Cryptococcus neoformans capsular polysaccharide component galactoxylomannan induces apoptosis of human T-cells through activation of caspase-8
Summary
The major virulence factor of Cryptococcus neoformans is its polysaccharide capsule composed of glucuronoxylomannan (GXM), galactoxylomannan (GalXM) and mannoproteins. A variety of immunomodulating activities have been described for GXM and mannoproteins but little is known about possible interactions of GalXM with the immune system. In the present article, we investigate the effect of purified soluble GalXM on human T lymphocytes. The results indicate that, GalXM (i) can affect selected immune responses; (ii) causes significant impairment of T cell proliferation and increases interferon-γ and interleukin-10 production; and (iii) induces apoptosis of T lymphocytes through activation of caspase-8 that terminates with fragmentation of DNA. These results are the first to suggest a role for GalXM in C. neoformans virulence by demonstrating that it can target human T cells, and that it may impair the development of an effective specific T cell response.
Introduction
Cryptococcus neoformans is a fungus relatively frequently responsible for meningoencephalitis in immunocompromised hosts (Vecchiarelli, 2000; Perfect and Casadevall, 2002). The major virulence factor of C. neoformans is its polysaccharide capsule which interferes with immune function and allows survival in the mammalian host. The capsular polysaccharide is composed of glucuronoxylomannan (GXM) (Vecchiarelli, 2000) and two other minor components, galactoxylomannan (GalXM) and mannoproteins (Turner et al., 1984; Pietrella et al., 2004). Previously we have described a variety of immunosuppressive effects triggered by purified GXM (Monari et al., 2005a). We have recently observed that GXM is able to induce FasL in antigen-presenting cells such as monocytes/macrophages, resulting in apoptosis of T cells expressing Fas. This effect was also observed in T cells in the presence of related or unrelated stimuli such as phitoemoagglutinin (PHA), and in Jurkat cells that constitutively express Fas (Monari et al., 2005b).
The immune system uses apoptosis as a mechanism to eliminate immune effector cells that have aged or threaten immune homeostasis. Apoptosis of T cells might be a central feature in the induction of tolerance in the setting of allogeneic transplantation (Li et al., 2000; Chiffoleau et al., 2003). It has long been recognized that T cell deletion is an important mechanism for inducing immune tolerance (Li et al., 2000; Chiffoleau et al., 2003). Thymic tolerance to self-antigens is primarily mediated through apoptosis of autoreactive T cells. This mechanism is extremely efficient, and participates in the development of healthy T cell pool outside the spleen and thymus. Moreover, thymic tolerance is the process by which potentially autoreactive T cells are removed through negative selection (Sykes, 1996). In addition, some potentially autoreactive thymocytes, can exit the thymus autoimmunity but autoimmunity is prevented by peripheral deletion mediated through death receptor-mediated apoptosis and death induced by cytokine withdrawal (Chiffoleau et al., 2003; Herndon et al., 2005).
Galactoxylomannan (GalXM) is a minor component of capsular material of C. neoformans and there is little or no information on its immunoregulatory properties. In particular, TNF-α inductions by peripheral blood mononuclear cells have been reported (Chaka et al., 1997). In the present article, we investigate the effect of purified soluble GalXM on T cells and describe a new suppressive effect on T cell proliferation by a C. neoformans product that is, at least in part, mediated by induction of apoptosis signalling.
Results
Proliferation of PBMC and cytokine production
We evaluated the influence of GalXM on the proliferation of peripheral blood mononuclear cells (PBMC) stimulated with PHA, mAb to CD3 or heat-inactivated yeasts such as C. neoformans or C. albicans. Different doses of GalXM were added to cell culture at the same time as the proliferative stimuli and the blastogenic response was measured after 5 days of culture. GalXM-treated cells manifested a significant reduction in cell proliferation relative to control cells (Fig. 1). The inhibitory effect of GalXM was dose-dependent and was evident at doses as low as 1 µg ml−1. It is noteworthy that the suppressive effect exerted by GalXM was approximately 50-fold greater than that observed with GXM on a mass per volume basis (Fig. 1). It should be noted that stimulation with heat-inactivated C. neoformans could conceivably result in further addition of GalXM present in its fungal capsule. However, we believe that any GalXM from dead organisms would be an extremely small amount relative to the amount of purified GalXM added, as it is a minor component of the capsule and dead cells would not be secreting soluble polysaccharides.
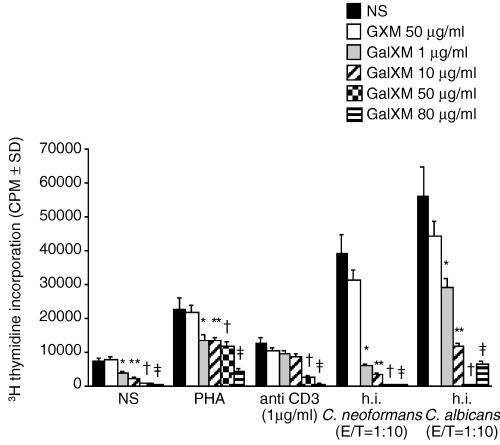
Proliferative response of human PBMC to PHA, mAb to CD3, heat-inactivated C. neoformans or C. albicans in the presence or absence of different doses of GalXM. PBMC (1 × 106 ml−1) were incubated alone or with 5 ng ml−1 PHA or 1 µg ml−1 mouse anti-human CD3 mAb, or heat-inactivated (h.i.) C. neoformans or C. albicans cells in the presence or absence of various doses of GalXM (1, 10, 50, 80 µg ml−1) or 50 µg ml−1 GXM. Proliferation was measured by [3H] thymidine incorporation. *P < 0.01 GalXM (1 µg ml−1)-treated versus GalXM-untreated cells. **P < 0.01 GalXM (10 µg ml−1)-treated versus GalXM-untreated cells. †P < 0.01 GalXM (50 µg ml−1)-treated versus GalXM-untreated cells. ‡P < 0.01 GalXM (80 µg ml−1)-treated versus GalXM-untreated cells.
Given the drastic decrease of proliferation observed in the presence of GalXM, we hypothesized the occurrence of an altered cytokine production by these cells. To exploit this issue, experiments were performed, using PBMC treated with or without lipopolysaccaride (LPS) (10 µg ml−1) and heat-inactivated yeast cells (E/T 1:10) alone or in combination with different doses of GalXM. The results show increased levels of interferon (IFN)-γ and IL-10 in supernatants of cells cocultured with GalXM compared to untreated cells (Fig. 2). In particular, the maximum IFN-γ production was observed in the presence of 10 µg ml−1 GalXM. This latter dose was also optimal in conferring a positive regulation of IL-10 production under the same experimental conditions. A concentration of 1 µg ml−1 GalXM induced a significant increase of IFN-γ but was insufficient for regulating IL-10 secretion.
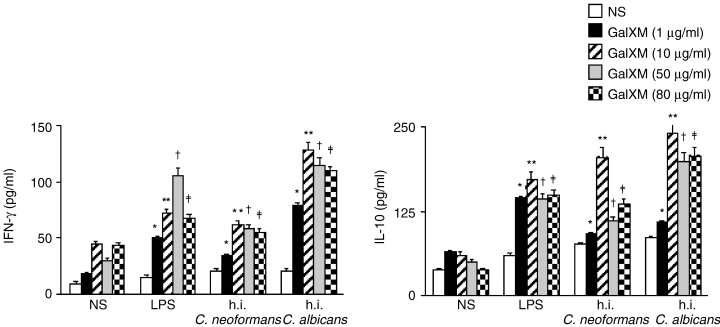
IFN-γ and IL-10 secretion by PBMC in response to LPS or heat-inactivated C. neoformans or C. albicans in the presence or absence of different doses of GalXM. PBMC (1 × 106 ml−1) were incubated overnight in complete medium alone or with LPS (10 µg ml−1), heat-inactivated (h.i.) C. neoformans and C. albicans cells (E/T 1:10) in the presence or absence of various doses of GalXM (1, 10, 50, 80 µg ml−1). After incubation, supernatants were collected and the levels of IL-10 and IFN-γ were determined as described in Experimental procedures. *P < 0.01 GalXM (1 µg ml−1)-treated versus GalXM-untreated cells. **P < 0.01 GalXM (10 µg ml−1)-treated versus GalXM-untreated cells. †P < 0.01 GalXM (50 µg ml−1)-treated versus GalXM-untreated cells. ‡P < 0.01 GalXM (80 µg ml−1)-treated versus GalXM-untreated cells.
Proliferation of purified human CD3 T lymphocytes
To ascertain whether the inhibition of proliferation was due to the GalXM immunosuppressive effect on purified human T cells, purified CD3 T lymphocytes were cultured in presence of blastogenic stimuli such as interleukin (IL)-2, mAb to CD3, Concanavalin A (ConA) and PHA, in presence or absence of 1 or 10 µg ml−1 GalXM or 50 µg ml−1 GXM. A dramatic decrease of cell proliferation was observed when CD3+ lymphocytes were cultured with mAb to CD3, PHA or ConA in the presence of either 1 or 10 µg ml−1 GalXM. When CD3+ lymphocytes were cultured with IL-2, a significant decrease in proliferation was observed only at 10 µg ml−1 GalXM. This evidences a direct effect of GalXM on human T cells and the possibility that GalXM binds to T cell surface molecules.
The treatment of T cells with GXM did not significantly influence their blastogenic response (Fig. 3).
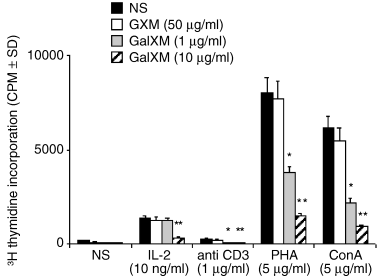
Proliferative response of purified human CD3+ T lymphocytes to IL-2, mAb to CD3, PHA, ConA in the presence or absence of different doses of GalXM. CD3+ T lymphocytes (5 × 105 ml−1) were incubated alone or with 10 ng ml−1 IL-2 or 5 µg ml−1 PHA or 1 µg ml−1 mAb to CD3 or 5 µg ml−1 ConA in the presence or absence of GalXM (1 or 10 µg ml−1) or 50 µg ml−1 GXM. Proliferation was measured by [3H] thymidine incorporation. *P < 0.01 GalXM (1 µg ml−1)-treated versus GalXM-untreated cells. **P < 0.01 GalXM (10 µg ml−1)-treated versus GalXM-untreated cells.
Apoptosis of purified human CD3 T lymphocytes and Jurkat cells
The strong reduction of T cell proliferation mediated by GalXM prompted us to investigate a possible role for apoptosis in the above mentioned inhibitory effect. For this purpose we tested the percentage of apoptotic CD3 T lymphocytes after 18 h of incubation with 10 µg ml−1 GalXM in the presence of different stimuli including IL-2, PHA, ConA and mAb to CD3. A similar experimental design was used for Jurkat cells that are routinely used for apoptosis studies, given their constitutive Fas expression (Juo et al., 1999; Liu et al., 1999). As shown in Fig. 4A, a significant increase in the percentage of apoptotic CD3 T lymphocyte cells was observed after 18 h of incubation with indicated stimuli. When Jurkat cells were used to measure GalXM-induced apoptosis, an increase of constitutive apoptosis was observed irrespective of stimuli such as IL-2, PHA, ConA and mAb to CD3 (Fig. 4B). The percentage of apoptotic cells was significantly increased after 18 h of incubation.
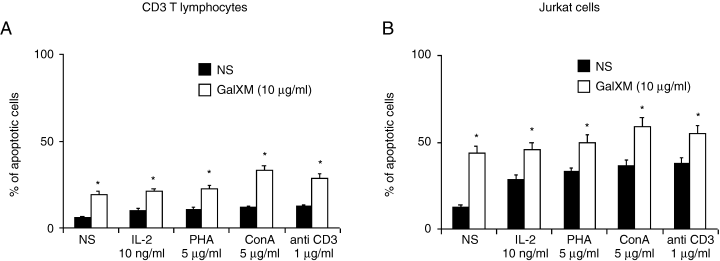
Apoptosis of purified human CD3+ T lymphocytes and Jurkat cells induced by GalXM. Purified human CD3+ T lymphocytes (5 × 105 ml−1) (A) or Jurkat cells (B), preactivated or not with 10 ng ml−1 IL-2 or 5 µg ml−1 PHA or 5 µg ml−1 ConA or 1 µg ml−1 mAb to CD3 were incubated for 18 h in complete medium at 37°C and 5% CO2 alone or in the presence of 10 µg ml−1 GalXM. *P < 0.01 GalXM-treated versus untreated cells.
Expression of Fas/FasL on purified human CD3 T lymphocytes and Jurkat cells
There is compelling evidence that surface molecules Fas/FasL are involved in the induction of apoptosis and that their reciprocal interaction inevitably leads to programmed cell death (Griffith et al., 1995). Thus the surface expression of Fas/FasL on CD3 T lymphocytes and Jurkat cells after GalXM addition was evaluated. The results showed a dramatic increase of Fas/FasL-positive cells in GalXM treated CD3 T lymphocytes and Jurkat cells (Fig. 5). Overall, the results indicate (i) upregulation of Fas/FasL in CD3 T lymphocytes unstimulated or stimulated with ConA; (ii) similar increase in expression of Fas and FasL in Jurkat; (iii) greater increase for Fas compared to FasL in CD3 T lymphocytes.
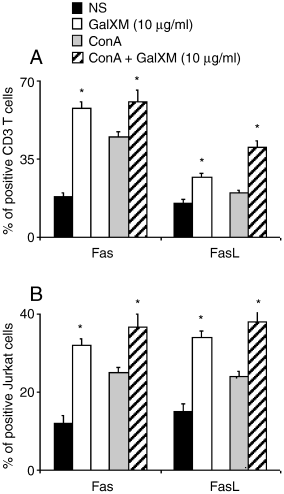
Fas and FasL expression on purified human CD3+ T lymphocytes or Jurkat cells. Purified human CD3+ T lymphocytes (5 × 105 ml−1) or Jurkat cells (5 × 105 ml−1) were incubated alone or with 5 µg ml−1 ConA in the presence or absence of GalXM (10 µg ml−1) for 18 h. Fas and FasL expression was evaluated by cytofluorimetric analysis as described in Experimental procedures. *P < 0.01 GalXM-treated versus untreated cells.
DNA fragmentation and activation of caspase-8
Fas/FasL are considered death receptors and natural triggers of extrinsic apoptosis pathways. The pathway is initiated by recruitment and activation of caspase-8, an initiator caspase, in the death-inducing signalling complex (Kennedy et al., 1999). Conversely the intrinsic apoptosis pathway starts from within the cell either by direct activation of caspases or through intracellular changes such as DNA damage resulting in the release of pro-apoptotic factors from the intermembrane space of the mitochondria. The release of these factors results in the activation of another initiator caspase, caspase-9 (Hakem et al., 1998). Cells that die through CD95 (Fas) without help from mitochondria are called Type I cells, whereas cells in which CD95-mediated death relies mostly on the intrinsic pathway are called Type II cells. To verify the involvement of caspase-8 in the induction of GalXM-mediated T cell apoptosis, we performed experiments in purified CD3 T lymphocytes and Jurkat cells. The results show a clear activation of caspase-8 (Fig. 6, upper panels) manifested in CD3 T lymphocytes and in Jurkat cells incubated for 18 h with GalXM, stimulated or unstimulated with ConA.
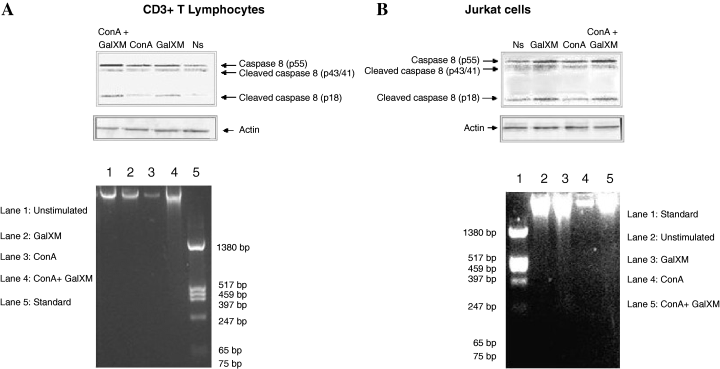
GalXM-induced apoptosis results in cleavage of caspase-8 of CD3+ T lymphocytes (Fig. 6A, upper panel) and Jurkat cells (Fig. 6B, upper panel). CD3+ T lymphocytes and Jurkat cells (5 × 106) were incubated in the presence or absence of GalXM (10 µg ml−1) and ConA (5 µg ml−1) in RPMI plus 10% FCS for 18 h at 37°C with 5% CO2. After incubation, cell extracts were analysed for the presence of the cleavage forms of caspase-8 (p43/41 and p18) by immunoblotting as described in Experimental procedures. An anti-actin antibody was added as a loading control. DNA fragmentation in CD3+ T lymphocytes (Fig. 6A, lower panel) and Jurkat cells (Fig. 6B, lower panel). Genomic DNA was obtained from 4 × 106 CD3+ T lymphocytes and Jurkat cells incubated for 18 h in RPMI 1640 plus 10% FCS at 37°C and 5% CO2 alone or in the presence of GalXM (10 µg ml−1) and ConA (5 µg ml−1) and analysed using 1% agarose gel electrophoresis followed by staining with ethidium bromide as described in Experimental procedures.
In a subsequent experiment we investigated DNA-fragmentation of CD3 T lymphocytes and Jurkat cells after 18 h of in vitro culture in the presence or absence of GalXM and ConA. The results showed that CD3 T lymphocytes stimulated with ConA and GalXM manifested a more pronounced DNA fragmentation with respect to ConA-treated cells (Fig. 6A, lower panel, lane 4 versus 3). Consistent with these results, DNA fragmentation in Jurkat cells exposed to GalXM resulted more evident with respect to GalXM-untreated cells (Fig. 6B, lower panel, lane 3 versus 2 and lane 5 versus 4 respectively).
Discussion
The role of T cell activation and T cell apoptosis on the acquisition of immune tolerance and the development of active immune regulation is very complex. Compelling evidence shows that GXM, a major capsular component of C. neoformans, is able to induce a myriad of suppressive effects on innate and adaptive immunity that culminate in induction of apoptosis (Monari et al., 2005b). In this article, we investigated the contribution of another capsular component of C. neoformans, GalXM, in influencing the immune response to C. neoformans or to unrelated stimuli. Here we describe for the first time a variety of effects induced by GalXM on purified human T cells that could have significant consequences for immunosuppression and characterize mechanisms involved in this phenomenon. In particular we demonstrate that GalXM: (i) strongly inhibits PBMC activation that coincides with significant increase of IFN-γ and IL-10; (ii) affects the functions of purified human T cells; (iii) promotes suppression of T cell proliferation at low dose such as 10 µg ml−1; (iv) upregulates Fas/FasL; and (v) initiates the apoptosis pathway by recruitment and activation of caspase-8 leading to DNA fragmentation. Overall these results indicate that GalXM is a potent immunomodulator.
GalXM has generally been considered a minor component of C. neoformans capsular material and there are only a few reports on its immunoregulatory activity. GalXM was reported to induce TNF-α production by PBMC (Delfino et al., 1996; Chaka et al., 1997) and to bind to human neutrophils via CD18 (Dong and Murphy, 1997). Biochemical studies reveal that GalXM and GXM are structurally different (Vaishnav et al., 1998). In this report we describe a new role for GalXM as an inducer of T cell apoptosis. It is noteworthy that the inhibition of proliferation observed in PBMC was reproduced by using purified CD3 T lymphocytes, thus implying a direct interaction of GalXM with T cells, thereby controlling Fas/FasL upregulation and ultimately cell death.
Two distinct apoptotic pathways have been proposed for Fas signalling. Type I pathway involves the efficient recruitment of caspase-8, which then acts directly on downstream caspases such as caspase-3 that are responsible for apoptosis execution. Type II pathway begins from within the cell with subsequent release of various apoptotic factors from the mitochondrial membrane leading to activation of caspase-9 (Barnhart et al., 2003). As GalXM induces death receptors such as Fas and subsequent activation of caspase-8, it is most likely that the type I apoptotic pathway is involved through the cleavage of ‘death substrates’. Our results suggest the possibility of a putative receptor for GalXM on T cells that is involved in triggering apoptotic signals. We recently described that GXM, the major capsular component of C. neoformans, is able to induce apoptosis. Indeed we observed profound differences between GXM- and GalXM-induced T cell apoptosis. Unlike GalXM, GXM appears unable to directly interact with T cells, and consequently, the GXM-mediated T cell apoptosis involves the induction of FasL on antigen-presenting cells such as monocytes/macrophages (Monari et al., 2005b). Moreover, Fas induction capability is a property of GalXM and not of GXM. Hence, the two capsular polysaccharides appear to have fundamentally different mechanisms of action in interfering with the cellular-immune system. Finally, the dose of GalXM used to produce a comparable effect is fivefold lower than that of GXM. As the molecular weight of GalXM is approximately 10-fold lower than that of GXM, this implies that GalXM and GXM have comparable pro-apoptotic properties.
Galectins are a family of carbohydrate-binding proteins found in various organisms including mammals and fungi (Cooper and Barondes, 1999; Muller, 2001). Galectins have been subdivided into prototypes (galactin 1, 3, 7, 8, 9 and 12) consisting of one carbohydrate recognition domain (Liu, 2000).
T cell glycoproteins including CD2, CD3, CD4, CD7, CD43 and CD45 function as counter-receptors. Given that they recognize galactose-containing oligosaccharides, the direct interaction of GalXM with T cells strongly suggests an involvement of galectin-like binding on T cells. However, a specific subset of T cell glycoproteins such as CD7 is essential to induce cell death. Indeed CD7 is essential for signalling galectin-1-mediated T cell death (Pace et al., 1999; 2000; Walzel et al., 2000) and could possibly participate in the observed induction of apoptosis.
Galactoxylomannan promotes apoptosis in Jurkat cells and CD3 T lymphocytes through Fas/FasL interaction, which is considered an extrinsic apoptosis pathway. It should also be mentioned that, in contrast to GXM, GalXM induces a strong upregulation of Fas, considered a death domain-containing receptor (Barnhart et al., 2003).
Remarkably, the overexpression of Fas is strongly induced in unstimulated CD3 T lymphocytes and Jurkat cells. This suggests that GalXM may function to arrest the initial growth process of cells by directly inducing Fas on CD3 T lymphocytes.
Galactoxylomannan (GalXM) or a GalXM-like compound could conceivably be useful to: (i) induce apoptosis in cancer cells, as suggested by the GalXM-induced apoptotic signal directly on Jurkat cells; or (ii) abrogate the deleterious response of T cells (GVH) in transplant recipients. GalXM-mediated effects include a cascade activation of caspase-8 that in turn leads to activation of caspase-3 and fragmentation of DNA. However, we cannot rule out the activation of other apoptotic pathways by GalXM in T lymphocytes or other cells. In addition, GalXM-mediated cell death could reinforce the suppressive effect of GXM and contribute to the suppression of T cell response during cryptococcosis.
By induction of T cell apoptosis, GalXM could restrict T cell growth in a general context. This hypothesis is supported by the induction of apoptosis in T cell stimulated with IL-2 or mitogenic compounds such as PHA, ConA or mAb to CD3. Several factors including cytokines such as IFN-γ and IL-10 could participate in the process and their release in supernatants of PBMC could be involved in the induction of cell death. This is consistent with the activation of pro-apoptotic genes, and proteins including caspases attributed to IFN-γ (Lakhani and Flavell, 2003). It is moreover conformable to the involvement of IL-10 in termination of an immune response, through upregulation of FasL (Barreiro et al., 2004), and in tolerance maintaining (Li et al., 2000; Hara et al., 2001).
The first encounter of a T cell with an antigen determines the outcome of a subsequent response. If antigen is first perceived in tolerogenic form, ‘no response’ will ensue. If apoptotic self-antigen is perceived in the presence of foreign antigen, subsequent exposure to the antigen will be controlled. Possibly it is the default pathway, i.e. the presence of apoptotic self leads to regulation, not to immunity. Moreover, it is conceivable that apoptosis of activated T cells not only reduces the mass of potentially cytopathic T cells, but also promotes active immune regulation that is essential for long-lasting tolerance.
In the light of this evidence, further studies will be focused on the mechanisms involved in the apoptotic effect of GalXM on CD3 T lymphocytes that can lead to tolerance induction.
Experimental procedures
Reagents and media
RPMI 1640 with l-glutamine and FCS were obtained from Gibco BRL (Paisley, Scotland). Penicillin-streptomycin solution was obtained from Sigma-Aldrich (St Louis, MO). R-phycoerythrin (RPE)-conjugated mouse mAb to human CD178 (FasL, IgG1) was purchased from Ancell (Bayport, MN). Fluorescein isothiocyanate (FITC)-conjugated mouse mAb to human CD95 (Fas, IgG1) was purchased from Immunological Sciences (Rome, Italy). Mouse mAb to caspase-8 (IgG1) was purchased from Cell Signaling Technology (Beverly, MA). Mouse isotype control IgG1 was purchased from Sigma-Aldrich. LPS, PHA, ConA, IL-2, mouse mAb to human CD3 were obtained from Sigma-Aldrich. All reagents and media were negative for endotoxin, as assessed by Limulus amebocyte lysate assay (QCL-1000, BioWhittaker, Walkersville, MD).
Microorganisms and cryptococcal polysaccharide
A thinly encapsulated strain of C. neoformans var. neoformans (CBS no. 6995) was obtained from Centraalbureau voor schimmelcultures (Delft, the Netherlands). The cultures were maintained by serial passages on Sabouraud agar (bioMerieux, Lyon, France). The agerminative Candida albicans strain used (PCA-2) was supplied by D. Kerridge (University of Cambridge, Cambridge, UK). Yeasts were killed by heating at 60°C for 30 min. The purified C. neoformans capsular polysaccharide GalXM was purified as described elsewhere (James and Cherniak, 1992). To eliminate LPS contamination in the GalXM preparation, this polysaccharide was dialysed extensively versus LPS-free water and the dialysate changed until negative by Limulus amobocyte assay. GXM was obtained from TR Kozel (Department of Microbiology and Immunology, University of Nevada, Reno, NE) and was isolated and purified as described elsewhere (Cherniak et al., 1980; Houpt et al., 1994). Purified soluble GXM contained < 125 pg LPS/mg of GXM as detected by Limulus amebocytes lysate assay.
Jurkat cells
The human Jurkat T-cell line was obtained from the American Type Culture Collection. Cells were maintained in RPMI 1640 with l-glutamine plus 10% FCS (complete medium) and antibiotics (100 U ml−1 penicillin and 100 µg ml−1 streptomycin) at 37°C in 5% CO2 atmosphere.
Cell separation
Heparinized venous blood was obtained from healthy donors. PBMC were separated by density gradient centrifugation on Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). For CD3+ T lymphocytes purification, PBMC were plated on culture flasks for 1 h in RPMI plus 5% FCS at 37°C in 5% CO2. After incubation the non-adherent fraction was reacted with anti-human CD3 mAbs-conjugated MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and CD3+ T lymphocytes were purified by magnetic separation according to the manufacturer's instructions.
Proliferation assay
Peripheral blood mononuclear cells (1 × 106 ml−1) were incubated in complete medium in a 96-well plate at 37°C in 5% CO2 for 5 days, alone or with 1, 10, 50, or 80 µg ml−1 GalXM, 50 µg ml−1 GXM, 5 µg ml−1 PHA, 1 µg ml−1 mouse anti-human CD3 mAb, or heat-inactivated C. neoformans or C. albicans cells (E/T 1:10), in a final volume of 200 µl. In selected experiments, purified CD3+ T lymphocytes (5 × 105 ml−1) were incubated in complete medium for 5 days in a 96-well plate at 37°C in 5% CO2, alone or with 1 or 10 µg ml−1 GalXM, 50 µg ml−1 GXM, 5 µg ml−1 PHA, 10 ng ml−1 IL-2, 1 µg ml−1 mouse anti-human CD3 mAb or 5 µg ml−1 ConA in a final volume of 200 µl. The concentrations of ConA and IL-2 (Monari et al., 1997) and PHA (Vecchiarelli et al., 1994) were chosen according to previous studies. The concentration of mouse anti-human CD3 mAb was determined by preliminary dose-dependency studies (not shown). Eighteen hours before harvesting, cells were pulsed with 0.5 µCi of [3H] thymidine per well. Incorporation into cellular DNA was measured by liquid scintillation counting. The results are expressed as mean counts per minute (CPM) ± SE of triplicate cultures.
Cytokine determination
Peripheral blood mononuclear cells (1 × 106 ml−1) were incubated overnight in complete medium in a 96-well plate at 37°C and 5% CO2 alone or in the presence of various concentrations of GalXM (1, 10, 50, 80 µg ml−1), LPS (10 µg ml−1, Sigma), heat-inactivated C. neoformans and C. albicans cells (E/T 1:10) in a final volume of 200 µl. After incubation, culture supernatants were collected and the levels of IL-10 and IFN-γ were determined using a commercially available specific ELISA kit (Biosource, Camarillo CA).
Evaluation of apoptosis by PI stain
Isolated T cells (5 × 105 ml−1), treated with or without with IL-2 (10 µg ml−1), PHA (5 µg ml−1), ConA (5 µg ml−1) or monoclonal antibody to human CD3 (1 µg ml−1) were incubated for 18 h in complete medium at 37°C and 5% CO2 alone or in the presence of GalXM (10 µg ml−1). In selected experiments Jurkat cells were stimulated as above. The percentage of T lymphocytes and Jurkat cells undergoing apoptosis was quantified by staining with propidium iodide (PI) (50 µg ml−1; Sigma-Aldrich). Briefly, cells were centrifuged, resuspended in hypotonic PI solution, and kept for 1 h at room temperature. Apoptosis was evaluated as previously described (Migliorati et al., 1993).
Detection of apoptosis by DNA fragmentation
Genomic DNA was obtained from either 4 × 106 Jurkat cells or CD3 T lymphocytes. After 18 h of incubation in complete medium at 37°C and 5% CO2 alone or in the presence of GalXM (10 µg ml−1) and ConA (5 µg ml−1). Jurkat cells and CD3 T lymphocytes were washed twice in PBS and genomic DNA was extracted using the Apoptotic DNA Ladder Detection Kit (Chemicon Int., Temecula, CA) according to the manufacturer's instructions. Ethidium bromide-stained DNA fragments were detected by 1% agarose gel electrophoresis.
Flow cytometry analysis of Fas and FasL molecules on Jurkat cells and T lymphocytes
Freshly isolated T lymphocytes (5 × 105 ml−1) and Jurkat cells (5 × 105 ml−1) were incubated in the presence or absence of GalXM (10 µg ml−1) and ConA (5 µg ml−1) in complete medium at 37°C and 5% CO2 for 18 h. After incubation the cells were collected, fixed with 10% paraformaldehyde and stained with FITC-conjugated mouse mAb to human CD95 (Fas) 10 µl/tube, IgG1 isotype in fluorescence buffer (FB) for 40 min on ice, washed twice with FB and labelled with RPE-conjugated mouse mAb to human CD178 (FasL) (dilution 1:50; IgG1 isotype) in FB for 40 min on ice. After incubation the cells were washed twice, resuspended in FB. A total of 10 000 events were analysed by flow cytometry using a FACScan flow cytofluorometer (BD Biosciences, Franklin Lakes, NJ). Control staining of cells with irrelevant antibody was used to obtain background fluorescence values. Data are expressed as percentage of labelled positive cells.
Caspase-8 activation
CD3+ T lymphocytes and Jurkat cells (5 × 106) were incubated in the presence or absence of GalXM (10 µg ml−1) and ConA (5 µg ml−1) in complete medium for 18 h at 37°C with 5% CO2.
After culture, the cells were washed, treated with 250 µl of M-PER in the presence of protease inhibitors (Pierce Rockford, IL) and the lysates were collected by removing particulate material through centrifugation for 10 min at 12 000 g. Lysate proteins were separated by sodium dodecyl-sulphate-10% polyacrylamide gel electrophoresis and 30 µg of each sample were transferred to a nitrocellulose membrane (Pierce) for 1 h at 100 V in a blotting system (Bio-Rad, Hercules, CA) for Western blot analysis. Membranes were then placed in blocking buffer, incubated overnight at 4°C with mouse monoclonal Ab to caspase-8 (dil. 1/1000; Cell Signalling Technology, Beverly, MA) and rabbit antibody to actin (dil. 1/200; Santa Cruz Biotechnology, Santa Cruz, CA) in blocking buffer and stained according to the manufacturer's directions (WesternBreeze Chemiluminescent Western Blot Immunodetection Kit, Invitrogen Corporation, CA). Immunoreactive bands were visualized and quantified by Chemidoc Instrument (Bio-Rad).
Statistical analysis
Statistical analysis was performed with the Primer of Biostatistics software program. Data are reported as the mean ± standard error from replicate experiments. Data were evaluated by one-way analysis of variance. Post-hoc comparisons were done with Bonferroni's test.
Acknowledgements
The authors thank Gabriella F. Mansi for editorial and secretarial assistance and Roberto Castronari for technical assistance. This work was supported by the National Institute of Health AIDS Project, Contract no. 50F.