Cortical interneurons, immune factors and oxidative stress as early targets for schizophrenia
Abstract
Schizophrenia is a common disorder in which strong genetic predisposition is combined with environmental factors. Despite the widely recognized developmental nature of the disease, symptoms do not emerge until late adolescence. Current therapeutic approaches are therefore employed too late, as brain alterations may have been present earlier than symptom onset. Here I review the developmental trajectory of the cortical circuits responsible for excitation–inhibition balance, which are at the center of current pathophysiological views, and propose that oxidative stress in cortical interneurons may be a final common pathway by which several different etiological factors can yield the cortical dysfunction characteristic of schizophrenia. If this scenario is correct, redox modulators may be beneficial for the disease. It is critical that the developmental trajectories of the factors yielding oxidative stress are taken into account for those approaches to succeed.
Introduction
Schizophrenia (SZ) affects about 1% of the population worldwide, yet current therapies have limited impact on many aspects of this devastating disorder. For decades, dopamine was central to pathophysiological views for schizophrenia, and all effective pharmacological approaches have in common a D2 dopamine receptor blockade (Seeman, 1987). However, although first- and second-generation antipsychotic drugs improve positive symptoms, they are marginally effective for negative symptoms and not at all effective for cognitive deficits (Carpenter & Koenig, 2008). Cognitive deficits in SZ have received increasing attention, as they are a core feature of the disease and perhaps the strongest predictor of outcome (Green, 1996). The lack of efficacy of current therapies on cognitive deficits is troubling, and has prompted several groups to seek other tools. As neuroleptics are ‘dirty drugs’ that target multiple receptors and their efficacy against psychotic symptoms was discovered serendipitously (Delay & Deniker, 1955), the past decades have witnessed the search for new pharmacological approaches based on preclinical research. GABA (gamma-aminobutyric acid) and glutamate neurotransmission emerged as critical elements in SZ pathophysiology (Coyle, 2004; Lewis et al., 2005; O’Donnell, 2011) and gained prominence in such efforts (Heresco-Levy et al., 1996; Patil et al., 2007). Both amino acid transmitters are critical for the balance between excitation and inhibition necessary for proper prefrontal cortical activity and adequate cognitive functions (Lewis & Moghaddam, 2006). Therefore, it makes sense to target excitation–inhibition balance to treat cognitive deficits in SZ. Although some attempts have been made to develop novel agents that target glutamate or GABA, the results have not been impressive (Lewis et al., 2008). SZ is a disorder in dire need of a new approach, and it is critical that any new direction is based on solid preclinical science for it to be effective. Most importantly, these new approaches must consider the developmental trajectories of brain circuits implicated in SZ. Indeed, SZ has a clear genetic component and is widely accepted as a neurodevelopmental disorder (Harrison & Weinberger, 2005), but symptoms do not become full blown until late adolescence. Here, I review evidence of altered excitation–inhibition balance in SZ, the possible role of oxidative stress as a source of interneuron dysfunction and the developmental nature of these factors. Although most of the work reviewed relates to the prefrontal cortex (PFC), these alterations are likely to be present in other cortical areas, including allocortical regions such as the hippocampal formation. Novel therapeutic approaches could therefore include agents that target glutamate, GABA or oxidative stress, but the highest impact will be achieved with early treatment, including the prodromal stage.
GABA and glutamate – the cortical interneuron story
A remarkable convergence of data from preclinical and clinical studies identifies cortical GABA interneurons as a critical element in SZ pathophysiology. Non-competing NMDA (N-methyl-d-aspartate) receptor antagonists are widely used as a pharmacological model of the disease. These agents yield a state of cortical disinhibition (Coyle, 2004; Homayoun & Moghaddam, 2007) that is thought to be related to cognitive deficits (Lewis & Moghaddam, 2006). It has been proposed that NMDA antagonist-induced disinhibition is due to these compounds acting with higher affinity for NMDA receptors located in inhibitory interneurons (Coyle, 2004; Homayoun & Moghaddam, 2007; Behrens & Sejnowski, 2009), but recent evidence indicates NMDA currents in cortical interneurons may be negligible (Rotaru et al., 2011). Although it is possible that cortical disinhibition is elicited by effects of NMDA antagonists at a circuit level, it is evident that NMDA blockade can induce cellular changes in fast-spiking interneurons (Behrens et al., 2007), and knocking out NMDA receptors from parvalbumin (PV) interneurons yields mice with several signs of a disinhibited cortex (Belforte et al., 2010). These data strongly suggest that altered interneuron function is a critical component of the consequences of non-competing NMDA antagonists. Another pharmacological model, sensitization to repeated amphetamine, produces loss of PV interneuron labeling in the prelimbic PFC (Morshedi & Meredith, 2007). Amphetamine treatment and NMDA receptor antagonism are interesting and useful models, but the developmental aspects of the disease cannot be reproduced with pharmacological treatments in adult animals.
Among the several widely used developmental models, the neonatal ventral hippocampal lesion (NVHL) stands out by producing diverse neurochemical, physiological and behavioral deficits resembling phenomena observed in SZ with an onset during late adolescence (O’Donnell, 2011). The PFC in this model is also disinhibited due to impaired GABA interneuron activation, as cortical interneurons cannot be efficiently activated by dopamine in this model (Tseng et al., 2008). PFC pyramidal neurons of adult NVHL rats present excess firing along with loss of interneuron-dependent beta oscillations during a choice task (Gruber et al., 2010), indicating the disinhibition is evident during behaviorally critical epochs. A medial PFC lesion reverses abnormal behaviors and altered physiological changes in adult NVHL rats (Lipska et al., 1998; Goto & O’Donnell, 2004), strongly suggesting the PFC is the critical brain region responsible for adult outcomes in the NVHL model.
Other developmental and genetic models also show evidence of altered interneuron function. Perinatal immune activation yields adult animals with SZ-related anomalies. Treatment with the viral mimic poly I:C or the bacterial endotoxin lipopolysaccharide (LPS) during gestation results in offspring with behavioral deficits when they become adults, including reduced prepulse inhibition of the acoustic startle response and latent inhibition (Borrell et al., 2002; Shi et al., 2003; Meyer et al., 2006). Although interneuron function has not been assessed in these models, neonatal LPS injections in the ventral hippocampus altered adult cortical interneuron responses to dopamine (Feleder et al., 2010). Prenatal treatment with the antimitotic agent methylazoxymethanol acetate (MAM) yields a reduction in PV immunoreactivity in the PFC and ventral hippocampus of adult rats (Penschuck et al., 2006; Lodge et al., 2009) along with a reduction in interneuron-dependent high-frequency electroencephalographic (EEG) oscillations (Lodge et al., 2009). Among genetic models, expression of a truncated disrupted-in-schizophrenia 1 (DISC1) gene that acts as a dominant negative during development resulted in SZ-relevant abnormal behaviors and reduction in PV levels in adult mice (Hikida et al., 2007). Knocking down DISC1 in the medial PFC via in utero electroporation of siRNA also resulted in abnormal behaviors with adult-onset and electrophysiological data consistent with loss of adult interneuron function (Niwa et al., 2010). Dysbindin is another candidate gene for SZ, and dysbindin knock-out mice exhibit fast-spiking interneurons with reduced excitability (Ji et al., 2009). Several of these models result in reduced PV staining in cortical and hippocampal regions. These observations could be due to either neuronal loss or reduced PV expression in neurons that are still present. The latter is more likely, as reduced PV is not accompanied by loss of the GABA-synthesizing enzyme GAD67 in many of these studies (Behrens & Sejnowski, 2009; Lodge & Grace, 2009) and PV is an activity-dependent calcium-binding protein. Thus, several different rodent developmental models have in common altered interneuron function, and whenever the timing was explored, this alteration emerged during adolescence.
A causal role of interneuron dysfunction in SZ-relevant phenomena was ascertained with genetic and pharmacological tools. A simple but clever experiment testing whether intra-PFC blockade of GABA-A receptors revealed that cognitive functions become impaired (Enomoto et al., 2011). Furthermore, selectively knocking down NMDA receptors in PV interneurons resulted in a wide array of SZ-relevant altered behaviors and electrophysiological changes (Belforte et al., 2010). These data indicate that altered interneurons in animal models of SZ can be causal to behavioral deficits, reinforcing the notion that interneuron dysfunction may be a central element in SZ pathophysiology.
It is remarkable that the quite different manipulations involved in the diverse SZ models have in common a deficit in interneuron function and that this deficit emerges during adolescence. This convergence indicates that interneurons, and in particular PV interneurons, are somewhat susceptible to insult from a variety of deleterious factors; the diversity of manipulations that can yield interneuron dysfunction and their different timing may reflect the various factors that may play a role in SZ etiology, which include combinations of gene variations that alone confer a very small risk with environmental factors. NMDA antagonism, gene manipulations or developmental manipulations are useful tools to alter interneuron development, but they should be considered as just tools. Although the notion that NMDA receptors are abnormal in SZ has become popular due to the effects of NMDA antagonists, there is no unambiguous support for an NMDA deficit in the disease. NMDA blockade may actually cause a downstream condition that mimics SZ pathophysiology without the need to have an actual NMDA deficit in the disorder. Also, the neonatal hippocampal lesion is a tool to yield altered PFC interneurons and should not be interpreted as modeling hippocampal pathology. Genetic models are also reproducing risk factors, not the disease. Not all SZ patients exhibit a truncated DISC1 gene, but when DISC1 function is impaired during development, PFC circuits become altered. All these manipulations are tools that yield adult interneuron dysfunction. Why are interneurons affected by such a diverse set of conditions? Understanding the mechanisms that may affect this vulnerable neuronal population at specific developmental points could open new possibilities on alternative approaches to treat SZ.
Immune factors and oxidative stress
Oxidative stress could play an important role in the biochemical basis of SZ as the mechanism that yields altered cortical interneurons. Oxidative stress results from an imbalance between reactive oxygen species (ROS) and antioxidants. Typical ROS include O2− (superoxide), H2O2 (hydrogen peroxide), NO (nitric oxide) and ONOO− (peroxynitrite). Key enzymes that generate superoxide and NO are nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and NO synthase (NOS) (Babior, 2004). Glutathione (GSH) and its associated enzymes are critical for the degradation of hydrogen peroxide (Meister & Anderson, 1983). GSH is a free radical scavenger and an inhibitor of lipid peroxidation that protects the brain from oxidative stress (Janaky et al., 2007) and helps to regenerate other antioxidants. Environmental factors, such as viral infections, inflammation and obstetrical complications, as well as psychological stress are tightly associated with an increase in oxidative stress (Liu et al., 1996; Lante et al., 2007) and are considered risk factors for SZ (Watson et al., 1984; Brown, 2006, 2011). It is therefore possible that disturbances of the mechanisms that maintain cellular homeostasis against oxidative damage may be involved in SZ pathology. It will be beneficial to determine whether perinatal immune activation or conditions that yield oxidative stress during development result in altered interneuron function.
There is indeed some evidence of oxidative stress in SZ. Redox alterations are associated with positive, negative and cognitive symptoms in SZ (Do et al., 2009), and GSH synthesis is impaired in SZ patients (Do et al., 2000; Yao et al., 2006; Gysin et al., 2007). Furthermore, GSH levels were reduced in neuroleptic-free and treated SZ patients and were inversely correlated with symptom severity (Raffa et al., 2009). The evidence indicates that oxidative stress correlates with SZ symptom clusters, but it needs to be determined whether this is a causal link and which brain circuits are affected by oxidative stress yielding such deficits.
Animal models are useful tools to explore hypotheses such as whether oxidative stress affects SZ-relevant behavioral outcomes. Studies indicate that markers of oxidative stress may be increased in several models of SZ; for example, PV-positive interneurons exhibit oxidative stress in the presence of NMDA receptor blockade (Behrens et al., 2007). Furthermore, it is likely that such oxidative stress is related to altered immune responses, as it cannot be observed in mice deficient in interleukin-6 (Behrens et al., 2008). Emerging evidence from our lab indicates that PV interneurons in NVHL rats exhibit oxidative stress prior to symptom onset. NVHL rats, but not sham controls, show increased levels of 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dG), a marker of oxidized DNA. Most PV interneurons exhibit this marker in adult NVHL rats, and the increase in 8-oxo-dG precedes the loss of PV cell count (O’Donnell et al., 2011). Furthermore, adult NVHL rats show increased cytochrome-oxidase I staining in the PFC (Tseng et al., 2006), indicating enhanced mitochondrial activity. Thus, several animal models indicate the possible overlap of interneuron pathology and oxidative stress. Fast-spiking interneurons are highly active and therefore may be more vulnerable to oxidative damage than other cell populations. Interneuron oxidative stress may be responsible for the altered behaviors in NVHL rats, for example, as treatment with the GSH precursor N-acetyl cysteine (NAC) during juvenile and adolescent periods reverses the loss of PV cell counts and restores deficits in prepulse inhibition of the acoustic startle response (O’Donnell et al., 2011). Animal models not only reveal the presence of oxidative stress in interneurons, but are providing evidence of a causal role of oxidative stress in abnormal behaviors.
A combination of gene variations conferring risk and environmental factors may thus alter the developmental trajectory of cortical interneurons. Oxidative stress may be a common final mechanism by which different developmental disturbances may affect cortical PV interneurons (Fig. 1), and perhaps other cell types as well. As interneurons do not mature until late adolescence (Tseng & O’Donnell, 2007), the deleterious effects of these factors on cortical function (i.e. disinhibited cortical circuits) will not become evident until the transition to adult stages. If PV interneuron oxidative stress is present at early stages and even prior to symptom onset, agents that modulate redox balance may be beneficial.
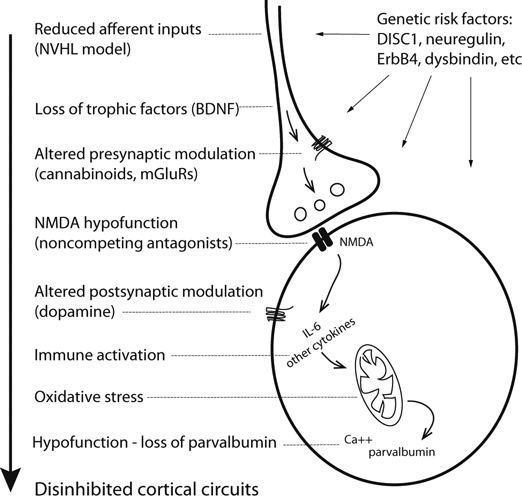
Cartoon illustrating a cortical interneuron and a representative glutamatergic afferent indicating where vulnerability factors can affect interneuron function during development, and possible events occurring in animal models that ultimately yield disinhibited cortical circuits. A reduction in NMDA receptor function in GABAergic interneurons could be driven by NMDA antagonists, reduced inputs, reduced trophic factors, etc. Reduced NMDA activation in this cell population induces cytokine expression and redox alterations, and eventually lower levels of PV. PV is a calcium-buffering protein that may not be needed if neurons are hypoactive; therefore, PV loss may not indicate cell death, but lack of sufficient activity. Genetic risk factors may contribute to this scenario both at the pre- and post-synaptic level. BDNF, brain-derived neurotrophic factor. Reproduced from O’Donnell (2011), with permission.
Antioxidants in SZ
Multiple studies have examined the efficacy of drugs with antioxidant properties on neuropsychiatric disorders. Ginkgo biloba is a potent antioxidant that reduced symptoms when used as an adjuvant (Zhang et al., 2001a,b; Atmaca et al., 2005; Doruk et al., 2008). GSH levels are reduced in SZ (Do et al., 2000; Gysin et al., 2007; Gawryluk et al., 2010), suggesting the possibility that increasing GSH levels may be useful in the treatment of SZ. Preclinical studies suggest that the GSH precursor NAC may minimize cognitive impairments associated with prenatal exposure to bacterial infections and pro-inflammatory cytokines (Lante et al., 2007). NAC treatment in people with multi-episode schizophrenia improves mismatch negativity (Lavoie et al., 2008) and global psychopathology (Berk et al., 2008). Finally, a series of studies examined omega 3 fatty acids in people with SZ, with mixed results (Fenton et al., 2001; Peet et al., 2001, 2002; Emsley et al., 2006, 2008; Berger et al., 2007). When omega 3 fatty acids were used in people who met criteria for high-risk of psychotic disorder, however, they significantly reduced the rate of conversion to full-blown psychosis (Amminger et al., 2010). This may be a crucial observation. Antioxidant treatment seems more efficient at early stages and mostly in ultrahigh-risk subjects. Remarkably, animal model data indicate that interneurons exhibit the highest levels of oxidative stress prior to full symptom onset (O’Donnell et al., 2011). In summary, these studies suggest that antioxidant treatment may reduce measures of oxidative stress, reduce positive and negative symptoms, and improve function. However, for this approach to work most efficiently, treatment at early stages is crucial.
What may come – stem cell-based approaches; gene therapy
If interneuron pathology during development is a critical element in SZ pathophysiology, a major goal should be to restore interneuron function as early as possible and even prevent the loss. Besides reducing oxidative damage, other approaches that may eventually ameliorate interneuron deficits include stem cell-based replacement and gene therapy. These ideas are still in their early stages, but are likely to evolve in the next few years. Although one may question the feasibility of replacing dysfunctional neurons in a tightly interconnected population such as PV interneurons, some data suggest it could be possible. Recent evidence suggests that altered neuronal migration and adult neurogenesis may play a role in SZ. A post-mortem study revealed a high number of white matter (WM) neurons in SZ (Fung et al., 2011), interpreted as interneuron precursors that failed to migrate, being stuck in the WM. Another interpretation of such a finding could be that the increased number of WM neurons in SZ patients reflects enhanced neuronal migration in response to a signal from a damaged cortical neuronal population. Neurons generated in the subventricular zone typically travel towards the olfactory bulb via the rostral migratory stream, even in the human brain (Curtis et al., 2007). Some newly generated neurons have been reported to reach the cortex in primates (Gould et al., 1999). It could be speculated that if those neurons were to become interneurons, their role would be to replenish a neural population that may be vulnerable to a number insults, such as fast-spiking interneurons. In that case, a cell replenishment approach could be feasible by enhancing the rescue mechanism perhaps provided by normal adult neurogenesis.
Conclusion
Restoring excitation–inhibition balance may be critical to address cognitive deficits in SZ. Several approaches have been attempted in the past decade. A small trial with an agonist for metabotropic glutamate receptors containing alpha 2/3 subunits (mGluR2/3) showed some promise (Patil et al., 2007), but subsequent trials showed an equally high effect of placebo. Other trials with inconclusive results included GABA-A agonists (Lewis et al., 2008). Several studies addressed the possibility of increasing NMDA receptor efficacy with modulators (Javitt et al., 1994; Heresco-Levy, 2005). Results have been encouraging, but far from conclusive. As GABA–glutamate interactions mature during adolescence (O’Donnell, 2011), it is critical that approaches attempting to restore excitation–inhibition balance in SZ are applied at early stages. In addition, antioxidant treatment may also be effective at early stages, and could even prove helpful to prevent conversion in subjects at high risk for SZ. Regardless of the approach, the field needs to establish biological mechanisms that could connect altered immune activation with the disease and identify proper targets at the optimal time for treatment. This is the time for translational approaches and bold, large-scale, long-term trials with sufficient power to be conclusive.
Abbreviations
-
- 8-oxo-dG
-
- 8-oxo-7,8-dihydro-2′-deoxyguanosine
-
- GABA
-
- gamma-aminobutyric acid
-
- GSH
-
- glutathione
-
- LPS
-
- lipopolysaccharide
-
- NAC
-
- N-acetyl cysteine
-
- NMDA
-
- N-methyl-d-aspartate
-
- NVHL
-
- neonatal ventral hippocampal lesion
-
- PFC
-
- prefrontal cortex
-
- PV
-
- parvalbumin
-
- ROS
-
- reactive oxygen species
-
- SZ
-
- schizophrenia
-
- WM
-
- white matter




