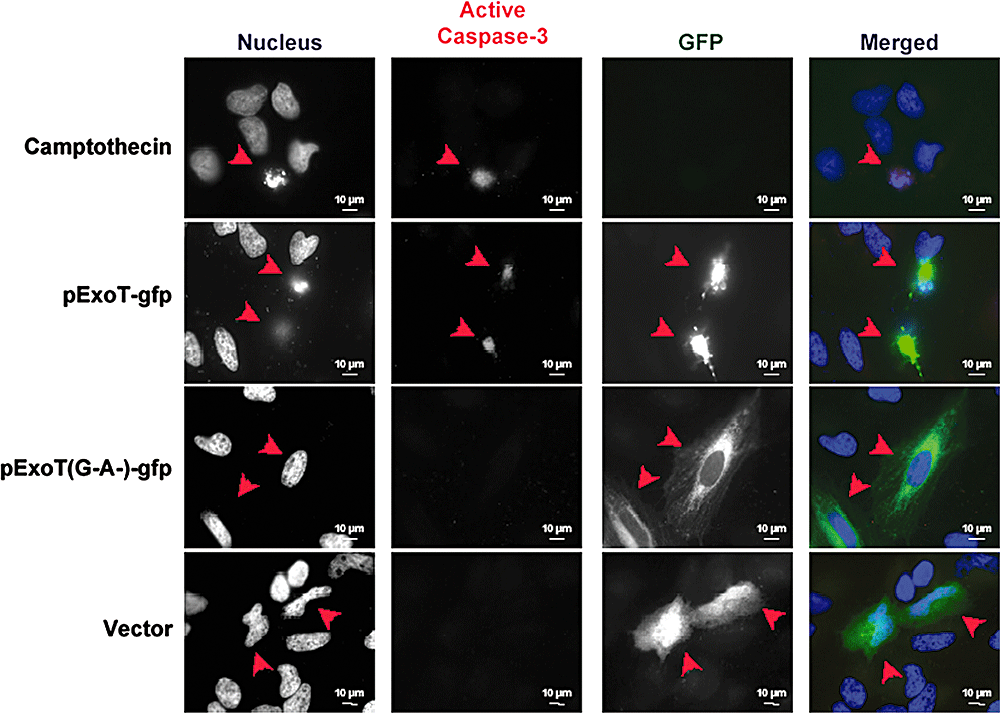
Transient transfection of ExoT is sufficient to activate caspase-3 in HeLa cells. HeLa cells, transiently transfected with the indicated constructs fused to GFP, were fixed and stained with DAPI (nucleus, blue) and an antibody to activated caspase-3 (red). Representative ExoT-transfected cells are indicated with an arrow. ExoT-transfected cells exhibit caspase-3 activation, whereas vector-transfected or ExoT(G−A−) cells do not. Camptothecin-treated cells serve as a positive control.
ExoT-mediated apoptosis is primarily mediated by its ADPRT activity
To determine the contribution of the GAP and the ADPRT activities in ExoT-mediated cell death, we examined the ability of isogenic mutants of PA103ΔU defective in either GAP or ADPRT activity to induce cell death as assessed by IF time-lapse microscopy in the presence of PI. As indicated in Fig. 7A, infection with P. aeruginosa expressing a functional ADPRT domain, ΔU/T(G−A+), resulted in nearly 100% cell death by 10 h post infection. Similar to infection with an ExoT-producing strain, PA103ΔU (see Fig. 1A), cell death was blocked by ZVAD (Fig. 7B). The GAP-expressing strain PA103ΔU/T(G+A−) also induced cell death, but the kinetics were slower (Fig. 7A) and it was only partially rescued by ZVAD. These data suggested that the ADPRT activity was likely responsible for the majority of ExoT-mediated cytotoxicity and that it was also capable of blocking the T3SS-dependent cell death that is observed in the presence of ZVAD. Our results also suggested either (i) that the GAP activity did not induce cell death but partially inhibited the T3SS-mediated necrotic cell death or (ii) that the GAP activity was able to induce apoptotic cell death but only partially prevented the T3SS-induced necrosis.
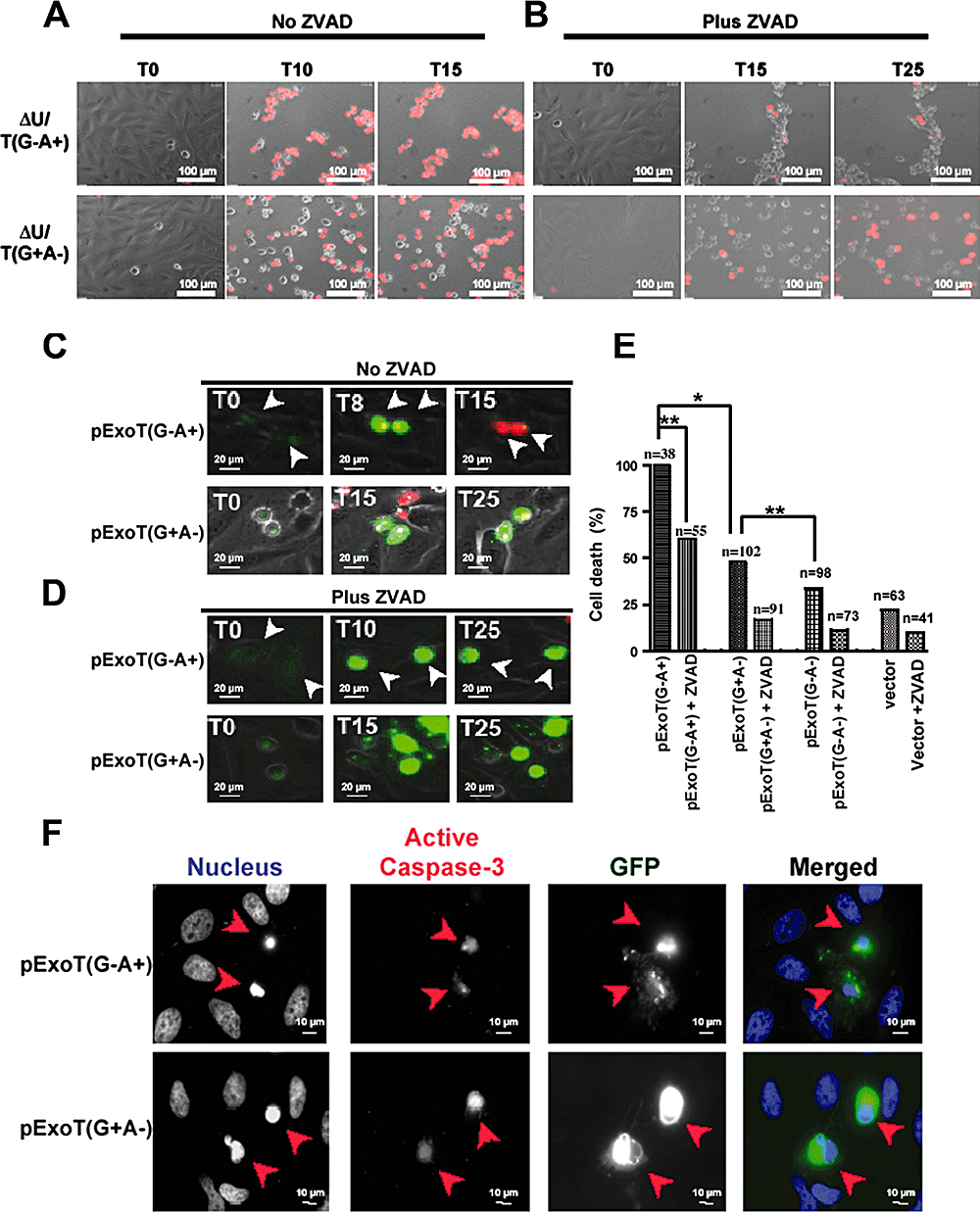
ExoT induction of cell death is primarily due to its ADPRT activity.A and B. HeLa cells were infected in the absence (A) or presence (B) of ZVAD (60 μM) with PA103ΔU/T(G−A+) or PA103ΔU/T(G+A−) at an moi of ca. 10 for the indicated time. Host cell death was assessed by uptake of PI (red) by simultaneous time-lapse phase and fluorescent videomicroscopy. Note that while infection with PA103ΔU/T(G−A+) results in rapid, ZVAD-sensitive cell death, in the presence of PA103ΔU/T(G+A−) cell death is delayed and is partially ZVAD-sensitive.C–E. HeLa cells were transiently transfected in the absence (C) or presence (D) of ZVAD with a mammalian expression vector expressing either ExoT(G+A−) or ExoT(G−A+) fused to GFP at the C-termini. Cell death was analysed by time-lapse videomicroscopy in the presence of PI. Video images were captured every 15 min. The arrows show representative transfected cells. The tabulated results, collected from multiple movies, are shown in (E). The numbers above each column in (E) indicates the total number of transfected cells that were scored (*P < 0.001; **P < 0.01, χ2 test).F. Nuclear condensation or caspase-3 activation of pExoT(G−A+)- or pExoT(G+A−)-transfected cells are assessed by IF microscopy at 1000× magnification. Note that expression of mutant GAP but wild-type ADPRT, ExoT(G−A+), results in nearly 100% cell death whereas the ExoT(G+A−) with wild-type GAP and mutant ADPRT induces only 48% cell death. ZVAD significantly inhibits both the GAP- and the ADPRT-induced cell death. Also worth noting is that both GAP and ADPRT expression results in nuclear condensation and caspase-3 activation.
To distinguish between these possibilities and to further assess the involvement of the ADPRT and GAP in ExoT cytotoxicity, we transfected HeLa cells with expression vectors harbouring either a functional GAP domain, pExoT(G+A−), or a functional ADPRT domain, pExoT(G−A+), C-terminally fused to GFP. Cell death was assessed by IF time-lapse microscopy in the presence of PI. As shown in Fig. 7 and Movie S9, 100% of HeLa cells transfected with pExoT(G−A+) took up PI and succumbed to death with a MTD = 8.6 + 0.77 (n = 32) in a manner that mirrored ExoT both phenotypically and kinetically (Fig. 4 and Movie S4). pExoT(G+A−) expression also caused cell death but only in 48% of transfected cells (n = 102) (Fig. 7B and Movie S10) and with a significantly longer MTD (16.5 ± 3.3 h, P < 0.005). The GAP-induced cell death was about 15% higher than pExoT(G−A−) double mutant (P < 0.05). The cell death of either the transfected ADPRT-active or GAP-active ExoT was apoptotic in nature, as they were substantially blocked by ZVAD (Fig. 7D and E, Movies S11 and S12), resulted in characteristic nuclear condensation (Fig. 7F, nucleus panel) and led to caspase-3 activation (Fig. 7F). Collectively, these data suggest that both the GAP and ADPRT domain contribute to ExoT-mediated cell death, although the ADPRT domain is more potent and induces death more rapidly in a manner that phenocopies ExoT.
Discussion
Our previous studies demonstrated that PA103, an ExoU- and ExoT-producing strain, induces apoptotic-like death in a T3SS-dependent manner that does not require ExoU (Hauser and Engel, 1999). We now demonstrate that intoxication by ExoT can account for this apoptotic death. Using various approaches, we show that in HeLa cells: (i) ExoT is necessary and sufficient to cause apoptosis, (ii) both the GAP and the ADPRT activities can contribute to ExoT cytotoxicity, (iii) the T3SS can also cause cell death which is primarily necrotic in nature and (iv) ExoT effectively prevents the T3SS-induced cell death.
Specifically, we find that infection of cells with PA103ΔU leads to membrane damage and uptake of PI, nuclear blebbing and formation of apoptotic bodies, loss of the mitochondrial membrane potential and activation of caspase-3. Likewise, transfection of HeLa cells with ExoT, the ADPRT, and to a lesser extent the GAP, leads to PI uptake, changes in nuclear morphology consistent with apoptosis and activation of caspase-3. All of these outcomes can be inhibited by ZVAD, a broad spectrum pan-caspase inhibitor, supporting the notion that ExoT-mediated cell death occurs primarily through apoptosis.
Previous studies employing PAK, a strain that encodes ExoY, ExoS and ExoT, did not show a role for ExoT in host cell cytotoxicity at early times, 2–5 h post infection (Lee et al., 2005). In fact, this study demonstrated that ExoT protects against early cytotoxicity associated with ExoS and with the T3SS. Moreover, Kaufman et al. (2000) reported that early during infection (2–5 h), PAK induces apoptosis in an ExoS-dependent manner and that PA103 strain causes necrotic cell death in its host. These apparent discrepancies could be due to the genetic differences between PAK and PA103 strains, host cell types, moi, or to differences in the methods used to detect cell death. Under our experimental conditions, cell death induced by ExoT appears to proceed with slower kinetics compared with ExoS, occurring at times later than 5 h post infection.
Our data indicate that each domain of ExoT contributes to host cell death, although the ADPRT activity appears to predominate under the conditions of our experiments. The ADPRT function of ExoT, whether bacterially translocated or delivered into host cells by transient transfection, resulted in apoptotic cell death, as evidenced by ZVAD-sensitive PI uptake, characteristic changes in nuclear morphology and activation of caspase-3. The GAP activity of ExoT was also sufficient to cause cell death, though with slower kinetics and less potency. It is possible that the GAP and ADPRT domains may target different apoptotic pathways or that the GAP activity is not as efficient as the ADPRT domain in triggering apoptosis. More studies are needed to address these questions.
ExoS is a highly homologous T3SS protein produced by ∼75% of strains that also encode ExoT (Feltman et al., 2001). Like ExoT, ExoS-induced cell death is also mediated by its ADPRT activity (Kaufman et al., 2000). However, the observation that ExoS-mediated early cell death is substantially reduced in the presence of ExoT (Lee et al., 2005) and the fact that ExoS and ExoT ADP ribosylate different, non-overlapping substrates (Sun et al., 2004) suggest that these toxins likely use different mechanisms to induce apoptosis. We speculate that the ADPRT activity of ExoT, by interfering with host Crk function, may at least in part account for the apoptotic activity of ExoT. Consistent with this notion, interference with Crk/p130Cas interaction and cell migration has been shown to lead to apoptosis (Cho and Klemke, 2000). Our preliminary data support this notion as they indicate that expression of dominant negative alleles of Crk in HeLa cells results in apoptosis, phenocopying the ExoT-mediated apoptotic cell death (our unpublished data). Experiments to test this hypothesis and further probe the role of the ADPRT and GAP and the mechanism(s) of ExoT-dependent cell death are underway.
Induction of apoptosis is a new and distinct virulence function of ExoT. The fact that ZVAD effectively blocks ExoT cytotoxicity without affecting its ability to cause cell rounding (Fig. 1B and Movie S4) or to inhibit cytokinesis (Shafikhani and Engel, 2006) suggests that these ExoT-associated virulence functions are distinct and can be uncoupled.
Our studies further reveal that PA103ΔUΔT can also cause necrotic cell death in a T3SS-dependent manner, adding to a growing body of data that suggest that insertion of the T3SS apparatus of Gram-negative pathogens can result in pore formation and subsequent cell death (Viboud and Bliska, 2001; Stockbauer et al., 2003; Lee et al., 2005). Interestingly, the T3SS-induced necrosis is effectively blocked by ExoT. In Yersinia pseudotuberculosis, a number of virulence factors including YopE, an orthologue of the GAP domain of ExoT, have also been shown to inhibit the T3SS-induced pore-forming activity and cytotoxicity (Viboud and Bliska, 2001). Our data indicate that both the GAP and the ADPRT activities can contribute to ExoT inhibition of T3SS-induced necrosis, although the ADPRT appears more potent in this regard as well. Whether they target the same or different pathways to suppress T3SS-induced necrosis remains to be determined. In either case, these data suggest that Gram-negative pathogens may have evolved multiple strategies to prevent T3SS-associated cytotoxicity.
Intriguingly, PA103ΔUΔT does induce caspase-3 activation, albeit substantially less than the ExoT-expressing PA103ΔU strain. ZVAD however only protects against less than 20% of the T3SS-induced cell death, leading us to conclude that apoptosis may not significantly contribute to the T3SS-induced cell death under these experimental conditions. What triggers this ExoT-independent but T3SS-dependent caspase-3 activation remains unknown. It is tempting to postulate that caspase-3 activation may occur as an indirect consequence of caspase-1 activation in response to insertion of T3SS. Consistent with this hypothesis, it has been recently postulated that the T3SS can act as a conduit to deliver the pathogen-associated molecular pattern molecules, such as LPS, into the host cytosol where they are recognized by the NALP3 inflammasome, culminating in activation of caspase-1, the main caspase required for processing of pro-IL1β and induction of pro-inflammatory response (Kanneganti et al., 2007). Moreover, caspase-8 and caspase-9, the closest homologues of caspase-1 (Mariathasan and Monack, 2007), are known activators of caspase-3 (Philchenkov, 2004), suggesting that activated caspase-1 could potentially activate caspase-3. More studies are needed to test this hypothesis and to determine the mechanism that underlies the T3SS-induction of caspase-3 in the absence of ExoT.
Inhibition of wound healing is paramount to the success of this opportunistic pathogen especially in the settings of acute infections. P. aeruginosa has evolved multiple strategies to inhibit the repair of injured epithelium and ExoT appears to play a fundamental role in these virulence strategies. ExoT inhibits cell migration by altering actin cytoskeleton (Geiser et al., 2001; Barbieri and Sun, 2004; Garrity-Ryan et al., 2004); it blocks cell division by inhibiting cytokinesis (Shafikhani and Engel, 2006); and it induces cell death, a new virulence function as demonstrated in this report, while effectively preventing the T3SS-associated necrotic cell death, an event that could potentially lead to the induction of pro-inflammatory response.
Experimental procedures
Reagents and supplies
Propidium iodide (Sigma; St Louis, MO) was used at 7 μg ml−1. ZVAD (60 μM final concentration), from R&D Systems (Minneapolis, MN), was added at least 1 h prior to bacteria. Camptothecin (4 μg ml−1; Sigma; St Louis, MO) was added for at least 10 h.
Bacterial strains and media
All strains and plasmids are shown in Table 1. Except where noted, P. aeruginosa strains used in these studies were grown in LB without shaking at 37°C overnight. Bacterial counts were determined by optical density and confirmed by plating serial dilutions for colony counts.
Growth and infection of epithelial cell lines
In all experiments, wells or coverslips were pre-coated with poly l-Lysine (Sigma; St Louis, MO) for 5 min at room temperature, followed by human fibronectin (Chemicon International; Temecula, CA) at 40 μg ml−1 for at least 30 min prior to cell seeding. HeLa epithelial cells were grown and maintained in minimal essential Eagle's medium (MEM) supplemented with 10% heat-inactivated FBS (Gibco BRL), l-glutamine (0.292 g l−1), glucose (1.0 g l−1) and NaHCO3 (2.2 g l−1), incubated at 37°C in the presence of 5% CO2, as described previously (Garrity-Ryan et al., 2004). Infections were performed at an moi of ca. 10. Bacteria were either present throughout the experiment (Fig. 1) or removed 5 h post infection (2-5), at which time medium containing amikacin was added to kill remaining bacteria.
Cell death analysis by videomicroscopy
Time-lapse videomicroscopy was performed as described (Shafikhani and Engel, 2006) with the following modifications. For experiments involving IF time-lapse videomicroscopy, HeLa cells were grown in medium lacking phenol red for 3 days. After trypsin digestion, 1 × 105 HeLa cells per well were seeded and incubated overnight in the same medium without phenol red. PI, at 7 μg ml−1, was added prior to filming and kept throughout.
Transient transfection
Transient transfection studies were performed as described (Shafikhani and Engel, 2006). Briefly, HeLa cells were seeded at 2 × 104 per well in a 24-well dish and transfected by Effectene (Qiagen; Valencia, CA), using the vendor's protocol.
IF microscopy
Fifteen to 24 h post transfection, the attached cells were fixed with 10% Trichloroacetic acid (Fisher Scientific; Fair Lawn, NJ) for 10 min at room temperature, washed three times with PBS containing glycine (30 mM), permeabilized with PBS + 0.2% Triton X-100 for 15 min at 37°C, and blocked with PBS + 0.7% fish scale gelatin for 1 h at 37°C. Images were captured at a magnification of 1000× under oil immersion with a Nikon Eclipse TE 2000-E fluorescence microscope, using a CCD camera and Simple PCI imaging software.
Analysis of mitochondrial/cytochrome c-mediated cell death
Involvement of mitochondrial apoptotic pathway in ExoT-mediated cell death was determined by IF microscopy, using MitoCapture™ Mitochondrial Detection Kit (BioVision; Mountain View, CA). At 5 h post infection, live HeLa cells were stained with MitoCapture stain and imaged without fixation by IF microscopy, using the vendor's protocol.
Analysis of caspase-3 activation
Caspase-3 activation was measured by either flow cytometry or IF imaging, using APO ACTIVE 3TM (Cell Technology; Mountain View, CA) and vendor's protocol. For flow cytometry experiments, 4 × 105 HeLa cells per well were seeded in a six-well dish. Bacteria were removed 5 h post infection and fresh media containing antibiotics were added to kill the remaining bacteria. After another 15 h, cells were harvested, stained and fixed, using vendor's protocol. For IF imaging, HeLa cells were transfected as described above. Fifteen to 20 h post transfection, transfected cells were fixed and stained for active caspase-3.
Statistical analysis
The statistical analyses (two-tailed P-value ± SEM) were based on the Mann–Whitney test from either six random fields (Fig. 3B) or flow cytometry experiments performed in triplicates (Fig. 2C), using GraphPad Instat 3.0 software. For 4-6, web Chi-square calculator (http://www.graphpad.com/quickcalcs/chisquared1.cfm) was used to determine the pair-wise statistical significance by χ2 test. P≤ 0.05 was taken as significant.
Acknowledgements
We thank Dr Andy Finch and the Mt. Zion Cancer centre for technical assistance on videomicroscopy. We also like to thank members of Engel lab for their advice and suggestions. This work was supported by the NIH Grants F32 AI054056 (to S.H.S.), R01 AI053194 and R01 AI42806 (to J.E.).




