Factors driving pathogenicity vs. prevalence of amphibian panzootic chytridiomycosis in Iberia
Abstract
Ecology Letters (2010) 13: 372–382
Amphibian chytridiomycosis is a disease caused by the fungus Batrachochytrium dendrobatidis (Bd). Whether Bd is a new emerging pathogen (the novel pathogen hypothesis; NPH) or whether environmental changes are exacerbating the host-pathogen dynamic (the endemic pathogen hypothesis; EPH) is debated. To disentangle these hypotheses we map the distribution of Bd and chytridiomycosis across the Iberian Peninsula centred on the first European outbreak site. We find that the infection-free state is the norm across both sample sites and individuals. To analyse this dataset, we use Bayesian zero-inflated binomial models to test whether environmental variables can account for heterogeneity in both the presence and prevalence of Bd, and heterogeneity in the occurrence of the disease, chytridiomycosis. We also search for signatures of Bd-spread within Iberia using genotyping. We show (1) no evidence for any relationship between the presence of Bd and environmental variables, (2) a weak relationship between environmental variables and the conditional prevalence of infection, (3) stage-dependent heterogeneity in the infection risk, (4) a strong association between altitude and chytridiomycosis, (5) multiple Iberian genotypes and (6) recent introduction and spread of a single genotype of Bd in the Pyrenees. We conclude that the NPH is consistent with the emergence of Bd in Iberia. However, epizootic forcing of infection is tied to location and shaped by both biotic and abiotic variables. Therefore, the population-level consequences of disease introduction are explained by EPH-like processes. This study demonstrates the power of combining surveillance and molecular data to ascertain the drivers of new emerging infections diseases.
Introduction
Patterns of infection are informative about the epidemiology and spatio-temporal spread of pathogens, and the role of environmental factors in forcing infection dynamics. Emergence of the chytrid fungus Batrachochytrium dendrobatidis (Bd) is now widely recognised as a proximate driver of amphibian declines across the globe (Berger et al. 1998; Fisher et al. 2009b). Following the relatively synchronous discovery of Bd in Australia (1993–1994), Panama (1996–1997) and Europe (1997), the occurrences of global amphibian declines attributed to chytridiomycosis have been accounted for by two non mutually-exclusive hypotheses. The novel/spreading pathogen hypothesis (NPH) states that the timing of disease-driven declines have resulted from the spread of Bd subsequent to its introduction into naïve host populations (Rachowicz et al. 2005; Skerratt et al. 2007). In Central America, for example, the sequential pattern of declines across a NW–SE trajectory are consistent with an introduction of Bd in the north of the region (Lips et al. 2008). The hypothesis of recent global spread is supported by the low genetic diversity exhibited by Bd and the lack of any strong support for phylogeographical clustering of genotypes collected from Africa, the Americas, Australia and Europe (James et al. 2009).
In contrast, the endemic pathogen hypothesis (EPH) states that the timing of declines is a consequence of changes in key environmental conditions that drive patterns of disease (Rachowicz et al. 2005). In the Neotropics and Europe, an increase in air temperatures has been linked to the timing of amphibian extinctions and declines (Pounds et al. 2006; Bosch et al. 2007). Climatic conditions can create key constraints on the distributions of pathogens and the epidemic potential of disease. The signature of this influence is typically highest for those infectious organisms with an environmental life history stage (such as the Bd zoospore) and a definitive host which is ectothermic; amphibian chytridiomycosis fulfils both these criteria. Akin to other ectotherms, the fitness and immune response of amphibians is particularly sensitive to the ambient temperature (Raffel et al. 2006). The survival, growth and reproduction of Bd is also temperature-dependent with a growth maximum between 17 and 25 °C (Piotrowski et al. 2004).
Disentangling the relative causality of NPH vs. EPH-type processes has proven problematic given the multiple temporal and spatial scales over which chytridiomycosis occurs. Given that Bd exhibits extremely low host specificity (http://www.spatialepidemiology.net/bd-maps; Fisher et al. 2009b) as well as distinct intra- and interspecific differences in the distribution of infection and disease in amphibians (Briggs et al. 2005; Woodhams et al. 2007), the understanding of external forcing factors on infection dynamics is best addressed using ecological studies of single species. Our research here focuses on Alytes obstetricans. This species of amphibian is ecologically and developmentally plastic and, given a permanence of water, is found across a wide range of ecotypes throughout much of Europe. The species is found from sea-level to altitudes exceeding 2500 m (Vences et al. 2003). At high altitudes, larvae undergo a prolonged period of development and may over-winter for at least one season. The potential for A. obstetricans to be highly susceptible to chytridiomycosis has been demonstrated by the near extirpation of this species by Bd in the Peñalara Natural Park, Spain. This is the first site in Europe where the pathogen and the disease were discovered to be affecting native amphibians (Bosch et al. 2001).
In this study, we detail the surveillance and analysis of the distribution of Bd and the occurrence of chytridiomycosis in populations of A. obstetricans across the Iberian Peninsula. We assess first, whether there is heterogeneity in the distribution of infection and second, whether environmental variables account for heterogeneity in (1) the presence/absence of infection (i.e. whether infection occurs at a site) and (2) the prevalence of infection (i.e. what is the proportion of Bd-infected individuals that are present at a site). We then determine whether these factors are linked to outbreaks of chytridiomycosis. Subsequently, we analyse multi-locus genotypes to assess whether there is evidence for single- or multiple- strains of Bd occurring in the Iberian Peninsula and whether these can be used to identify local patterns of introduction and spread. To conclude, we consider the implications of these ecological and molecular signatures on the novel and endemic pathogen hypotheses in Iberia.
Materials and methods
Field data
A total of 74 populations of Alytes obstetricans in the Pyrenees (PY) and a further 52 populations from within the Iberian Peninsula were surveyed for Batrachochytrium dendrobatidis (Fig. 1). Site selection was based upon the whereabouts of known populations of A. obstetricans (including distribution data from the Asociación Herpetológica Española) and explorations into areas considered to be suitable for A. obstetricans. Our studies are centred on two long-term Bd study sites: the Bd outbreak site at the Peñalara Natural Park (768 ha, 1800–2430 m), an alpine region in the Sierra de Guadarrama mountains of central Spain and Ibon Acherito, a site in the PY (1867 m). Fatal outbreaks of chytridiomycosis were first detected in these localities in 1997 and 2002 respectively.
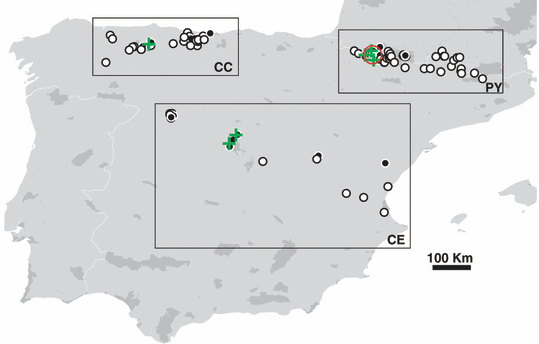
Distribution of surveillance sites. (a) Black circle, Bd detected; white circle, Bd not detected; green cross, outbreak of fatal chytridiomycosis. In the Pyrenees the spatial cluster detected by SaTScan v8 software is shown by a red circle. The heavily shaded regions are national parks.
Each study site was geo-referenced using GPS and sampled at least once during the study period (2003–2008 inclusive). The two long-term sites were visited annually from 2003 to 2008 inclusive. Sampling was conducted at each site during the summer months (June–September). During each site visit up to 30 larvae (L), up to 30 metamorphs (RM) and up to 30 adults (A) of A. obstetricans were collected. In localities where A. obstetricans was rare, the observed population was often sampled exhaustively. In an infinite population, a sample size of 30 is sufficient to detect at least one infected individual with a 95% probability if the underlying prevalence is 10% (Cannon & Roe 1982).
The keratinised mouthparts of larval anuran stages and the skin of post-metamorphic stages were tested for Bd infection using the protocols of Hyatt et al. (2007). Wearing disposable gloves, the mouthparts of larvae between Gosner stages 26 and 41 were swabbed using sterile cotton-tipped swabs (MW100-100; Medical Wire & Equipment Co, Corsham, UK) (Boyle et al. 2004). Post-metamorphic animals were either sampled by toe-clipping (years 2003–2005, predominantly) or by swabbing the lower ventral surface and hind-limbs (years 2006–2008, predominantly). Tissue samples were stored in 70% ethanol and swabs stored dry. Upon return to the laboratory samples were stored at 4 °C until processing (Hyatt et al. 2007). The presence/absence of infection was then assessed using the quantitative PCR (qPCR) protocol described by Boyle et al. (2004).
Among infected sites, ‘outbreak sites’ were defined as sites where fatal chytridiomycosis affected over 15% of the total number of metamorphs. At sites where mortality among amphibians was observed, counts of dead and live recent metamorphs (RM) were made by walking the circumference of the target waterbody and searching beneath stones. Any carcasses were collected and stored in either 70% ethanol (for qPCR) or 10% formalin (for histopathology).
Environmental data
Summary climatic statistics (as detailed in Supporting information methods) were calculated by using the modelling framework outlined previously (Ninyerola et al. 2007a,b). Climatic grids had a 200 m spatial resolution and covered the period 1950–2000. In addition to the climatic variables, altitude (m), geographical coordinates (latitude/longitude) and broad geographical region was specified. Three arbitrary geographical regions were recognised: the Cordillera-Cantabrica (CC) in the north (CC), the Pyrenees in the north-east (PY) and a diffuse NW–SE band running across central Spain including the Sierra de Guadarrama mountain range (CE) (Fig. 1). All of the quantitative covariates were standardised [(x-mean)/SD] prior to their use in the statistical models. Environmental correlates of outbreaks were assessed using the univariate non-parametric Wilcoxon test/Mann–Whitney test constrained to the outbreak period (June, July and August).
Heterogeneity in patterns of infection and detection of disease clusters
In the two main sampled regions where sample sizes were adequate, PY and CC (not CE), the Potthoff Whittinghill test statistic (Potthoff & Whittinghill 1966) was used to assess the null hypothesis of homogeneity among all the relative risks. Under the null hypothesis, the number of cases in each site is Poisson distributed whereas the alternative hypothesis is one of over dispersion, represented by the negative binomial distribution. Subsequently, the spatial scan statistic implemented in SaTScan v8 software was used to detect spatial clusters with high levels of infection (Kulldorff 1997). For this analysis a Bernoulli model was defined.
Statistical modelling using the Bayesian zero-inflated binomial distribution
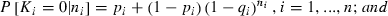
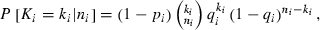
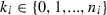 , and ni is the total number of amphibians observed in site i, while n is the total number of sites in the region of interest. The presence of Bd at a site is represented by p and the conditional prevalence of infection is represented by q. Here, conditional prevalence is defined as the prevalence of infection given an underlying assumption that the site is infected (i.e. the site is either known to be infected, or is estimated by the bayesian joint-distributions to have an associated probability of being infected). As explained in the supplementary information, we extend this model to incorporate the host’s developmental stages [larva (l), recent metamorph (RM) and adult (a)] as a further variable which is nested within site.
, and ni is the total number of amphibians observed in site i, while n is the total number of sites in the region of interest. The presence of Bd at a site is represented by p and the conditional prevalence of infection is represented by q. Here, conditional prevalence is defined as the prevalence of infection given an underlying assumption that the site is infected (i.e. the site is either known to be infected, or is estimated by the bayesian joint-distributions to have an associated probability of being infected). As explained in the supplementary information, we extend this model to incorporate the host’s developmental stages [larva (l), recent metamorph (RM) and adult (a)] as a further variable which is nested within site.Selected covariates were subsequently used in the full nested model. The Bayesian nested versions of the minimum adequate model (MAM) were implemented in the software WinBUGS, version 1.4.3 (Lunn et al. 2000). In each model two chains with different initial values for each of the parameters were specified. Given that the covariates and the intercepts act at the logit (Log-Odds Unit) scale, sets of initial values of 0 and 1 were used. In both of the chains for Yi (the infection status of a site), zero was used if Bd had not been detected at the site and NA was used if Bd had been detected. Non-informative priors were specified for the beta coefficients (normal priors with mean = 0 and precision = 0.0001) and the alpha intercepts (normal prior with mean = 0 and precision 0.01). Following an initial burn-in of 1000 runs the models were run until acceptable convergence and autocorrelation had been obtained (Gelman et al. 2000). In the Bayesian framework, all the parameter estimates are treated as random variables, thus enabling probability statements to be made about them. The Markov Chain Monte Carlo simulation technique used in WinBUGS provides a joint distribution of the parameters in the model given the observed data. This is particularly useful allowing us to provide intervals for the conditional prevalence at a site.
Culturing and multilocus genotypes
Isolates of Bd from infected sites were recovered using protocols provided by Joyce Longcore. In some cases, Bd was recovered from co-habiting conspecific species. Following culture in mTGhL media, DNA was extracted from cultures following the protocol of Boyle et al. (2004). Subsequently, six polymorphic loci (8702 × 2, 8392 × 2, CTSYN, BdC5, BdC18 and BdC24), were amplified by PCR and scored according to the methods and conditions detailed by James et al. (2009). Phylogeny was inferred using parsimony with (1) a model of evolution that made loss of heterozygosity three times more likely than either gain of heterozygosity, or change from one homozygous allele to another through relatively rare sexual recombination and (2) a model that weighted movement from any state equally.
Results
Distribution of infection and outbreaks of fatal chytridiomycosis
In total, 3016 individuals from across 126 sites were sampled. Of these sites, 25% (n = 31) were shown to be infected with Bd (Fig. 1). In the PY, the CC and the diffuse NW–SE band running across central Spain which included the Sierra de Guadarrama (CE), the proportion of infected sites was 23% (n = 74), 24% (n = 34) and 33% (n = 18) respectively (Table S1). There was no significant difference in the prevalence of infection between these regions (Fisher’s exact two-sided test, P = 0.643). In the PY, all of the infected sites were found occupying a small region in the western half of the mountain range. Here, the prevalence of infection exceeded 0.6 in 35% (n = 6) of these sites. At infected sites among all three regions, the prevalence of infection (aggregated across developmental stages) varied from a median of 0.12 in the PY, to 0.72 and 0.90 in the CC, and CE respectively. Across all Bd-positive sites in Iberia the median prevalence of infection was 0.63.
Heterogeneity in patterns of infection and detection of disease clusters
In both PY and CC, the Potthoff Whittinghill test statistic (T) was highly significant and the null hypothesis of homogeneity of relative risks was rejected (PY, T = 22091.77, P < 0.0001; CC, T = 1385326, P <0.0001). The spatial scan statistic detected clusters of infection in PY though no clusters were detected in CC. In the most likely spatial cluster [coordinates/radius (42.880200 N, 0.640133 W)/25.17 km] in the PY, 15 of the 33 sites were actually infected (Fig. 1); this was almost twice that expected (Loglikelihood ratio = 7.79, relative risk = 6.36, P = 0.019). A secondary cluster in the east of the Pyrenees National park was also identified [Coordinates/radius (42.862767 N, 0.143433 E)/1.13 km], though was not statistically significant (P = 0.997). Sample sizes were too small to analyse region CE using these tests. Outbreaks of fatal chytridiomycosis among recently metamorphosed animals (RM) were reported at seven of the 31 positive sites across the whole of Iberia (Fig. 1). The prevalence of Bd among RM in these localities consistently exceeded 0.65.
Statistical signature of zero-inflation
The lack of fit of the standard binomial and the presence of zero inflation in our dataset was ascertained by two different means. Firstly, the predicted-vs.-fitted plot from a standard binomial fitted at the site level was shown to produce very poor predictions that were close to zero. Secondly, when no covariates were considered, the P-value from the zero-inflated score test was shown to be highly significant (with Z = 427.68 and P = 5.2 × 10−95).
Environmental covariates
Summary statistics for the environmental and climatic factors considered are shown in Table 1. The altitude of infected sites ranged from 82 to 2409 m above sea-level and the STempMin and STempMax at these sites ranged from 1.9–11.9 and 14.5–30.1 °C respectively (Table 1). Among the Bd-positive sites (n = 31) the localities with known outbreaks (n = 7) of fatal chytridiomycosis were significantly associated with Alt [altitude: Wilcoxon Mann–Whitney test, W = 36, P = 0.022 (+)], STempMin [summer temperature minimum: W = 41, P = 0.045 (−)], STempAv [summer temperature mean: W = 0, P = 7.97 × 10−05 (−)] and TempMin [minimum temperature during the coldest month: W = 41, P = 0.045 (−)] (Fig. 2).
| Variable | All sites | Infected | Fatal | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Min. | Max. | Mean | Min. | Max. | Mean | Min. | Max. | |
| Altitude (Alt; m) | 1468.4 | 82.1 | 2588.0 | 1552.7 | 82.1 | 2409.0 | 1922.8 | 1602.0 | 2196.0 |
| Summer temperature minimum (STempMin; °C) | 6.6 | 1.7 | 15.7 | 6.2 | 1.9 | 11.9 | 4.4 | 4.1 | 4.9 |
| Summer temperature mean (STempAv; °C) | 14.5 | 8.6 | 23.7 | 14.4 | 8.6 | 20.5 | 13.2 | 11.7 | 15.3 |
| Summer temperature max (STempMax; °C) | 22.2 | 14.5 | 32.6 | 22.3 | 14.5 | 30.1 | 21.9 | 19.2 | 26.3 |
| Summer cumulative precipitation (SPrecCum; mm) | 231.2 | 51.4 | 354.7 | 225.1 | 55.8 | 334.8 | 232.8 | 107.1 | 324.4 |
| Summer radiation average (SRadAv; kJm−2) | 2950.5 | 2504.4 | 3119.6 | 2970.5 | 2640.1 | 3119.6 | 2904.7 | 2640.1 | 3113.1 |
| Seasonality (Seas; °C) | 547.8 | 431.3 | 699.5 | 556.8 | 435.0 | 699.5 | 588.5 | 518.5 | 697.9 |
| Mean diurnal range (MDR; °C) | 10.5 | 8.4 | 14.8 | 10.5 | 8.5 | 13.7 | 10.9 | 9.5 | 12.5 |
| Temperature annual range (TempAr; °C) | 25.8 | 21.7 | 33.6 | 26.1 | 22.4 | 33.6 | 27.6 | 24.8 | 32.4 |
| Isothermality (Iso; °C) | 40.7 | 35.6 | 48.7 | 40.1 | 36.1 | 48.7 | 40.1 | 37.7 | 44.6 |
| Minimum temperature during the coldest month (TempMin; °C) | −3.6 | −9.1 | 5.9 | −3.9 | −8.4 | 3.1 | −5.7 | −7.0 | −4.1 |
- The acronyms used in the text for each of these variables and the units involved are shown in parentheses.
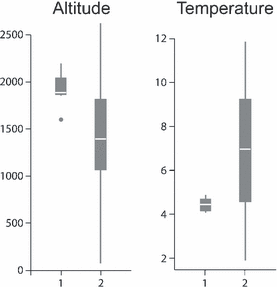
Box-plots displaying the altitude (m) and temperature minima (°C) during the warmest quarter at localities associated with known outbreaks of fatal chytridiomycosis, 1, and at localities from which Bd-infected amphibians occur though no outbreaks have been recorded, 2. In both cases, the differences between 1 vs. 2 are significant.
In the univariate site-level Bayesian models the following variables were found to have a significant effect on the conditional prevalence of infection (q): StempAv, SradAv (summer radiation average), Seas (seasonality), MDR (mean diurnal range), TempAr (temperature annual range), TempMin and Longitude. By contrast, only a single variable, ‘TempMin’ was found to have a significant effect on the probability of Bd being present at a site (P). From these variables, four pairs of highly correlated variables were identified (STempAv–TempMin 0.91, Seas-TempAr 0.93, MDR–TempAr 0.85 and TempMin–TempAr 0.85) and the following variables with the lower P-value within the pair were dropped: Seas, MDR, TempAr and STempAv. A backwards stepwise regression with TempMin specified as a covariate for P and TempMin, Longitude and SRadAv specified as covariates for q was used to identify the MAM. In this MAM, the conditional prevalence of Bd was negatively associated with SRadAv [mean = −0.22, 95% Bayesian credible intervals (BCI) = −0.31 to −0.13], TempMin [mean = −0.39, 95% BCI = −0.57 to −0.22] and Longitude (mean = −0.33, 95% BCI = −0.43 to −0.24). The 95% BCI did not include zero and were considered to be significant. Note that these analyses were conducted for the site-level model, though subsequent cross-checks were made with the nested version of the model which accommodated developmental stage.
Outputs from Bayesian nested models
In the expanded ZIB with developmental stage nested within site and environmental covariates specified for q (model 4TLR; Table S2) the prevalence of infection among larvae (mean = −1.611, 95% BCI = −1.86 to −1.362) and adults (mean = −1.077, 95% BCI = −2.103 to −0.02085) was significantly depressed relative to metamorphic animals (Table S3). As in the non-nested MAM, the conditional prevalence of Bd was negatively associated with Longitude (mean = −0.2424, 95% BCI = −0.3496 to −0.1445), SRadAv (mean = −0.2563, 95% BCI = −0.356 to −0.5191) and TempMin (mean = −0.04549, 95% BCI = −0.2391–0.1476), though the significance of the relationship only held for the former two covariates (Table S3). In this model the underlying conditional prevalence and probability of Bd presence was 0.7613 (95% BCI = 0.5007–0.7612) and 0.2575 (95% BCI = 0.1850–0.3425) respectively. By contrast, in the nested model where intercepts only were specified, the conditional prevalence was 0.6031 (95% BCI = 0.5784–0.6277).
The infection status and conditional prevalence of Bd at sites across the Iberian Peninsula (according to model 4TLR), is shown in Fig. S1, with insets for PY and CC in Figs 3 and S2 respectively. The accompanying caterpillar plots in these latter figures display the 95% BCI associated with each estimate of the conditional prevalence, at infected sites. Across all sites the conditional prevalence of infection was significantly elevated (i.e. lower 95% BCI greater than the underlying value of 0.7612) at 16 of the 31 sites from which Bd had been recorded. Beyond these sites, the estimated mean conditional prevalence of Bd was above zero (mean = 0.01912–0.1253) at 19 sites. However the 95% Bayesian credible intervals were large and included zero in all cases.
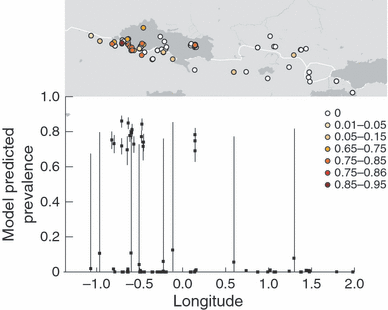
Map and caterpillar plot showing site infection status and conditional prevalence as predicted by the best model 4TLR (Table S2) in the Pyrenees (PY). This model contains environmental covariates (TempMin, Longitude and SradAv) and nested developmental stage effects. 95% Bayesian credible intervals are shown. The equivalent figure for the Cordillera-Cantabrica (CC) is shown in Fig. S2.
Model fit
In the nested model with intercepts only (Model 1; Table S2), the values of the AIC (Akaike Information Criterion) and BIC (Bayesian Information Criterion) were 1433.174 and 1825.994 respectively. Relative to this null model the greatest reduction in AIC, albeit very marginal (< 1 decimal place) was achieved by the addition of both developmental stage and all three environmental covariates on q (model 4TLR, Table S2). By contrast the greatest reduction in BIC was achieved when TempMin was omitted from q (model 4RL; Table S2); this omission is consistent with the lack of significance of this variable in the full model (Table S2). In the full version of model 4 (model 4TLR) the predictive check showed overestimation of infection in 26 of the 159 site:stage combinations. This overestimation is likely to be related to the presence of some sites with small sample sizes.
Results from the analysis of multilocus genotypes
All of the alleles found in Iberia had been previously documented by James et al. (2009) and are included here (Supporting information). Three trees were recovered with the weighted model and they differed only in the placement of the UK isolate relative to the Mallorca isolates, and in the placement of one Valencia isolate. Twenty-seven unresolved trees were recovered with the unweighted model. For both models, unique multilocus genotypes were found between each of the regions surveyed (Table S4 and Fig. 4). In the cluster of infection in the PY, all isolates were of a single identical genotype. The isolate recovered from the original outbreak site of Peñalara was heterozygous at all six loci in this dataset, and at 12/14 loci in the James et al. (2009) dataset; this heterozygosity puts this first European ‘outbreak’ isolate at the centre of the dendrogram. Maximum genotypic diversity was found in Valencia at a site 400 km distant from Peñalara, where three distinct genotypes were recovered from a single pond and from two species of amphibian, A. obstetricans and Pelophylax perezi.
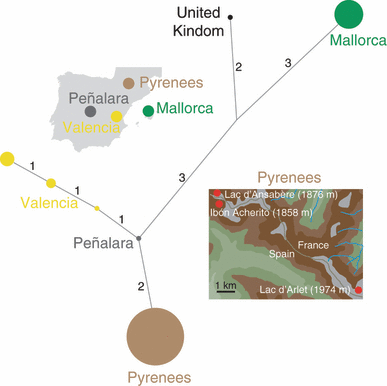
Phylogeny showing relationships among Bd strains within Iberia based on six locus genotypes. The tree shown is one of the three generated by a weighted model; these differ only in the placement of the UK isolate relative to Mallorcan isolates. The radius of the circles is relative to the numbers of identical genotypes. Insets show where samples were taken, and the different watersheds that occur in the Pyrenees.
Discussion
The widespread yet heterogeneous distribution of Bd reported here illustrates the potential risk of chytridiomycosis to populations of A. obstetricans across the Iberian Peninsula and perhaps across the host’s entire European range. This risk surface is three- tiered, accommodating first, the actual distribution of Bd, second, the modelled conditional prevalence of infection (without necessarily observing infection), and third, the risk of a disease outbreak. In our analyses we find: (1) no evidence for any relationship between the occupancy of Bd and environmental variables, (2) a weak relationship between environmental variables (average/minimum temperature of the warmest quarter, minimum temperature of the coldest month, mean solar radiation and longitude) and the conditional prevalence of infection, (3) stage-dependent heterogeneity in the risk of infection, (4) an association between high altitudes and outbreaks of fatal chytridiomycosis, (5) multiple Iberian genotypes and (6) recent introduction and spread of a single genotype of Bd in the Pyrenees.
In this study, evidence for weak environmental effects on the establishment of Bd per se is consistent with the broad spatial distribution and environmental envelope of Bd identified in previous studies (Ron 2005; Fisher et al. 2009a). Together, these data are suggestive of Bd being an ecological generalist that is able to persist wherever susceptible amphibians occur. The Bd-infected localities reported here traverse a broad altitudinal range (82–2588 m a.s.l.). Above 2000 m in our study region, infected sites are typically associated with air temperature minima and maxima below the limits currently recognised for the optimal growth of Bd. This disparity is not unprecedented and highlights the need for an improved understanding of the hypervolume of space characterising the fundamental niche of Bd. Notably, the highest record of Bd, at an altitude of 5400 m, is known from the Peruvian Andes where soil surface temperatures may fall to −13.5 °C and diurnal variability of 30 °C has been recorded (Seimon et al. 2007); it is clear from these, and ours, data that the breadth of environments within which Bd persists is wider than was previously thought. In addition to not supporting the concept of refugia from infection at high altitudes (Muths et al. 2008) our findings motivate the need to: (1) consider any incongruity of scale between the (often coarse) summary climatic statistics used in models and the actual microenvironment occupied by Bd and (2) assess whether the physiological thresholds which have been defined for strains of Bd from various regions across North America (Piotrowski et al. 2004) are applicable to the Bd genotypes that are found infecting Europe and elsewhere (Fisher et al. 2009a).
Our models present evidence for a negative relationship between prevalence and the minimum temperature during the coldest month (TempMin), radiation (SRadAv) and longitude. However, this relationship is weak and a far greater improvement in model fit is achieved by the specification of developmental stage as an individual-level risk factor (Table S2). This finding is consistent with observations that recently metamorphosed amphibians are predisposed to infection, perhaps owing to transient immunosuppression owing to metamorphosis (Robert et al. 1995; Garner et al. 2009). The negative relationship between conditional prevalence and increasing (+) longitude is consistent with a signature of spread, with a bias to the west of Iberia; this is weighted to a large degree by the Bd hotspot in the western Pyrenees. The negative relationship between conditional prevalence and temperature of the minimum month is somewhat equivocal since, although its significance is lost when developmental stage is not accommodated in the model, when either radiation or longitude is omitted from the full model, this variable’s significance is regained. In terms of model fit, the greatest reduction in AIC/BIC in the full model is achieved by the addition of the variable radiation. Elevated levels of UVB are known to be harmful to amphibians (Nagl & Hofer 1997; Blaustein et al. 2005) and may increase susceptibility to pathogens such as Bd. However, this finding needs to be interpreted with caution as, in addition to the technical shortcomings in the air-based measurements of UVR (Ninyerola et al. 2007b), differential attenuation of ultraviolet wavelengths in water (Laurion et al. 2000; Blaustein et al. 2004) risks distorting the ecological significance of this variable.
The results of our study do not refute the frequently cited hypothesis regarding the predilection of Bd for cooler temperatures (Berger et al. 2004; Drew et al. 2006; Kriger & Hero 2007) however they do emphasize the context-dependent nature of fatal chytridiomycosis as all occurrences of disease were found at altitudes above 1600 m. The environmental forcing of many infectious diseases is well-documented (Rogers et al. 2002; Clements et al. 2007; Liang et al. 2007) and are often related to the prevalence of the infectious organism in the environment. However, in diseases where the outcome of infection is dependent more on the host’s ability to tolerate an infection, environmental forcing of pathogenicity rather than prevalence per se may dominate the fitness landscape. In addition to the occurrence of fatal outbreaks at high altitudes, the interannual variability in the mortality rate at one of our ‘outbreak’ sites, at which the prevalence of infection was consistently high (Ibon Acherito), is strongly consistent with the importance of context-dependent host tolerance of infections. Specifically, the association of mean temperature minima during the summer months and mortality is a prediction borne by our knowledge that: (1) the maintenance/clearance of infection and the survival of the host amphibian is temperature dependent (Woodhams et al. 2003; Andre et al. 2008) and (2) in the field, temperature-dependent immunity may be forced by seasonal patterns (Raffel et al. 2006).
Temporal data documenting the rapid spread of fatal chytridiomycosis and the subsequent collapse of Rana muscosa populations in the Sierra Nevada, as well as the decline of A. obstetricans in Peñalara, serve as a cautionary note for the potential fate of Alytes populations in the Pyrenees (Bosch et al. 2001; Briggs et al. 2005). Akin to A. obstetricans, the larval stage of R. mucosa is typically prolonged in high altitude montane lakes. The prolonged period of exposure to Bd and the associated window of time for the accumulation of fitness costs may be pivotal to explaining the susceptibility of these montane species and populations to fatal chytridiomycosis. In our study, although the configuration of infected sites containing identical genotypes in the Pyrenees is strongly suggestive of a pathogen in the early stages of its emergence [in contrast with that observed for R. muscosa (Morgan et al. 2007)], the widespread distribution of Bd across the Iberian Peninsula suggests that Bd has been introduced into this wider region some time ago. Such a conclusion is consistent with the broad distribution of Bd across Europe (Garner et al. 2005). That Bd is in relative equilibrium with its environment and not undergoing a period of range expansion is a critical assumption of landscape epidemiology (Pavlovsky 1966) since spatial heterogeneity in the environmental extent of disease is attributed directly to the underlying variation in the ecological conditions that support the pathogen, its vectors, its reservoirs and its hosts (Ostfeld et al. 2005). Given that the relative endemicity of infection in our study region is debated, the ZIB model is particularly suited as it models separately occupancy and conditional prevalence. The signature of zero-inflation in our data has not been reported from previous Bd-surveillance in other systems and it is plausible that, if Bd is not at equilibrium within Iberia, this signature may be lost with time. Notably, the proportion of sites occupied by Bd in each of our regions (23–33%) is considerably lower than that reported by Muths et al. (Muths et al. 2008) in their study on Bufo boreas in the Rockies (north 84% clusters occupied n = 49; south 42%, n = 48) and, more generally, across the United States. This observation suggests that Bd is at an earlier phase of it’s emergence within Iberia relative to North America.
Our finding of distinct genotypes within each of the sampled regions is consistent with either a history of multiple introductions or of a single relatively ancient introduction of Bd into Iberia (Table S4). James et al. (2009) using 14 marker loci show that Iberian genotypes from Peñalara (C2A) and the Pyrenees (IA042/043) co-occur within a clade of genotypes that are exclusively found in North America, raising the hypothesis that a transatlantic introduction of Bd into mainland Iberia has ocurred. The high levels of heterozygosity found in Peñalara C2A (Fig. 4) are concordant with this hypothesis as this site is arguably the first European outbreak site, and the most heterozygous isolate yet found comes from a North American bullfrog in Maine (JEL404). However, owing to low genetic diversity and global sampling, the loci utilised by James et al. (2009) lack the power to accurately describe potential global sources of infection and a definitive identification of the original source of the Iberian infections will likely require a next-generation sequencing approach.
On the other hand, the Iberian genotypes clearly show local patterns of introduction and spread. Principally, in the Pyrenees the identical nature of each of the isolates from three lakes in three different drainage basins (Fig. 4) is strongly supportive of a single introduction and subsequent spread, or multiple introductions of an identical clone from an unknown source population. We believe that the former is the most parsimonious interpretation of our data, as evidenced by the discovery of a recent single introduction of Bd into an endemic species of Alytes in Mallorca (Walker et al. 2008).
Our findings have shown that the expression of an NPH-like process (the introduction of Bd into Iberian biomes) is dependent on its EPH-like environmental context (the expression of chytridiomycosis). We have shown that these processes have likely not reached their spatial equilibria within Iberia, and that further spread (both of the pathogen and the disease) across this region is likely. It is also possible that future changes in these environments will further modify the Alytes/Bd host/pathogen relationship. Montane systems are particularly vulnerable to climate change (Catalan et al. 2006; Nogues-Bravo et al. 2007) and the global trends in changes between minima and maxima temperatures reported by Pounds et al. (2006), are supported by temperature trends in the Pyrenees (Dessens & Bucher 1995). For this region, an increase of between 2.8 and 4 °C in the mean annual temperature and a decrease of between 10.7% and 14.8% in the annual precipitation is predicted for the next century (Lopez-Moreno et al. 2008). However, beyond the need to assess the impact of climate change on the physiology of Bd and the host amphibian per se, there is a need also to consider changes in: hydrological patterns, phenology, community composition and changes in the amphibian epidermal microfauna. All of these variables are likely to impact on the epidemiology of Bd and chytridiomycosis, greatly increasing the challenge of developing a predictive understanding of the emergence and occurrence of this increasingly devastating infectious disease.
Acknowledgements
This work was supported by grants from the United Kingdom Natural Environmental Research Council (NERC) and European Union ERAnet project RACE. We wish to thank colleagues and students who have contributed to this work in many ways – specifically Nicky Best, Christl Donnelly, Frances Clare, Jon Bielby, Mat Perkins, Amanda Duffus, Laia Ribas, Joyce Longcore and Ana Díaz-Guerra. We also extend our particular thanks to the Director and employees of both Peñalara Natural Park and the Pyrenees National Park and the governing departments for the Environment in Madrid, Aragón, Asturias, Cantabria, Cataluña, Valencia, Castilla-La Mancha, Islas Baleares, Castilla-León, Andorra, Aquitaine and the Midi-Pyrénées.




