The Steinernema carpocapsae intestinal vesicle contains a subcellular structure with which Xenorhabdus nematophila associates during colonization initiation
Summary
Steinernema carpocapsae infective juvenile (IJ) nematodes are intestinally colonized by mutualistic Xenorhabdus nematophila bacteria. During IJ development, a small number of ingested X. nematophila cells initiate colonization in an anterior region of the intestine termed the vesicle and subsequently multiply within this host niche. We hypothesize that efficient colonization of a high percentage of S. carpocapsae individuals (typically > 85%) is facilitated by bacterial adherence to a site(s) in the nematode intestine. We provide evidence that the adherence site is a structure in the lumen of the IJ vesicle that we have termed the intravesicular structure (IVS). The IVS is an untethered cluster of anucleate spherical bodies that colocalizes with colonizing X. nematophila cells, but does not require X. nematophila for its formation. Colocalization with the IVS is readily apparent in IJs colonized by X. nematophila mutants that initiate intestinal colonization but fail to proliferate normally, suggesting that bacterial–IVS interaction occurs early in the colonization process. Treatment with insect haemolymph induces anal release of X. nematophila from colonized IJs and induces release of the IVS from uncolonized S. carpocapsae IJs. Released IVS were probed with several carbohydrate-specific lectins. One lectin, wheat-germ agglutinin, reacts strongly with a mucus-like substance that is present around individual spheres in the aggregate IVS. Potential roles for the IVS in mediating X. nematophila colonization of the nematode intestine are discussed.
Introduction
The entomopathogenic nematode Steinernema carpocapsae and its mutualistic symbiont, the γ-proteobacterium Xenorhabdus nematophila, parasitize various insect species that they kill and use for reproduction (Forst and Clarke, 2002). Within the insect cadaver, S. carpocapsae feeds on accumulated X. nematophila cells and insect-derived nutrients. Progeny S. carpocapsae develop into environmentally resistant and non-feeding infective juveniles (IJs) that emerge from exhausted insect cadavers to serve as vectors of X. nematophila into new insect hosts. The anterior intestine of this vector IJ stage is colonized at a discrete location, termed the vesicle, by > 40 viable X. nematophila cells (Poinar, 1966; Popiel et al., 1989; Heungens et al., 2002). This colonization is specific: closely related Xenorhabdus spp. are unable to colonize S. carpocapsae IJ intestinal vesicles (Poinar and Thomas, 1966; Grewal et al., 1997; Sicard et al., 2004a). Viable X. nematophila cells persist in the IJ vesicle until they are released, by nematode defecation, into a new insect host (Lewis et al., 1995; Poinar, 1966; Martens et al., 2003a; Sicard et al., 2004b). The mechanism by which X. nematophila specifically colonizes S. carpocapsae IJ vesicles serves as a model to understand general aspects of horizontal transmission of stable, beneficial symbionts by their hosts (Vivas and Goodrich-Blair, 2001; Martens et al., 2003a).
Colonization of S. carpocapsae IJs is initiated by very few X. nematophila cells that are retained in the intestinal vesicle of developing nematodes. Once the environmentally resistant IJ stage has formed, these initial colonizers undergo limited proliferation until the vesicle niche is filled (Martens et al., 2003b). Although only one or few X. nematophila cells initiate colonization of individual IJs, the overall rate of IJ colonization in a population is typically high (≥ 85% of individuals colonized) (Akhurst and Boemare, 1990; Martens et al., 2003b; Sicard et al., 2004b). How is such efficient and specific colonization of individual IJs achieved by only a few initiating X. nematophila cells per nematode? One possibility is that the nematode presents an X. nematophila-specific receptor before or during IJ development (Martens et al., 2003b). Specific binding and retention of X. nematophila with this hypothetical receptor could ensure efficient colonization. Furthermore, a scenario in which such receptors are present transiently and/or in limited numbers is consistent with few initiating X. nematophila cells. Few attachment sites, or infrequent encounters with those sites, would effectively limit the number of X. nematophila cells retained.
Microbial attachment to host tissues plays a well-established role in the initiation of many pathogenic and non-pathogenic microbe–host interactions (Smit et al., 1989; Hultgren et al., 1996; Soto and Hultgren, 1999; Toleman et al., 2001). Proteins and carbohydrates present on host and microbe surfaces mediate adhesive interactions, and both protein–protein and protein–carbohydrate interactions can occur (Krukonis and Isberg, 2000; Swords et al., 2001; Hung et al., 2002). Specific interaction between bacterial and host molecules provides a basis for host or tissue tropisms and is an important determinant of the ability of some bacterial species to colonize certain hosts and/or host sites, but not others (Hultgren et al., 1996).
General classes of bacterial outer surface structures that mediate adherence to host tissue receptors include fimbriae, non-fimbrial adhesins and polysaccharides. X. nematophila factors that mediate attachment to tissue(s) within the IJ vesicle have not been described, although some candidates have been suggested. For example, immunogold labelling experiments, using antibody generated against X. nematophila F1 fimbriae, indicated that these surface structures are expressed in the IJ vesicle and therefore may be involved in IJ colonization (Binnington and Brooks, 1993). However, a potential role for F1 fimbriae in nematode colonization has not been explored further. Two X. nematophila surface proteins, NilB and NilC, are required for colonization (Heungens et al., 2002) and may serve as adhesins. NilB is predicted to be a β-barrel type outer membrane protein (Heungens et al., 2002) and NilC is an outer membrane lipoprotein with probable orientation towards the periplasmic space (Cowles and Goodrich-Blair, 2004). X. nematophila nilB and nilC mutants are completely deficient for IJ colonization (Heungens et al., 2002; Cowles and Goodrich-Blair, 2004) suggesting NilB and NilC function early during colonization, before bacterial retention in the vesicle. This idea is consistent with the possible function of NilB and NilC as adhesins, although their biochemical activities remain unknown.
The hypothetical nematode receptor to which X. nematophila binds also has not yet been identified. In fact physical aspects of the IJ vesicle are only partially described. An examination of the morphology of the intestinal vesicle in several Steinernema spp. was conducted using differential interference contrast (DIC) light microscopy and transmission electron microscopy (Bird and Akhurst, 1983). This study concluded that the vesicle is a modification of the anterior nematode intestine and forms independently of bacterial colonization. The authors noted that the epithelial cells lining the intestinal vesicle contain microvilli in some Steinernema spp. and the vesicular lumen appears to contain an amorphous matrix in which mutualistic Xenorhabdus spp. are closely packed (Bird and Akhurst, 1983). The IJs microscopically examined in this study were likely mature (i.e. they contained fully grown bacterial populations). Therefore, this report did not provide insight into the mechanism(s) through which mutualistic Xenorhabdus spp. interact with either the nematode intestinal epithelium or lumenal contents of the IJ vesicle during colonization initiation and subsequent bacterial growth.
In this report, we present evidence of an intestinal substructure, the intravesicular structure (IVS) that occupies the IJ vesicle and colocalizes with initiating X. nematophila within the nematode intestine. These observations support the hypothesis that X. nematophila colonization of the IJ intestine occurs through specific bacterial–IVS interaction. Observation of structures similar to the S. carpocapsae IVS in the intestinal vesicles of two other Steinernema spp. suggests these structures may perform a conserved function in this genus, and may in fact play a role in dictating the species specificity of Steinernema–Xenorhabdus interactions.
Results
Observation of axenic S. carpocapsae IJ vesicles
Previous isolation of several colonization-defective X. nematophila mutants (Vivas and Goodrich-Blair, 2001; Heungens et al., 2002; Martens et al., 2003c) facilitated the production of large numbers of axenic (intestinally uncolonized) S. carpocapsae IJs. We used these axenic IJs to better understand the morphology of the S. carpocapsae intestinal vesicle. DIC microscopy was used to observe the vesicles of IJs grown on the colonization-deficient rpoS mutant, HGB151 (Vivas and Goodrich-Blair, 2001). In these vesicles we noted the presence of a conspicuous structure moving visibly (Fig. 1 and Movie S1 in Supplementary material). This IVS appears as an irregularly shaped, < 7 µm diameter structure that is an aggregate of several smaller (<2 µm diameter) spherical lobes, which may themselves vary in diameter (Fig. 1). Observations of greater than 100 axenic IJs revealed that each vesicle contained either one (Fig. 1A) or two (Fig. 1B) IVS.

IVS in S. carpocapsae intestinal vesicle. The IVS is a multilobed structure that occurs in the intestinal vesicle of S. carpocapsae. A and B. DIC images of axenic IJs, oriented with IJ head facing upward. Basal bulb (b), vesicle (v), posterior intestine (int) and IVS are indicated. Note the ‘hour-glass’ morphology of the vesicle in both (A) and (B). We have observed that the vesicle assumes this morphology when IJs are paralysed with the cholinergic receptor agonist levamisole (see Discussion and Experimental procedures). IJ vesicles are observed to contain either one (A) or two (B) IVS. The IVS moves visibly within the vesicular lumen and images present in (A) and (B) are frames taken from time-lapse videos documenting IVS movement. C. Schematic diagram of an S. carpocapsae IJ, with the head facing rightward, summarizing the position of the IVS relative to other IJ body features, pharynx (ph), basal bulb (b), vesicle (v) and posterior intestine (int). Magnification is ×600; bar = 10 µm.
The IVS was observed in axenic S. carpocapsae IJs resulting from nematode cultivation with each of the currently described X. nematophila colonization-deficient mutants (with the exception of rpoE, which was not tested because this mutant grows poorly) (Vivas and Goodrich-Blair, 2001; Heungens et al., 2002; Martens et al., 2003c). Furthermore, IJs grown on sterile liver-kidney medium (Poinar and Thomas, 1966) in the absence of bacteria also contained IVS that were indistinguishable in morphology from those of IJs grown on colonization-deficient mutants (data not shown). In addition, the IVS was observed in the ≤ 15% of IJs that typically remain uncolonized after growth in the presence of wild-type X. nematophila (HGB007 and HGB081).
Colonizing X. nematophila localize to the IVS surface
The conspicuous presence of the IVS in the IJ vesicle raises the possibility that this structure is involved in the colonization process. Visualization of the IVS in maturing IJs (those between 1 and 6 d old) is rare due to the presence of X. nematophila aggregates that occupy the same space. However, in such IJs X. nematophila aggregates display movement that is similar to that of the IVS in uncolonized IJs (Fig. 2A–F and Movie S2A–E). These IJs also harbour either one or two bacterial aggregates (compare Fig. 2A and D) reminiscent of the fact that axenic IJs contain one or two IVS per vesicle (Fig. 1). Furthermore, in those rare instances in which the IVS can be seen in maturing IJs, it appears directly adjacent to, and moves in synchrony with, X. nematophila aggregates (Fig. 2C and D and Movie S2A and D). It should be noted that non-aggregated X. nematophila cells are frequently observed in the vesicular lumen of maturing IJs (Fig. 2D–E and Movie S2D–E). These cells appear to be peripheral to the aggregated bacteria and move independently from these clusters.
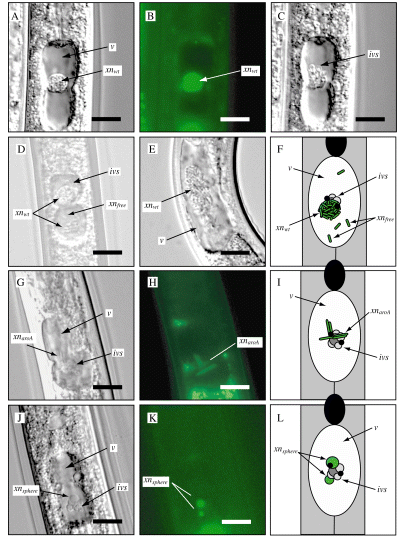
Xenorhabdus nematophila association with the IVS. Each image is a sample frame from a time-lapse movie depicting X. nematophila/IVS movement in the vesicular lumen (Movie S2A–K). IJs are oriented with heads facing upward. Images A–E depict wild-type X. nematophila (xnwt) within maturing IJs. A. DIC image of a maturing IJ showing a cluster of HGB338 (xnwt) cells occupying the vesicular lumen (v). B. Epifluorescence micrograph of the IJ shown in (A), showing green fluorescence associated with HGB338 cells (xnwt). C. DIC image of IJ shown in (A) and (B) in a different focal plane, revealing proximity of HGB338 cluster to the IVS (ivs). D. Maturing IJ showing two clusters of HGB338 as well as multiple unattached X. nematophila cells (xnfree) occupying the vesicle. E. Maturing IJ showing a large bacterial cluster (xnwt) and numerous unattached wild-type, non-fluorescent HGB007 cells which become apparent when viewing Movie S2E. Note that the IJ depicted in (E) was paralysed with sodium azide (Experimental procedures) and does not display the ‘hour-glass’ vesicle morphology that is typically observed with levamisole paralysis. F. Schematic diagram of X. nematophila colonization–maturation process summarizing observations in (A)–(E). Most X. nematophila cells (green rods) cluster tightly with each other and those that are clustered appear to move synchronously with the IVS. G. IJ colonized by HGB644 (xnaroA). H. Epifluorescence image of the IJ shown in (G). Filamentous HGB644 cells localize on, and move synchronously with the IVS (Movie S2G and H). I. Schematic representation of the HGB644 cells (green filaments) shown in (G) and (H) relative to the IVS. J. DIC micrograph of an IJ grown on HGB645, showing spherical X. nematophila serC cells (xnsphere) associated with the IVS. K. Epifluorescence micrograph of the IJ shown in (J), showing green fluorescence associated with HGB645 spheres. Abortive HGB645 cells localize on, and move synchronously with the IVS (Movie S2J and K). L. Schematic of the IJ shown in (J) and (K) depicting HGB645 spheroplasts (green circles) associating with the IVS. Magnification is ×600; bars = 10 µm.
The observations described above are consistent with the hypothesis that X. nematophila associates with the IVS during colonization initiation and maturation. To facilitate routine examination of bacterial interactions with the IVS, we examined IJs grown on previously described X. nematophila mutants (Heungens et al., 2002; Martens et al., 2005) which are retained within the IJ vesicle, but fail to subsequently proliferate within the lumen of the vesicle as do wild-type X. nematophila. The lack of proliferation in the vesicle is associated with two additional phenotypes. One is a spherical cell morphology that correlates with loss of bacterial viability (and therefore is termed ‘abortive’). This morphology within nematodes decreases in frequency over time, without a concomitant decrease in recoverable bacteria from these IJ populations (Martens et al., 2005). The second is a filamentous morphology that correlates with the presence of viable but non-growing or slowly growing cells (i.e. this morphology is persistent within an IJ population and correlates with recoverable bacterial counts) (Martens et al., 2005). Spherical, abortive cells are associated with mutants with defects in pyridoxine, l-threonine and para-aminobenzoate biosynthesis while the filamentous morphology has only been observed in some IJs colonized by para-aminobenzoate biosynthesis mutants (Martens et al., 2005).
We reasoned that these mutants might be proficient at interacting with the IVS but would not mask its presence by subsequent outgrowth. We therefore examined IJs grown on the para-aminobenzoate auxotroph HGB644 (aroA/gfp+) by DIC and epifluorescent microscopy and found that filamentous HGB644 cells consistently colocalize with the IVS (Fig. 2G–I). Time-lapse movies of these events show that HGB644 cells move in synchrony with the IVS (Movie S2G and H). Thus, we conclude that these bacteria are associated with the IVS surface and not with surfaces of surrounding epithelial cells. The pyridoxine auxotroph HGB645 (serC/gfp+) exhibits a high frequency of abortive colonization of the IJ vesicle. HGB645 spherical cells colocalized and moved synchronously with the IVS (Fig. 2J–L and Movie S2J and K). Similar colocalization between abortive cells and the IVS was observed for other abortive strains, including HGB644 (aroA/gfp+) and HGB692 (aroE/gfp+) (data not shown).
IVS characterization
To further explore the possibility that the IVS is an adherence site for X. nematophila, we wished to better understand its cellular and biochemical nature. S. carpocapsae IJ intestines were expelled using the previously described ‘guillotine technique’ (Vivas and Goodrich-Blair, 2001; see Experimental procedures) and examined to determine the relationship of the IVS relative to the surrounding intestinal epithelium (Fig. 3). The IVS moves within the vesicular lumen of disgorged IJ intestines as it does in intact IJ vesicles (Fig. 3B and Movie S3). To determine whether the IVS contains a nucleus, we stained disgorged IJ intestines with 4′-6-diamidino-2-phenylindole (DAPI), to fluorescently label DNA (Fig. 3C and D). The IVS did not colocalize with areas of DAPI staining (e.g. Fig. 3D), suggesting that the IVS does not contain a nucleus and therefore is subcellular.
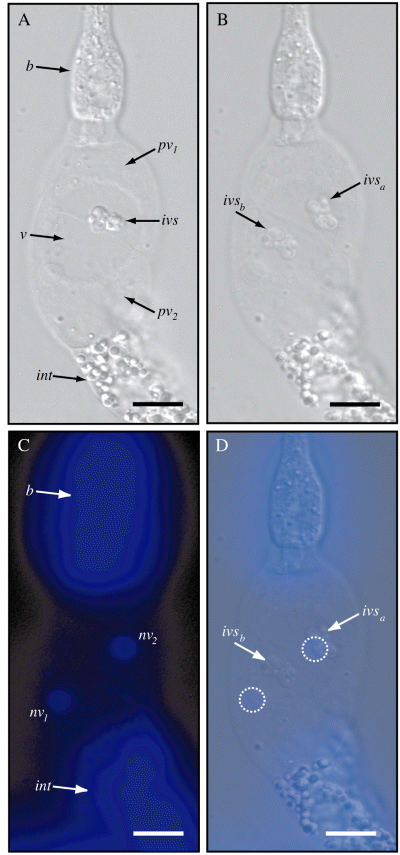
IVS observed in disgorged IJ intestines. A. DIC micrograph of axenic IJ intestine disgorged in dH2O. Basal bulb (b), vesicle (v), perivesicular cells (pv), posterior intestine (int) and IVS are indicated. Note the differential morphology between the transparent perivesicular cells (pv) and the heavily granulated posterior intestinal cells (int). B. The IVS is observed to move in the vesicular lumen of disgorged intestines. Two overlayed micrographs taken of the same specimen several minutes apart show that IVS has moved a substantial distance in this vesicle (positions ivsa to ivsb). A time-lapse movie of this specimen, provided as Movie S3, shows similar movement. C. Epifluorescence micrograph of the sample shown in (A) and (B) revealing DAPI staining of intestinal nuclei (nv1 and nv2). The basal bulb (b), containing numerous small muscle cells, fluoresces brightly. D. Overlay of images (B) and (C) showing relative sizes and positions of ivsa, ivsb, nv1 and nv2 (perivesicular nuclei from C are highlighted with dashed lines). During observation of this specimen and others, DAPI-stained regions did not co-migrate with the IVS suggesting that the IVS does not contain a nucleus. Magnification is ×600; bars = 10 µm.
DAPI staining also allowed us to further characterize the cellular nature of the vesicle region. The vesicle is surrounded by just two nucleated cells (perivesicular cells) (Fig. 3A and C) that are morphologically different from the heavily granulated posterior intestinal cells (Fig. 3A). The posterior IJ intestine comprises pairs of epithelial cells that surround the lumen of the intestine (data not shown).
IVS release by haemolymph-stimulated IJs
Colonized S. carpocapsae IJs release intestinal X. nematophila cells by defecation in response to an unknown signal(s) present in insect haemolymph (Poinar, 1966; Martens et al., 2003a; Sicard et al., 2004b). Based on this and the fact that the IVS is mobile and apparently unattached to vesicular epithelia, we speculated that axenic S. carpocapsae IJs might release the IVS into the external environment if stimulated with insect haemolymph. We treated, for 24–48 h, axenic, ex-sheathed (removal of the outer cuticle of the IJ that is retained after molting) IJs with 0.25× denatured Manduca sexta haemolymph diluted in PBS for (see Experimental procedures). After incubation under these conditions, IJs demonstrated the previously described expansion of the posterior intestinal lumen, pumping of the basal bulb and contraction of muscles surrounding the rectum and anus (Poinar, 1966; Martens et al., 2003a). During these events, the IVS moved up and down the entire length of the expanded intestinal lumen and was frequently observed to be present either at the posterior-most end of the intestinal tract or within the rectum itself (data not shown). In some instances individual spheres composing the IVS dissociated and moved independently of one another within the expanded nematode intestine, consistent with the hypothesis that these spheres are individual bodies that cluster to create the IVS (data not shown). Aside from the IVS, no other visible objects appeared to be present within the intestinal lumen. Treated IJs released IVS from the anus at a low frequency, allowing them to be observed in the external environment (Fig. 4). External IVS were most often observed to remain associated with the posterior end of IJs, although free IVS were also observed.
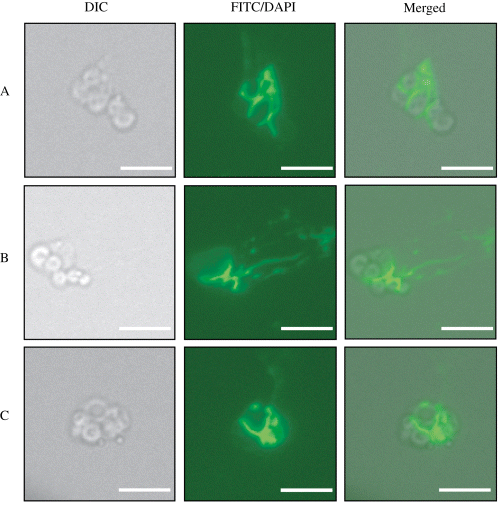
Staining of released IVS with FITC-WGA. Axenic IJs release the IVS into the external environment in response to denatured M. sexta haemolymph. Three individual released IVS are shown (A–C). The first image in each series shows a DIC micrograph of a single IVS. The middle image in each panel shows an epifluorescence micrograph taken with an FITC filter to detect FITC-WGA binding. The third image in each panel shows merged DIC and fluorescent images. FITC-WGA binds to a substance that appears to be localized between individual IVS spheres and also appears to be loosely associated with the IVS and capable of dissociating from the larger aggregate [e.g. IVS in (B) FITC and merged]. Magnification ×600; bars = 5 µm.
Attempts to purify sufficient amounts of IVS away from haemolymph-treated nematode mixtures for the purposes of in vitro biochemical characterization and X. nematophila binding experiments were unsuccessful. However, to begin to characterize the IVS surface, we probed haemolymph-treated IJ mixtures with a number of fluorescently conjugated lectins (see Experimental procedures). Wheat germ agglutinin (WGA), a lectin with binding specificity for N-acetyl glucosamine (GlcNac) and N-acetyl neuraminic acid (NeuNac) glycoconjugates (Burger and Goldeberg, 1967; Monsigny et al., 1980), bound to released IVS, particularly in the interstitial spaces located between the spherical IVS lobes (Fig. 4). This WGA-reactive material could dissociate from the IVS in streams (e.g. Fig. 4B) and resembles previously published images of mucosal secretions from other organisms (Nyholm et al., 2000). WGA binding to the IVS was not inhibited by addition of ∼45-fold excess GlcNac (20 µg ml−1) to binding reactions. This result may be explained by the fact that WGA exhibits 12-fold to 6000-fold higher affinity for GlcNac residues when they are present in the context of certain polymeric glycans rather than monomeric GlcNac alone (Goldstein and Poretz, 1986). No attempt was made to inhibit binding of WGA to the IVS by addition of the lower-affinity sugar NeuNac (Monsigny et al., 1980).
The lectins peanut agglutinin (PNA), soybean agglutinin (SBA), Ulex europeus agglutinin-1 (UEA-1) and concanavalin A (ConA) did not exhibit observable IVS binding but did exhibit punctate staining of an amorphous mass of secreted material released from the nematode anus in addition to the IVS (data not shown). This material may represent a mucosal secretion that is released from the nematode intestine along with the IVS, but did not stain similarly to the interstitial, WGA-reactive material associated with the IVS.
Presence of IVS in other Steinernema spp.
We sought to determine whether the IVS is a structure specific to S. carpocapsae or more generally present in Steinernema spp. and examined S. siamkaya, S. karii, S. glaseri, S. riobrave and S. feltiae. Phylogenetic analysis of Steinernema spp. has indicated they fall into two clades, one of which contains S. carpocapsae and S. siamkaya (Stock et al., 2001). The second clade is heterogeneous with several subclades represented by the remainder of the tested nematodes (Stock et al., 2001). We propagated axenic IJs of each of the selected species (see Experimental procedures) and examined the anterior intestine of each for the presence of a discernible vesicle and IVS. For at least two of these species, S. siamkaya and S. karii, IVS-like structures were apparent within the vesicles of axenic IJs (Fig. 5). Furthermore, in S. glaseri and S. riobrave structures that might be similar to the IVS were observed within the anterior intestine, but both the vesicle and potential IVS in these two species were very different in morphology to S. carpocapsae, S. siamkaya and S. karii, making it difficult to interpret these structures (data not shown). We failed to observe obvious vesicles in the anterior intestines of axenic S. feltiae IJs, precluding examining the presence or absence of IVS in these nematodes.

IVS-like structures in other Steinernema spp. The presence of IVS-like structures in S. siamkaya and S. karii. Specimens of both intact IJs and disgorged intestines are shown. IVS-like structures (ivs), basal bulb (b) and vesicle (v) are indicated. Magnification is ×600; bars = 10 µm.
Discussion
Entomopathogenic Steinernematid nematodes were discovered by Glaser (1932), but it was not until the 1960s that Poinar and Thomas (1966) revealed the crucial contributions made by X. nematophila bacteria to the life cycle of these insect parasitic nematodes. These investigators also reported that the IJ form of S. carpocapsae harbours only one species of bacterium, which is housed in the anterior region of the nematode's intestine (Poinar, 1966). Although some examination of the morphology of this region, the vesicle, have been reported (Poinar and Leutenegger, 1968; Bird and Akhurst, 1983; Endo and Nickle, 1995), details of its structure have remained mysterious. Use of our previously identified colonization-defective X. nematophila mutants (Vivas and Goodrich-Blair, 2001; Heungens et al., 2002; Martens et al., 2005) and green fluorescent protein (GFP)-labelled derivatives of these strains (Martens et al., 2003b; 2005) has facilitated a closer examination of the colonization site. Now, 40 years after the initial discovery of the vesicle, we report here for the first time that it is formed by two perivesicular cells (Fig. 3), and that the vesicular lumen contains a small structure, the IVS (Fig. 1). The IVS is the only physical structure apparent in the vesicle, and as such is likely to play a key role in mediating interactions with colonizing bacteria. Indeed, we provide evidence that this structure is a focal point for X. nematophila cells associating with the IJ vesicle (Fig. 2).
Structural characteristics of the IJ intestine, vesicle and IVS
Staining of disgorged IJ intestines with the nucleic acid stain DAPI revealed that the S. carpocapsae vesicular epithelium contains only two nuclei (Fig. 3C) and thus that this compartment is surrounded by two epithelial cells (perivesicular cells). The posterior intestine of S. carpocapsae is composed of adjacent pairs of epithelial cells, suggesting a morphogenic pattern similar to that of C. elegans that contains bilateral pairs of intestinal cells (http://www.wormatlas.org). Perivesicular cells exhibit a distinct morphology from those of the posterior intestine (Fig. 3A). Each perivesicular cell is homogenously transparent, while cells in the posterior intestine appear heavily granulated with spherical inclusions. This distinction supports the idea that the perivesicular cells are functionally different from the adjacent epithelium and raises the possibility that this difference contributes to the specific intestinal colonization tropism of X. nematophila for the IJ vesicle and not the posterior intestine. The junction between the two perivesicular cells constricts when nematodes are treated with the cholinergic receptor antagonist levamisole (Fig. 1), a compound known to cause muscle hypercontraction in nematodes (Gally et al., 2004). This contraction is not specific to levamisole treatment, as it is also infrequently observed in IJs paralysed using sodium azide (e.g. Fig. 2E). The ability of the perivesicular cells to contract suggests this process may play some biological role in defining physical restrictions of X. nematophila colonization (Martens et al., 2003b), or, not mutually exclusively, in mediating expulsion of bacteria and the IVS from the vesicle during infection. However, dynamic muscle contraction of the perivesicular cells is not necessary for movement of the IVS within the vesicle, as the IVS continues to move in IJs paralysed by levamisole (e.g. see Movie S1).
The only visible structure within the vesicle is the IVS, a mobile and < 7 µm diameter aggregate of individual < 2 µm diameter anucleate spheres. Axenic IJs can contain either one or two individual IVS, but the significance of this dimorphism is unknown. Interestingly, X. nematophila populations in the vesicle have been determined to be predominantly monoclonal or biclonal (Martens et al., 2003b) raising the possibility that the number of IVS present in the vesicle influences the number of initiating/surviving bacterial clones. The IVS is present in the vesicles of S. carpocapsae IJs reared for multiple generations on liver-kidney medium in the absence of bacterial contact, suggesting that exposure to X. nematophila or other bacterial spp. is not required for IVS development. This latter observation is consistent with a previous report that confirmed vesicle development in the anterior intestine of several Steinernema spp. in the absence of bacterial exposure (Bird and Akhurst, 1983), although these authors did not report the presence of the IVS. The ontogeny of the IVS could include derivation from a progenitor cell(s) through a programmed event such as apoptosis, or secretion of IVS component material(s) into the vesicular space. Developmental experiments with several Steinernema spp. are currently underway to determine when the IVS appears during IJ development and may provide insight into its origin within the nematode body (S.P. Stock, pers. comm.).
The IVS is also readily observable in intact, disgorged IJ intestines in which it still moves visibly despite the fact that the IJ intestine is no longer in contact with the underlying endothelium (Fig. 3). This observation suggests that IVS movement is either passive on the part of the nematode (i.e. Brownian movement of an unattached structure) or derives from a motive force intrinsic to the vesicular epithelium itself. Motive force for IVS movement could potentially derive from the motion of cilia present on the vesicular epithelium, although the presence of cilia within the digestive tracts of nematodes has little or no precedent (Andreassen, 1966). Of note, two previous reports on the structure of the intestinal vesicles of Steinernema spp. did not determine the presence of cilia in the IJ intestine in general (Poinar and Leutenegger, 1968), or on the vesicular epithelium in particular (Bird and Akhurst, 1983). However, the latter report described the presence of similarly sized structures interpreted to be microvilli (Bird and Akhurst, 1983). Thus, because epithelial extensions exist in the vesicle, the possible presence of cilia as a motive force in the IJ vesicle cannot be ruled out without additional studies of intestinal epithelium morphology and the nature of its cellular projections.
The movement of the IVS suggested to us that it is unattached to the perivesicular cells. Indeed, in response to insect haemolymph the IVS could be released into the posterior intestine and then into the external environment via defecation (Fig. 4). Interstitial regions of the IVS bound to fluorescein 5(6)-isothiocyanate (FITC)-labelled WGA suggesting that these regions harbour GlcNac- or NeuNac-containing glycans. Of note, the WGA cross-reactive IVS material can dissociate from the IVS (Fig. 4B) and separation of individual IVS spheres was observed in some experiments (data not shown). These data suggest that this material may be a soluble, mucus-like substance that holds the individual spheres in an aggregate, although we have not ruled out the possibility that this substance lines the intestine and associates with the passing IVS during release. If the mucus-like substance is associated with the IVS within the vesicle, it may serve as an adhesive molecule, a food source, or both. Mucus serves as a specific binding site for Vibrio fischeri during establishment of its symbiotic relationship with the squid Euprymna scolopes squid. In fact, mucus secretion is considered the first event in this symbiosis that ensures the specificity of the interaction (Nyholm and McFall-Ngai, 2003). Thus, during ingestion of bacteria, S. carpocapsae destined to develop into IJs may present the mucus-bathed IVS to specifically recruit and retain X. nematophila within the vesicle. Furthermore, the mucus may serve as a nutrient source that supports the growth of the one or two initial colonizing cells to fully populate the vesicle (Martens et al., 2003b).
Xenorhabdus nematophila–IVS interactions
Correlation between X. nematophila retention in the IJ vesicle and the IVS derives from the observation that bacterial cells colocalize with the IVS during both wild-type X. nematophila colonization (Fig. 2A–F) and during aberrant colonization by mutant X. nematophila strains (Fig. 2G–L). Examination of mutant colonization behaviours in particular provides what may be considered ‘snapshots’ of the colonization initiation process, when few X. nematophila cells are retained within the IJ vesicle and either grow slowly (aroA) or stop growing (aroA and serC). Regardless of their morphological defect, these mutant bacteria adhere to and move in synchrony with the IVS (Fig. 2 and Movie S2G–K). The fact that slow growing or spherical X. nematophila cells remain adherent to the IVS for protracted lengths of time indicates that X. nematophila–IVS interactions are stable over relatively long periods (days to weeks). Furthermore, previous evidence suggests that spherical X. nematophila cells are not viable (Martens et al., 2005) and therefore bacterial viability may not be required for IVS adherence.
The colonization pattern of wild-type X. nematophila during the maturation process is noteworthy. As initiating bacteria grow in the IJ vesicle during the first several days of IJ colonization (Martens et al., 2003b), single or double aggregates of densely packed X. nematophila cells move within the vesicular lumen as individual masses (Fig. 2A–E and Movie S2A–E) and localize near the IVS (Fig. 2C and D). The fact that the position of each bacterium is static relative to neighbouring bacteria in this nascent microcolony (see in particular Movie S2E) corroborates our previous report that bacterial growth in the vesicle is spatially restricted (Martens et al., 2003b). The cause of this spatial restriction during outgrowth remains unknown. However, our findings argue against the idea that a nematode-derived intestinal matrix limits bacterial movement and growth area, as free bacterial cells (which would presumably also be in contact with a hypothetical vesicular matrix) exhibit apparently unrestricted movement independent of bacterial aggregates (Fig. 2D–F). These unattached cells may move in response to the same mechanical forces that drive IVS movement (see above). Alternatively, these unattached X. nematophila could be displaying flagellar-based motility (Givaudan and Lanois, 2000).
Between one and two X. nematophila cells establish the vesicular colony of an individual colonized S. carpocpasae IJ, and the frequency of successful colonization is > 85% in an IJ population. Multiple overlapping mechanisms may underlie this specific and efficient colonization of S. carpocapsae IJs by X. nematophila cells. For example, entry into the vesicle may be restricted, while retention within the vesicle may be facilitated by adherence of bacterial cells. The X. nematophila–IVS association documented in this report represents the first evidence of this hypothetical adherence event, and suggests that X. nematophila expresses a factor(s) that mediates binding to the IVS. One candidate adhesin is F1 fimbriae, which are expressed within the IJ vesicle (Binnington and Brooks, 1993). Genes encoding the F1 fimbriae were recently identified (He et al., 2004) but mutational analysis to test the role of this putative adhesive organelle has not yet been reported. Two other potential IVS adhesins are NilB and NilC that are required for colonization (Heungens et al., 2002) and are likely associated with the cell envelope (Heungens et al., 2002; Cowles and Goodrich-Blair, 2004). gfp-labelled X. nematophila nilB cells are non-abortive; no bacterial cells are visible in the vesicle of an IJ grown on this strain (Martens et al., 2005). This result suggests that cells lacking NilB are not retained in the IJ vesicle, and supports the idea that this putative outer membrane protein could function as an initiating adhesin. Future experiments are required to determine whether the potential adhesins described above, or others that remain unidentified, are involved in adherence to the IVS. While purification of the IVS remains unlikely due to its inefficient release, its tendency to remain associated with surrounding nematode material, and its unstable structure, further examination of its biochemical nature through microscopy may provide information to facilitate its isolation.
Much progress has recently been made in the development of the S. carpocapsae–X. nematophila association as a model to understand stable, beneficial relationships between animals and microbes. However, the bulk of this progress has been focused on bacterial mechanisms of colonization, while the equally important host contributions to this mutualism are less well understood. The work described here has clarified details of the host environment that directly interacts with the bacterial symbiont. The identification of the IVS represents the first evidence of a direct physical interaction between S. carpocapsae by X. nematophila bacteria and may help explain how individual S. carpocapsae IJs each achieve efficient and specific colonization by few bacterial cells.
Experimental procedures
Bacteria and nematode strains and culture conditions
Bacteria and nematode strains used in this study are presented in Table 1. X. nematophila strains were grown and maintained as previously described (Martens et al., 2003b). Propagation of Steinernema spp. in Galleria mellonella larvae (Vanderhorst Wholesale, St Mary’s, OH), storage in dH2O and in vitro lipid-agar (LA) culture of S. carpocapsae were performed as previously described (Vivas and Goodrich-Blair, 2001). Liver-kidney medium was prepared according to Poinar and Thomas (1966). For rearing axenic IJs on liver-kidney medium, G. mellonella-grown IJs of each species were washed and resuspended in cell-free, filtered (0.22 µm) M. sexta haemolymph to which 5 mM glutathione (Sigma, St Louis, MO) was added as a preservative (Orchard and Goodrich-Blair, 2004). IJ suspensions were incubated in M. sexta haemolymph in the presence of an antibiotic mixture consisting of: chloramphenicol 60 µg ml−1, ampicillin 300 µg ml−1, gentamicin 60 µg ml−1, kanamycin 100 µg ml−1, streptomycin 100 µg ml−1, tetracycline 100 µg ml−1, rifampicin 200 µg ml−1, erythromycin 200 µg ml−1 and nalidixic acid 50 µg ml−1 (all antibiotics from Sigma). IJ suspensions were incubated in this mixture in 6 cm Petri plates in ∼2 ml volume for 16–20 h. This treatment induces recovery of IJs from the infective stage into feeding stages and the presence of antibiotics eliminates bacteria, including symbiotic Xenorhabdus spp., from the suspension. After 16–20 h, these suspensions were added to sterile liver-kidney plates (∼10 ml of liver-kidney medium per 6 cm plate) and dried in a laminar flow hood until most excess liquid had evaporated. Cultures of each species produced a new generation of IJs within 30 d (two to three nematode generations) and IJs were harvested by placing the original 6 cm Petri plate within a sterile 15 cm pyrex Petri dish filled with sterile dH2O.
| Bacterial or nematode strain | Features | Source or reference |
|---|---|---|
| X. nematophila strains | ||
| HGB007 | X. nematophila ATCC 19061 | ATCC |
| HGB081 | X. nematophila AN6 Rifr | Steve Forst |
| HGB151 | HGB007 rpoS::kan | Vivas and Goodrich-Blair (2001) |
| HGB644 | HGB081 aroA1::Tn5-kan, gfp+ | Martens et al. (2005) |
| HGB645 | HGB081 serC1::Tn5-kan, gfp+ | Martens et al. (2005) |
| HGB692 | HGB081 aroE5::Tn5-cml, gfp+ | Martens et al. (2005) |
| Steinernema spp. strains | ||
| S. carpocapsae strain All | Host of X. nematophila | Harry Kaya |
| S. siamkaya | Host of uncharacterized Xenorhabdus sp. | Harry Kaya |
| S. karii | Host of uncharacterized Xenorhabdus sp. | Harry Kaya |
| S. riobrave | Host of Xenorhabdus sp. USTX62 | Harry Kaya |
| S. glaseri | Host of X. poinarii | Harry Kaya |
| S. feltiae | Host of X. bovienii | Harry Kaya |
Microscopic observation of IJs
All Steinernema spp. IJs were prepared for microscopy as previously described for S. carpocapsae (Martens et al., 2003b). Levamisole (1 mg ml−1) was added to 2% agarose as a nematode paralisant in all experiments shown with the exception of Fig. 2E, where sodium azide (10 mM) was used instead. Microscopy was performed on a Nikon Eclipse TE300 inverted microscope. Fluorescence microscopy of GFP, DAPI, FITC and tetramethylrhodamine isothiocyanate (TRITC) containing samples was performed using FITC, TRITC and triple-band DAPI/FITC/TRITC filter sets (Chroma, Brattleboro, VT; items ♯31001, ♯31002 and ♯82000). Images were recorded electronically using either ORCA or Cool Snap HQ digital cameras (Hamamatsu, Hamamatsu City, Japan, and Roper Scientifics, Munich, Germany). Images and time-lapse movies were recorded on a personal computer using MetaMorph versions 4.5 and 6.2 software (Universal Imaging Corporation, West Chester, PA) and processed for publication using Adobe Photoshop 7.0 and Adobe Illustrator 10.0. Time-lapse movies presented as Movies S1–3 in Supplementary material are at ×6 speed.
Infective juvenile intestinal dissection and histochemistry
Disgorged IJ intestines were released from intact IJs using a modification of the previously described guillotine procedure (Vivas and Goodrich-Blair, 2001). Briefly, axenic IJs were washed in dH2O, resuspended to ∼1500 IJ ml−1 and 15 µl of this suspension was added to each well of a 10-well glass slide. A ♯11 disposable scalpel blade (Feather, Osaka, Japan) was used under a dissecting microscope to sever each IJ head within the pharyngeal region and release (disgorge) the nematode intestine. Slide wells containing disgorged IJ intestines were covered with coverslips and edges sealed with clear nail polish. For histochemical staining of disgorged intestines, DAPI (1 µg ml−1) was added directly to 1 ml of washed IJ suspension immediately before disgorging intestines.
IVS release from axenic IJs and histochemistry
Axenic S. carpocapsae IJs for IVS release experiments were produced in vitro on lawns of HGB151 grown on LA medium (Vivas and Goodrich-Blair, 2001). Emergent IJs were collected in distilled water and ex-sheathed by treatment with 0.25% HOCl for 1 min followed by washing with excess sterile dH2O (Vivas and Goodrich-Blair, 2001). Ex-sheathed IJs were resuspended in sterile 1× PBS to which antibiotics had been added at the same concentrations described above for liver-kidney culture, except that amphotericin B (Sigma) was also added at 10 µg ml−1 to retard fungal growth. IJs were stored in antibiotic-treated PBS (∼1–2 × 104 IJ ml−1) for at least 1 week to decrease the possibility of microbial contamination. In preliminary experiments, we determined that diluted and heat-denatured M. sexta haemolymph induces efficient recovery (induction of bacterial release via defecation and development into feeding stages) of S. carpocapsae IJs; similar to reports for the entomopathogenic nematode Heterorhabditis bacteriophora (Ciche and Ensign, 2003) (data not shown). M. sexta haemolymph was collected from fifth-instar larvae as previously described and preserved with 5 mM glutathione (Orchard and Goodrich-Blair, 2004). Aliquots of preserved haemolymph were individually heated at 100°C for 5 min in 1.5 ml eppendorf tubes, cooled on ice for 5 min, microcentrifuged at 16 000 g for 5 min to remove precipitated material and pooled before filtration through a sterile 0.22 µm filter.
To induce IVS release, IJs were concentrated ∼10-fold in their existing antibiotic-treated PBS by gravity sedimentation and mixed with denatured M. sexta haemolymph to achieve a final haemolymph concentration of 0.25×. IJ suspensions were incubated in diluted haemolymph in 50 ml tissue culture flasks at room temperature and with gentle agitation on an orbital shaker. Treated IJs generally demonstrated signs of recovery after 24 h, but IVS release increased with time and samples were collected at 48 h after induction.
For fluorescent-lectin histochemistry on released IVS, 1.5 ml samples of recovering IJs were removed from the flask and individually mixed with FITC- or TRITC-labelled lectins at a final concentration of 50 µg ml−1 (with the exception of FITC-WGA which was used at 25 µg ml−1). Lectins tested included FITC-WGA, FITC-PNA, FITC-UEA-1, FITC-ConA and TRITC-SBA (all lectins were from Sigma). Lectin-treated samples were incubated at room temperature for 15 min and microcentrifuged briefly at 1100 g, after which a 40 µl sample of the loosely pelleted material was added to a 2% agarose-levamisole slide. A coverslip was added, allowed to dry until coverslip was tight and microscopy performed.
Acknowledgements
We would like to thank Dr Harry Kaya (University of California-Davis) for providing isolates of S. siamkaya, S. feltiae, S. karii, S. riobrave and S. glaseri. We also thank Dr Walter Goodman (University of Wisconsin-Madison, Department of Entomology) for providing M. sexta eggs, and Charles Cowles for suggesting the IVS release in insect haemolymph experiment. This work was supported by NIH Grant GM59776, NSF Grant IBN0416783 and USDA/CREES Grant CRHF-0-6055 awarded to H.G.-B. E.C.M. was supported during some of this work by an Ira L. Baldwin Distinguished Predoctoral Fellowship, awarded by the Department of Bacteriology, University of Wisconsin-Madison.




