Enterohaemorrhagic and enteropathogenic Escherichia coli use different mechanisms for actin pedestal formation that converge on N-WASP
Summary
Enteropathogenic Escherichia coli (EPEC) and enterohaemorrhagic E. coli (EHEC), two closely related diarrhoeagenic pathogens, induce actin rearrangements at the surface of infected host cells resulting in the formation of pseudopod-like structures termed pedestals beneath intimately attached bacteria. We have shown previously that N-WASP, a key integrator of signalling pathways that regulate actin polymerization via the Arp2/3 complex, is essential for pedestal formation induced by EPEC using N-WASP-defective cell lines. Here we show that actin pedestal formation initiated by EHEC also depends on N-WASP. Amino acid residues 226–274 of N-WASP are both necessary and sufficient to target N-WASP to sites of EHEC attachment. The recruitment mechanism thus differs from that used by EPEC, in which amino-terminal sequences of N-WASP mediate recruitment. For EPEC, recruitment of N-WASP downstream of Nck has been postulated to be mediated by WIP. However, we find a direct interaction of N-WASP with WIP to be dispensable for EPEC-induced pedestal formation and present data supporting an F-actin-dependent localization of WIP to actin pedestals induced by both EPEC and EHEC. In summary, our data show that EPEC and EHEC use different mechanisms to recruit N-WASP, which is essential for actin pedestal formation induced by both pathogens.
Introduction
Enteropathogenic Escherichia coli (EPEC) and enterohaemorrhagic E. coli (EHEC) belong to the attaching and effacing family of enteropathogens that cause severe diarrhoeal disease in humans and animals and pose a significant risk to human health worldwide (Nataro and Kaper, 1998). Central to both EPEC- and EHEC-mediated disease is their striking mechanism of intestinal colonization termed attaching and effacing (A/E), which results in the formation of unique histopathological lesions. A/E lesions are characterized by a localized loss of microvilli (effacement), intimate adherence of the bacteria to the cell surface followed by recruitment of a cellular actin assembly machinery to sites of bacterial attachment, resulting in the formation of actin-rich pseudopod-like structures termed pedestals to which the bacteria intimately adhere (Ulshen and Rollo, 1980; Moon et al., 1983; Tzipori et al., 1986). A/E lesion formation is thought to be critical in mediating diarrhoea in the host, but its exact role in disease is unknown (Donnenberg et al., 1993; McKee et al., 1995; Tzipori et al., 1995; Abe et al., 1998; Dean-Nystrom et al., 1998; Marches et al., 2000; Tacket et al., 2000). Similar A/E lesions are produced by EPEC and EHEC in tissue culture cell lines, which allows the definition of the molecular mechanisms used by these pathogens to induce cytoskeletal rearrangements (Knutton et al., 1989).
The genes necessary for A/E lesion formation in EPEC and EHEC map to a 35 kb chromosomal pathogenicity island, designated the locus of enterocyte effacement (LEE) (McDaniel et al., 1995; McDaniel and Kaper, 1997; Perna et al., 1998). Although EPEC and EHEC produce highly similar lesions, EPEC in the small intestine and EHEC in the large intestine (Hart et al., 1993), pedestal composition (Goosney et al., 2001) and the molecular mechanisms of pedestal formation used by EPEC versus EHEC differ (Campellone and Leong, 2003).
By type III secretion, both pathogens deliver their own receptor, the translocated intimin receptor (Tir, EspE), which binds to the bacterial surface protein intimin, into the underlying host cell (Kenny et al., 1997; Deibel et al., 1998). Whereas EPEC-induced pedestal formation requires phosphorylation of Tir on tyrosine 474 (Kenny et al., 1997; Kenny, 1999), enabling recruitment of the cellular signalling adaptor protein Nck necessary for actin assembly (Gruenheid et al., 2001; Campellone et al., 2002), EHEC-initiated pedestal formation occurs independently of Tir tyrosine phosphorylation (Deibel et al., 1998; DeVinney et al., 1999) and Nck (Gruenheid et al., 2001).
The ubiquitously expressed N-WASP, a member of the Wiskott–Aldrich syndrome protein (WASP)/Scar family of cellular actin nucleation-promoting factors, regulates actin polymerization by activating the Arp2/3 complex, the best characterized nucleator of actin filaments, in response to multiple upstream signals (Caron, 2002; Welch and Mullins, 2002). N-WASP has a highly modular domain structure (see Fig. 2M). The C-terminal WA domain binds directly to and activates the Arp2/3 complex (Rohatgi et al., 1999), whereas the remaining domains are involved in targeting and regulating N-WASP activity. Thus, the WASP homology 1 (WH1) domain of N-WASP binds the proline-rich, F-actin- and Nck-binding protein WIP (WASP interacting protein) (Anton et al., 1998; Moreau et al., 2000; Martinez-Quiles et al., 2001; Vetterkind et al., 2002), which is thought to target N-WASP to vaccinia virus to drive actin-based motility of this pathogen (Moreau et al., 2000).
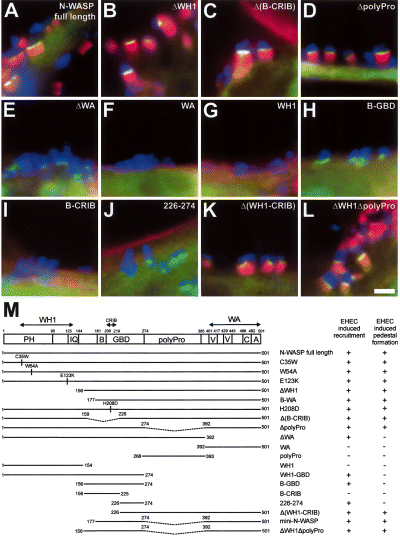
EHEC recruit N-WASP via amino acids 226–274 and depend on its WA domain for actin pedestal formation. A–L. Merged immunofluorescence micrographs showing EHEC O157:H7-infected N-WASP-defective (del/del) cells ectopically expressing GFP-tagged N-WASP proteins as indicated. EHEC are shown in blue, F-actin labelled with phalloidin in red and the GFP-tagged N-WASP proteins in green. Yellow colouration in (A–D), (K) and (L) indicates overlapping stainings of the respective GFP-tagged mutants and F-actin. The domain structure of N-WASP and an overview of the GFP-tagged N-WASP proteins used in this study are depicted in (M). Recruitment to sites of EHEC attachment and reconstitution of pedestal formation by the respective mutants are indicated. Pedestal formation in N-WASP-defective cells is restored upon expression of GFP-tagged full-length N-WASP (A, see also Fig. 1F), as well as by expression of GFP-tagged N-WASP mutants ΔWH1 (B), Δ(B-CRIB) (C), ΔpolyPro (D), Δ(WH1-CRIB) (K) and ΔWH1ΔpolyPro (L). Deletion of the WA domain (ΔWA) does not affect recruitment of N-WASP to sites of EHEC attachment, although the respective mutant is unable to restore pedestal formation (E). The WA domain (F), the WH1 domain (G) and the mutant B-CRIB (I) are not recruited to sites of EHEC attachment, whereas recruitment is clearly detectable for the mutant (B-GBD) (H) and for residues 226–274 of N-WASP (J). PH, pleckstrin homology domain; IQ, calmodulin-binding motif; B, basic domain; GBD, GTPase-binding domain; CRIB, Cdc42 and Rac interactive binding motif; polypro, polyproline domain, V, verprolin homology domain; C, cofilin homology domain; and A, acidic domain. Bar (2 µm) in (L) is valid for (A–L).
Intramolecular interactions cause autoinhibiton of N-WASP, which is relieved in in vitro experiments upon interactions with several upstream activators. Thus, the phospholipid phosphatidylinositol-(4,5)-bisphosphate (PIP2) and the small Rho family GTPase Cdc42 were shown in vitro to activate N-WASP synergistically by binding to the basic domain of N-WASP and to the CRIB (Cdc42 and Rac interactive binding) motif present within the GTPase-binding domain (GBD) of N-WASP respectively (Rohatgi et al., 1999; 2000; Prehoda et al., 2000). In addition, interaction of Src homology 3 (SH3) domain-containing proteins such as Grb2 or Nck with the polyproline domain (polyPro) of N-WASP also stimulates Arp2/3 activation by N-WASP in vitro (Carlier et al., 2000; Rohatgi et al., 2001).
We have shown previously that N-WASP is essential for pedestal formation induced by EPEC (Lommel et al., 2001), confirming earlier data implicating WASP/N-WASP in this process (Kalman et al., 1999). Given the aforementioned differences between EPEC- and EHEC-induced pedestal formation, the goal of the present study was to examine whether N-WASP mediates pedestal formation initiated by EHEC and, if so, to define the molecular mechanism used by EHEC for its recruitment, compared with EPEC-induced pedestal formation.
Results
N-WASP is essential for actin pedestal formation by EHEC O157:H7
We have shown previously that EPEC pedestal forma-tion depends on N-WASP using corresponding pairs of N-WASP-expressing and N-WASP-defective embryonic fibroblast cell lines. N-WASP-expressing so-called precursor lines (N-WASPflox/flox) were obtained by immortalization of embryonic fibroblasts isolated from mice carrying loxP-flanked N-WASP alleles allowing conditional inactivation of the N-WASP gene. From these precursor lines, corresponding N-WASP-defective fibroblast cell lines (N-WASPdel/del) carrying disrupted N-WASP alleles were derived by transient expression of Cre recombinase as described previously (Lommel et al., 2001). To determine whether EHEC-induced pedestal formation also requires N-WASP despite the differences between EPEC and EHEC regarding Tir tyrosine phosphorylation and requirement of Nck, we infected N-WASP-expressing precursor and their corresponding N-WASP-defective embryonic fibroblast cell lines with EHEC O157:H7 strains 86/24 or EDL933. As expected, both EHEC strains readily induced the formation of pedestals beneath adherent bacteria in the N-WASP-expressing precursor cells (Fig. 1A, C and E), and the endogenous N-WASP protein was found at the pedestal tips using a polyclonal antibody against N-WASP (Fig. 1E). In contrast, N-WASP-defective cells were unable to form pedestals upon attachment of these EHEC strains (Fig. 1B and D). However, ectopic expression of green fluorescent protein (GFP)-tagged full-length N-WASP in N-WASP-defective fibroblasts fully restored pedestal formation induced by EHEC (1, 2), and the GFP–N-WASP fusion protein was efficiently recruited to and focused at the very tip of the reconstituted pedestals (1, 2), identical to the recruitment pattern of endogenous N-WASP in pedestals of precursor cells (compare Fig. 1E and F). Collectively, these data demonstrate that, as for EPEC, N-WASP is essential for actin pedestal formation initiated by EHEC.
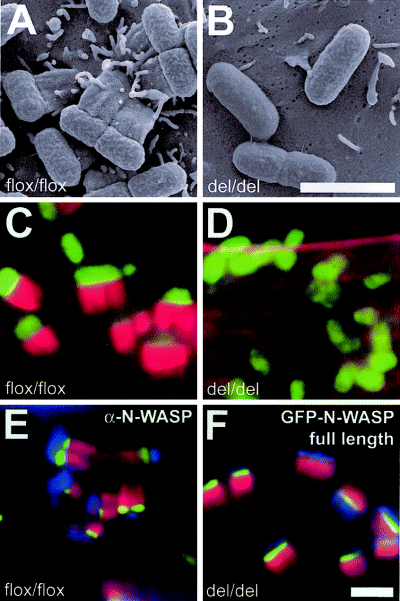
Actin pedestal formation by EHEC O157:H7 requires N-WASP. Scanning electron (A and B) and merged immunofluorescence micrographs (C–F) showing infection of N-WASP-expressing precursor (flox/flox) (A, C and E) and N-WASP-defective cells (del/del) (B, D and F) with EHEC O157:H7. For immunofluorescence, EHEC were labelled with anti-EspE antibody (C, D and F) or DAPI (E) and are shown in green in (C and D) and in blue (E and F). F-actin labelled with phalloidin is shown in red in (C–F). Endogenous N-WASP labelled with a polyclonal antibody (E) and GFP-tagged N-WASP (F) are shown in green. EHEC induces the formation of actin pedestals on the surface of N-WASP-expressing precursor cells (A, C and E), whereas N-WASP-defective cells are resistant to EHEC-induced pedestal formation, although the bacteria readily adhere to the cell surface (B and D). Pedestal formation in N-WASP-defective cells is restored upon expression of GFP-tagged full-length N-WASP, which localizes tightly focused at the tip of pedestals (F), identical to endogenous N-WASP in N-WASP-expressing cells (E). Bars (2 µm) in (B) and (F) are valid for (A) and (B) and (C–F) respectively.
Residues 226–274 of N-WASP mediate its recruitment to EHEC attachment sites
To establish which domain/s of N-WASP mediate (i) recruitment to the site of bacterial attachment and (ii) drive actin assembly leading to pedestal formation induced by EHEC, we expressed a series of point and deletion mutants of N-WASP tagged with GFP in N-WASP-defective cells (depicted and summarized in Fig. 2M) and assessed recruitment and the ability to restore pedestal formation for each mutant.
Besides full-length N-WASP (1, 2), N-WASP mutants unable to interact with WIP, such as the mutants ΔWH1 (Fig. 2B and M) or B-WA (Fig. 2M), both of which lack the WH1 domain necessary for WIP interaction, or WH1 point mutants that also abolish direct interaction with WIP (Moreau et al., 2000; Zettl and Way, 2002) (C35W, W54A, E123K; Fig. 2M), all targeted to and fully restored pedestal formation induced by EHEC in N-WASP-defective cells.
In addition, a mutant carrying a point mutation within the CRIB motif (H208D; Fig. 2M), previously demonstrated to be unable to interact with the small Rho family GTPase Cdc42 (Miki et al., 1998; Moreau et al., 2000; Lommel et al., 2001), as well as a mutant lacking both the basic domain (B) and the CRIB motif [Δ(B-CRIB); Fig. 2C and M] targeted to and fully reconstituted EHEC-initiated pedestal formation, demonstrating that a direct interaction of N-WASP with Cdc42 is dispensable for EHEC-induced pedestal formation, as was demonstrated previously for EPEC (Lommel et al., 2001). Pedestal formation was also completely restored by an N-WASP mutant lacking the polyproline region (ΔpolyPro; Fig. 2D and M). In contrast, C-terminally truncated N-WASP mutants lacking the WA domain were unable to restore pedestal formation (e.g. ΔWA; Fig. 2E and M), proving the requirement for N-WASP to interact with and activate the Arp2/3 complex to initiate pedestal formation. Nevertheless, the latter mutant proteins readily recruited to the sites of EHEC attachment as long as they contained amino acid residues 226–274 of N-WASP. Thus, the mutants ΔWA (Fig. 2E and M), WH1-GBD (Fig. 2M), B-GBD (Fig. 2H and M) and 226–274 (Fig. 2J and M) all clearly targeted to sites of EHEC attachment, whereas the mutants WH1 (Fig. 2G and M), B-CRIB (Fig. 2I and M) and polyPro (Fig. 2M) were not recruited to sites of EHEC adherence and displayed only diffuse cytoplasmic localization indistinguishable from that observed in control cells expressing GFP alone (not shown). As expected, although necessary for actin assembly, the WA domain itself was not recruited to sites of EHEC attachment (Fig. 2F and M). Finally, mutants containing residues 226–274 and the WA domain, but lacking either both the WH1 domain and the CRIB motif (ΔWH1-CRIB; Fig. 2K and M) or both the WH1 and polyproline domains [ΔWH1ΔpolyPro (Fig. 2L and M) and mini-N-WASP (Fig. 2M)] also restored pedestal formation induced by EHEC. Based on these data, we conclude that N-WASP is recruited to EHEC attachment sites via amino acid residues 226–274 and mediates actin polymerization by activating the Arp2/3 complex via its WA domain.
Differential recruitment of Nck1 and Nck2 to EPEC versus EHEC host cell interaction sites
Absence of both Nck family members (Nck1 and Nck2) had resulted in a failure of N-WASP to target to sites of EPEC attachment in experiments using Nck-deficient cells (Gruenheid et al., 2001), placing N-WASP downstream of Nck in EPEC-initiated pedestal formation. When we tested the localization of Nck1 (Fig. 3A and B) and Nck2 (Fig. 3E and F), both tagged with GFP in N-WASP-expressing (Fig, 3A and E) and N-WASP-defective fibroblasts (Fig, 3B and F) infected with EPEC O127:H6, we found that they both accumulated very prominently at sites of EPEC attachment in the presence and absence of pedestal-associated F-actin assembly, confirming Nck to be upstream of N-WASP in EPEC-initiated pedestal formation.
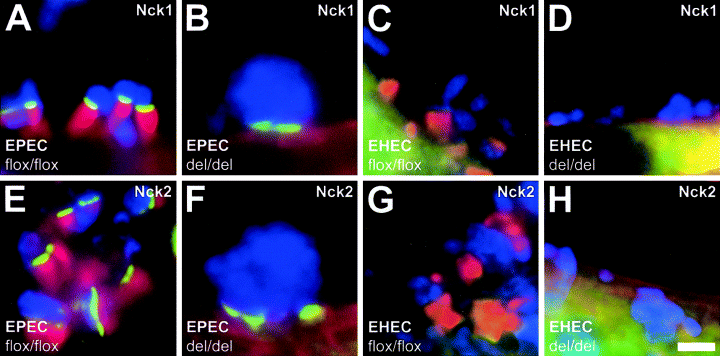
Differential recruitment of Nck1 and Nck2 in EPEC versus EHEC pedestal formation. A–H. Merged immunofluorescence micrographs showing EPEC O127:H6- and EHEC O157:H7-infected N-WASP-expressing (flox/flox) and N-WASP-defective (del/del) cells ectopically expressing GFP-tagged Nck1 or 2 as indicated. Bacteria are shown in blue, F-actin labelled with phalloidin in red and GFP–Nck fusion proteins in green. GFP-tagged Nck is strongly recruited to sites of EPEC attachment in the presence (A and E) and in the absence of pedestal-associated F-actin assembly (B and F) in N-WASP-expressing and N-WASP-defective cells respectively. In contrast, although GFP-tagged Nck recruits weakly to the tips of pedestals induced by EHEC in N-WASP-expressing cells (C and G), no recruitment to sites of EHEC attachment is detected in the absence of N-WASP in N-WASP-defective cells (D and H). Bar 2 µm in (H) is valid for (A–H).
In contrast, we did not detect any recruitment of either Nck1 or Nck2, both tagged with GFP, to sites of EHEC attachment in N-WASP-defective cells (Fig. 3D and H respectively), and only very weak recruitment to the tips of EHEC pedestals in N-WASP-expressing cells (Fig. 3C and G respectively). Together with the fact that EHEC-induced pedestals form robustly in cells deficient for both Nck1 and Nck2 (Gruenheid et al., 2001), these data argue against a significant role for Nck in pedestal formation by EHEC. The very weak recruitment to EHEC pedestal tips not observed earlier (Campellone et al., 2002; Campellone and Leong, 2003) must occur downstream of N-WASP recruitment, because no enrichment of Nck was detectable in the absence of N-WASP and pedestal formation (Fig. 3C, D, G and H).
Direct interaction of N-WASP with WIP is dispensable for recruitment of N-WASP to sites of EPEC attachment downstream of Nck
WIP was proposed to link Nck to N-WASP in EPEC-induced pedestal formation (Welch and Mullins, 2002; Campellone and Leong, 2003), by analogy with the sequence of events during N-WASP recruitment to vaccinia virus (Moreau et al., 2000). To test whether WIP functions downstream of Nck to mediate recruitment of N-WASP to sites of EPEC attachment, we examined the localization of GFP-tagged WIP in EPEC-infected N-WASP-expressing and -defective cells. We were unable to detect any specific enrichment of GFP-tagged WIP at sites of EPEC attachment in the absence of pedestal-associated F-actin assembly in N-WASP-defective cells (Fig. 4B). In N-WASP-expressing cells, GFP-tagged WIP was recruited prominently to EPEC pedestals, but localized along the entire pedestal length (Fig. 4A), with the GFP-WIP fluorescence mirroring phalloidin-stained F-actin. This is contrary to the tightly focused localization of GFP-tagged Nck (Fig. 3A and E) and N-WASP (Fig. 4F) at the very tip of EPEC pedestals. In addition, GFP-tagged WIP also localized along the entire length of pedestals initiated by EHEC (Fig. 4C), where neither Nck (Gruenheid et al., 2001) nor WIP (this study) seem to be involved in recruitment of N-WASP.
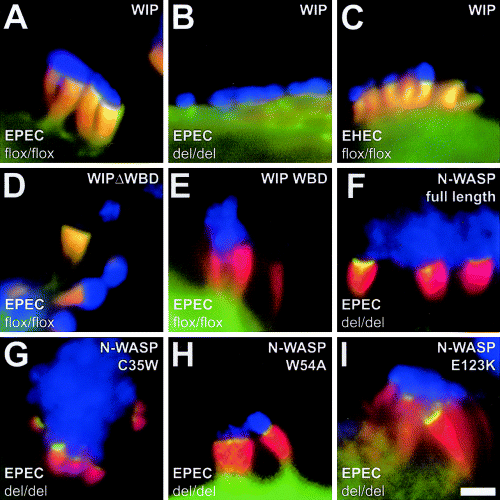
Direct interaction of N-WASP with WIP is dispensable for recruitment of N-WASP to sites of EPEC attachment downstream of Nck. A–I. Merged immunofluorescence micrographs showing EPEC O127:H6- and EHEC O157:H7-infected N-WASP-expressing (flox/flox) and N-WASP-defective (del/del) cells ectopically expressing GFP-tagged WIP or N-WASP fusion proteins as indicated. Bacteria are shown in blue, F-actin labelled with phalloidin in red and GFP fusion proteins in green. Yellow colouration indicates a complete overlap of the respective GFP-tagged protein and F-actin stainings along entire pedestals (A, C and D) or at the pedestal tips (F–I). GFP-tagged WIP is prominently recruited to pedestals induced by EPEC in N-WASP-expressing cells but, in contrast to the tightly focused localization of Nck at pedestal tips (Fig. 3A and E), it localizes along the entire length of pedestals (A). Note that its distribution within pedestals strongly resembles that of F-actin, with overlapping GFP-WIP and F-actin stainings indicated in yellow. In the absence of pedestal-associated F-actin assembly in N-WASP-defective cells, GFP-tagged WIP fails to target to sites of EPEC attachment (B). GFP-tagged WIP also localizes to entire pedestals induced by EHEC in N-WASP-expressing cells (C). Localization to entire pedestals induced by EPEC is also observed for GFP-tagged WIPΔWBD (D), in contrast to GFP-tagged WIP WBD (E), for which no enrichment is observed in pedestals compared with the cell body. Note that neither WIPΔWBD (D) nor WIP WBD (E) exerts any dominant-negative effect on pedestal formation induced by EPEC in N-WASP-expressing cells. EPEC-induced pedestal formation in N-WASP-defective cells is fully restored upon expression of GFP-tagged full-length N-WASP (F), as well as upon expression of N-WASP WH1 point mutants C35W (G), W54A (H) and E123K (I) that are unable to interact with WIP (see Fig. 2M for depiction of mutants). Bar in (I) equals 2 µm and is valid for (A–I).
In contrast to what might have been expected if WIP mediated recruitment of N-WASP to sites of EPEC attachment, pedestal formation induced by EPEC (Fig. 4D and E) and EHEC (not shown) was not affected by expression of either GFP-tagged WIP lacking its WASP-binding domain (WIPΔWBD) (Fig. 4D) or the isolated GFP-tagged WIP WASP-binding domain (WIP WBD) (Fig. 4E). In the vaccinia system, the linear recruitment cascade Nck–WIP–N-WASP is interrupted upon overexpression of the above mutant WIP WBD (Moreau et al., 2000). As observed for full-length WIP (Fig. 4A and C), WIPΔWBD localized along the entire length of pedestals induced by EPEC (Fig. 4D) and EHEC (not shown), whereas no significant enrichment in EPEC-induced pedestals compared with the cell body was detectable for WIP WBD (Fig. 4E).
Finally, to test more directly the proposed contribution of a direct interaction of N-WASP with WIP for recruitment of N-WASP and reconstitution of EPEC-induced pedestal formation in N-WASP-defective cells, we transfected these cells with GFP-tagged N-WASP constructs carrying point mutations within the WH1 domain (C35W, W54A and E123K; depicted in Fig. 2M) that are unable to interact with WIP (Moreau et al., 2000; Zettl and Way, 2002) and looked for reconstitution of pedestal formation upon infection with EPEC. We found that all three mutants [C35W (Fig. 4G), W54A (Fig. 4H) and E123K (Fig. 4I)] restored pedestal formation initiated by EPEC and recruited to and were tightly focused at the very tip of pedestals as GFP-tagged full-length N-WASP (Fig. 4F). We therefore conclude that a direct interaction of N-WASP with WIP is dispensable for EPEC-induced pedestal formation. Taken together, these data strongly suggest an F-actin-dependent localization of WIP to pedestals induced by both EPEC and EHEC, independent of direct interaction with Nck or N-WASP, and argue against a significant role for WIP in recruitment of N-WASP downstream of Nck during EPEC-induced pedestal formation.
WASP can compensate for N-WASP in actin pedestal formation by EPEC and EHEC
Wiskott–Aldrich syndrome protein (WASP), which is expressed exclusively in the haematopoietic system, has been reported to be able to substitute for N-WASP in actin-based motility of vaccinia virus, but not of Shigella flexneri (Snapper et al., 2001). As WASP is absent from the cell lines used in this study (Benesch et al., 2002), we ectopically expressed GFP-tagged human WASP in N-WASP-defective cells and tested whether WASP can compensate for N-WASP in actin pedestal formation induced by EPEC O127:H6 and EHEC O157:H7. Although the recruitment mechanism of N-WASP differs between the two pathogens, both EPEC- and EHEC-induced pedestal formation were restored upon expression of GFP-tagged WASP (Fig. 5A and B), demonstrating that WASP can compensate for N-WASP in EPEC- and EHEC-induced pedestal formation.
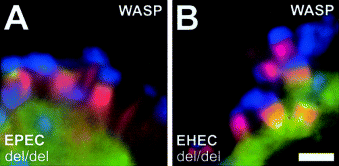
WASP can substitute for N-WASP in actin pedestal formation by EPEC and EHEC. A and B. Immunofluorescence micrographs showing EPEC O127:H6- (A) and EHEC O157:H7 (B)-infected N-WASP-defective (del/del) cells ectopically expressing GFP-tagged WASP. Bacteria are shown in blue, F-actin labelled with phalloidin in red and GFP–WASP in green. EPEC- and EHEC-induced pedestal formation in N-WASP-defective cells is restored upon expression of GFP-tagged WASP. Bar (2 µm) in (B) is valid for (A) and (B).
Discussion
Enterohaemorrhagic and enteropathogenic E. coli are examples of an increasing number of pathogens that have evolved mechanisms to overcome and subvert for their own benefit the cellular regulatory systems that control actin polymerization. Studying interactions of such pathogens with their host cells has enabled valuable insights into the regulation of cellular actin dynamics.
Most of these pathogens have evolved mechanisms to target signalling pathways that regulate actin assembly via the Arp2/3 complex by intervening at different steps of the signalling cascade leading to its activation. Whereas Listeria monocytogenes directly recruits and activates the Arp2/3 complex, S. flexneri and several other pathogens have found ways to target the cellular nucleation promoting factor N-WASP, either directly, as is the case for S. flexneri, or indirectly by exploiting cellular factors that function upstream of N-WASP in targeting and regulating its activity, as is the case for vaccinia virus and EPEC (reviewed by Frischknecht and Way, 2001).
In contrast to other intracellular pathogens, EPEC and EHEC remain largely extracellular and redirect the host actin assembly machinery to form elongated pedestals at the surface of infected cells. In this respect, they serve as model systems to analyse signalling to the actin cytoskeleton across the plasma membrane in response to external stimuli.
In the present study, we have demonstrated that EHEC-induced pedestal formation depends on N-WASP to drive actin assembly. This was not necessarily to be expected, given the known differences between EHEC- and EPEC-induced pedestal formation regarding Tir tyrosine phosphorylation, requirement of Nck and additional differences in pedestal composition (Campellone and Leong, 2003).
Using a series of N-WASP point and deletion mutants, we have identified the minimal domain requirements and targeting sequences of N-WASP during EHEC-induced pedestal formation. As for EPEC, the WA domain of N-WASP is essential to drive actin assembly during EHEC-induced pedestal formation, which proves the requirement for N-WASP to interact with and activate the Arp2/3 complex to restore pedestal formation. Nevertheless, the WA domain itself is not sufficient to reconstitute EHEC-induced pedestal formation, as it fails to recruit to sites of bacterial attachment.
In the case of EPEC, targeting of N-WASP involved sequences in the amino-terminal half of the protein (Lommel et al., 2001). In contrast, here, we have demonstrated amino acid residues 226–274 of N-WASP to be necessary and sufficient for its recruitment to sites of EHEC attachment. Thus, both EPEC and EHEC require N-WASP for actin pedestal formation, but use different mechanisms for its recruitment.
Amino acid residues 226–274 of N-WASP comprise the C-terminal half of the GTPase-binding domain (GBD). As Rho family GTPases such as Cdc42 require the CRIB motif (N-WASP residues 200–219) within the GBD of N-WASP/WASP for direct interaction (Symons et al., 1996; Rudolph et al., 1998; Abdul-Manan et al., 1999; Hoffman and Cerione, 2000; Prehoda et al., 2000), it seems unlikely that small GTPases are involved in targeting N-WASP to sites of EHEC attachment. In agreement with such a view, two N-WASP mutants carrying either a point mutation in or a deletion of the CRIB motif still targeted to and fully reconstituted EHEC-induced pedestal formation, suggesting that Rho family GTPases are dispensable for this process, as has been shown for EPEC-induced pedestal formation (Ben-Ami et al., 1998; Lommel et al., 2001).
Thus, the factor(s) interacting with amino acid residues 226–274 of N-WASP likely to mediate recruitment of N-WASP in EHEC pedestal formation still await identification. Tyrosine 253 in murine N-WASP (tyrosine 291 in human WASP) has been reported to be a site of tyrosine phosphorylation by src family kinases (Cory et al., 2002; Suetsugu et al., 2002; Torres and Rosen, 2003) and, upon phosphorylation, may serve as a docking site for SH2 domain-containing proteins. However, recruitment of src family kinases has not been demonstrated up to now for EHEC (Goosney et al., 2001), and the src family kinase inhibitors PP1 and PP2 did not affect EHEC-induced pedestal formation (our own unpublished results). Moreover, an N-WASP mutant lacking the phosphorylation site by exchange of tyrosine 253 for phenylalanine (Y253F) recruited and fully restored pedestal formation (our own unpublished results), and absence of antiphosphotyrosine immunolabelling beneath adherent EHEC (Ismaili et al., 1995; Deibel et al., 1998; DeVinney et al., 1999; our own unpublished results) suggests that EHEC-induced pedestal formation does not involve tyrosine phosphorylation.
As an alternative to recruitment of N-WASP via upstream cellular factors, EHEC may express a specific bacterial factor to mediate recruitment of N-WASP. This assumption is supported by the fact that EHEC Tir is unable to complement pedestal formation in an EPEC Tir deletion mutant (DeVinney et al., 2001), suggesting that EHEC-induced pedestal formation may require the delivery of one or more additional EHEC factors into the host cell. Thus, EHEC may use a bacterial factor that is able to interact directly with and recruit N-WASP via amino acid residues 226–274. Interestingly, we have previously delineated amino acid residues 226–274 as one of the two regions in N-WASP that mediate its targeting to surfaces of intracellular S. flexneri (Lommel et al., 2001). Shigella flexneri directly recruits and activates N-WASP via its surface protein IcsA/VirG (reviewed by Frischknecht and Way, 2001). This interaction is very specific, as WASP, the closest relative of N-WASP within the WASP/Scar family of nucleation promoting factors, the expression of which in vivo is restricted to the haematopoietic system (for a review, see Thrasher, 2002), has been unable to compensate for N-WASP in S. flexneri actin tail formation (Snapper et al., 2001). In contrast, in this study, we have demonstrated that WASP can substitute for N-WASP in EHEC-induced pedestal formation. Therefore, the bacterial factor, if existent, would have to be able to interact with both N-WASP and WASP, in contrast to S. flexneri IcsA/VirG. Amino acid residues 226–274 of murine N-WASP share 67% sequence identity and 81% sequence similarity with the corresponding sequences of human and murine WASP. Identification of the factor(s) that interact with amino acid residues 226–274 of N-WASP would broaden our understanding not only of EHEC pathogenicity, but also of the activation mechanisms of N-WASP and WASP in general.
In this study, WASP was also able to compensate for N-WASP in EPEC-induced pedestal formation. This is not surprising as EPEC target the signalling pathway leading to Arp2/3-mediated actin assembly upstream of N-WASP/WASP using Nck. Exactly how Nck mediates recruitment of N-WASP/WASP to sites of EPEC attachment is still unclear. In our previous experiments, targeting of N-WASP to sites of EPEC attachment required sequences in the amino-terminal half of the protein (Lommel et al., 2001), whereas the polyproline domain of N-WASP, capable of direct interaction with Nck in vitro (Rohatgi et al., 2001), did not by itself recruit to EPEC attachment sites (our own unpublished results). Therefore, in this study, we tested the hypothesis that WIP may serve to recruit N-WASP downstream of Nck during EPEC-induced pedestal formation by analogy with the sequence of events during recruitment of N-WASP to vaccinia virus (Frischknecht and Way, 2001). Although we confirmed Nck to be upstream of N-WASP in EPEC-induced pedestal formation, we did not find any evidence in support of a role for WIP in recruitment of N-WASP downstream of Nck. Instead, we found that the N-WASP WH1 mutants, C35W, W54A and E123K, which are unable to interact with WIP, recruited and restored EPEC-induced pedestal formation, demonstrating that a direct interaction of N-WASP with WIP is dispensable for pedestal formation induced by EPEC. This is contrary to the vaccinia system in which these N-WASP mutants were not recruited to the vaccinia surface (Moreau et al., 2000). Recently, two mammalian homologues of WIP, CR16 and WICH have been reported likewise to interact with N-WASP (Ho et al., 2001; Kato et al., 2002). As the N-WASP WH1 mutant W54A was demonstrated to block interaction not only with WIP, but also with CR16 and WICH (Zettl and Way, 2002), it is unlikely that these additional WIP family members interfered in our experiments.
In addition, whereas overexpression of the isolated WIP WASP-binding domain (WIP WBD) was found to block vaccinia actin tail formation (Moreau et al., 2000), neither WIP WBD nor WIP lacking its WASP-binding domain (WIPΔWBD) was observed to exert any dominant-negative effect on pedestal formation, contrary to what might have been expected if WIP mediated recruitment of N-WASP during EPEC-induced pedestal formation.
Importantly, we did not detect any specific recruitment of WIP to sites of EPEC attachment in the absence, and thus upstream, of N-WASP. In the presence of N-WASP, however, we found WIP to localize along the entire length of pedestals, comparable to the distribution of F-actin. Thus, its distribution differed completely from the tightly focused localization of Nck and N-WASP at the very tip of pedestals.
As WIP localized to pedestals only in the presence, and thus downstream, of N-WASP, one could speculate that localization of WIP to pedestals might be mediated by direct interaction with N-WASP, as has been reported for S. flexneri, in which the WIP WBD, but not WIPΔWBD, was recruited to actin tail-forming S. flexneri (Moreau et al., 2000). However, several of our observations argue against a recruitment of WIP downstream of N-WASP by direct interaction. The localizations of WIP and N-WASP within pedestals differed completely. Furthermore, WIP WBD was not recruited to pedestals. Moreover, deletion of the WIP WASP-binding domain did not affect WIP localization along the entire length of pedestals induced by both EPEC and EHEC. Instead, our data are consistent with the view that WIP prominently localizes along the entire length of pedestals induced by EPEC and EHEC dependent on its ability to bind F-actin and independent of direct interactions with N-WASP or Nck. Such an assumption is supported by the fact that WIP, as an F-actin-binding protein (Martinez-Quiles et al., 2001), can target to various actin cytoskeletal structures such as stress fibres (Vetterkind et al., 2002).
In summary, in this study using N-WASP-defective cells, we have demonstrated that EHEC-induced pedestal formation requires N-WASP, which is targeted to sites of EHEC attachment via amino acids 226–274 by an as yet unidentified cellular or bacterial factor. Furthermore, our data suggest an F-actin-dependent localization of WIP to pedestals independent of direct interactions with N-WASP or Nck and argue against a role for WIP in recruitment of N-WASP downstream of Nck in EPEC-induced pedestal formation. WIP prominently localizes to pedestals induced by both EPEC and EHEC. However, the potential role of WIP in these structures is still unclear. Given the fact that mammals have evolved three WIP family members, a better understanding of their potential functions may only come from multiple gene targeting experiments.
In conclusion, EPEC and EHEC represent further examples in the growing number of pathogens exploiting Arp2/3-mediated actin polymerization that have independently evolved distinct mechanisms to recruit the cellular nucleation promoting factor N-WASP to drive actin assembly.
Experimental procedures
Bacterial strains and infections
Strains used in this study were enteropathogenic E. coli strain E2348/69 (O127:H6) (Levine et al., 1978), enterohaemorrhagic E. coli strain 86/24 (O157:H7; isolated from an outbreak in Walla Walla, WA, USA; Griffin et al., 1988) and enterohaemorrhagic E. coli strain EDL933 (O157:H7) (American Type Culture Collection no. 700927; for genome sequence information, see Perna et al., 2001). Infections of cell monolayers with EPEC and EHEC were performed as described previously for EPEC (Lommel et al., 2001) with the modification that, for infections with EHEC, overnight cultures were diluted 1:50 into Dulbecco's modified Eagle medium (DMEM, low glucose) supplemented with 100 mM Hepes, pH 7.4, instead of plain DMEM for preactivation of bacteria before infection. GFP-tagged constructs were all tested in several independent infection experiments always using both strains 86/24 and EDL933 in parallel in the case of EHEC. In all analyses, the two EHEC strains always yielded the same results.
Cell lines, transfections and immunolabellings
N-WASPdel/del and their respective parental N-WASPflox/flox embryonic fibroblast precursor cell lines have been described previously (Lommel et al., 2001) and were cultured as described therein. For bacterial infection experiments and immunolabellings, cells were seeded onto 12 mm acid-washed glass coverslips coated with fibronectin (Roche Applied Science) at a concentration of 50 µg ml−1. Cells were transfected using FuGENE 6 (Roche Applied Science) for 14–24 h according to the manufacturer's instructions. Immunolabellings were performed essentially as described previously (Lommel et al., 2001). In brief, after infection, cells were fixed for 20 min with 4% paraformaldehyde in PBS and extracted with a mixture of 4% paraformaldehyde and 0.1% Triton X-100 in PBS for 1 min. Monoclonal antibody B51 against EspE (Deibel et al., 1998), kindly provided by T. Chakraborty (Giessen, Germany), was routinely used for detection of bacteria in combination with Alexa Fluor 350- or Alexa Fluor 488-conjugated goat anti-mouse antibodies (Molecular Probes). Affinity-purified anti-N-WASP rabbit polyclonal anti-body raised against residues 8–33 of murine N-WASP, the specificity of which was confirmed by immunoblotting (not shown), was used in combination with Alexa Fluor 488-conjugated goat anti-rabbit antibody for immunolabelling of N-WASP, in which case EHEC bacteria were detected by staining bacterial DNA with DAPI. Filamentous actin was stained with Alexa Fluor 594–phalloidin (Molecular Probes).
Expression constructs
EGFP-tagged murine full-length N-WASP and a set of mutants (see Fig. 2M), as well as EGFP-tagged human WASP and murine WIP, have been described previously (Lommel et al., 2001; Benesch et al., 2002). The EGFP-tagged N-WASP point mutants C35W, W54A and E123K were constructed with the QuikChange site-directed mutagenesis kit (Stratagene) and verified by sequencing, and correspond to mutants described by Moreau et al. (2000) and Zettl and Way (2002). GFP-tagged Nck1 and 2, the WASP-binding domain of human WIP (WIP WBD) and WIP lacking the WBD (WIPΔWBD) were kindly provided by Michael Way (London, UK) and have been described previously (Frischknecht et al., 1999; Moreau et al., 2000).
Fluorescence microscopy
Cells were observed with an inverted microscope (Axiovert 135TV; Zeiss) equipped with computer-controlled shutters (Optilas). Images were acquired with a back-illuminated cooled charge-coupled device camera (Princeton Research Instruments) driven by iplab software (version 3.1; Scanalytics) and processed on Macintosh G4 computers using iplab and Adobe photoshop 6.0 (Adobe Systems) software.
Scanning electron microscopy
Infected monolayers on coverslips were fixed with 5% formaldehyde, 2% glutaraldehyde in cacodylate buffer (0.1 M cacodylate, 0.01 M CaCl2, 0.01 M MgCl2, 0.09 M sucrose, pH 6.9) for 1 h on ice. After several washes with cacodylate buffer and TE buffer (20 mM Tris, 1 mM EDTA, pH 6.9), samples were dehydrated in serial dilutions of acetone (10%, 30%, 50%, 70%, 90% and 100%) on ice for 15 min each step. Samples were allowed to reach room temperature before another change of 100% acetone, after which they were subjected to critical-point drying with liquid CO2 (CPD030; Balzers). Samples were covered with an ≈ 10-nm-thick gold film by sputter coating (SCD040; Balzers) and examined in a field emission scanning electron microscope Zeiss DSM 982 Gemini using an Everhart Thornley SE detector and an in-lens detector in a 50:50 ratio at an acceleration voltage of 5 kV. Digital images were processed in Adobe photoshop 6.0 (Adobe Systems).
Acknowledgements
We thank Trinad Chakraborty for EPEC and anti-EspE antibody, Florian Gunzer for EHEC, Michael Way for Nck and WIP constructs, Brigitte Denker for excellent technical assistance, and Simon Mauch for critical reading of the manuscript. This work was supported in part by the Deutsche Forschungsgemeinschaft (SFB 621, Project B2) to K.R. and J.W. and the Fonds der Chemischen Industrie to J.W.




