An integrated approach to design novel therapeutic interventions for demyelinating disorders
Abstract
Therapeutic strategies are often based on two general principles: interference with the pathogenic process and repair of the damaged tissues. Recent studies, however, have suggested that several pathological conditions may result from the interplay between genetic susceptibility traits and environmental influences that, by modulating the epigenome, also affect disease onset and progression. Based on lessons from neural development, it is conceivable that new lines of preventive and possibly therapeutic intervention might be developed to modulate disease onset or decrease the severity of the symptoms. This review will discuss these concepts within the context of multiple sclerosis, the most common demyelinating disease of the central nervous system, and the leading cause of progressive neurological disability in young adults.
Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating disorder affecting the young adult population (Compston & Coles, 2008), typically affecting individuals between the ages of 20 and 45 years, although the occurrence of pediatric MS is on the rise. Clinical symptoms are variable, depending on the involvement of visual, sensory or motor pathways, and include but are not limited to: ataxia, fatigue, cognitive impairment and autonomic manifestations. A large proportion of patients (approximately 80%) develop a relapsing-remitting (RRMS) course of the disease, characterized by acute relapses and long stretches of symptom-free periods (called ‘remissions’), while a smaller proportion (approximately 10–15%) of the patients do not return to baseline conditions and accumulate disability over time (primary progressive form of the disease). Eventually, the relapsing-remitting course of the disease will enter a phase of incomplete recovery, when the symptoms persist and neurological function deteriorates over time, defining the secondary progressive (SPMS) phase of the disease (Thompson et al., 1997; Rovaris et al., 2006). Here we review current studies and propose an integrated view of disease pathogenesis, with the aim of opening a debate regarding the feasibility of future potential integrated approaches to therapy.
MS as an autoimmune disease
Most current knowledge on the etio-pathogenesis of MS is derived from a large number of epidemiological, histopathological, immunological and genetic studies. Inflammation, demyelination and axonal degeneration are the major pathologic features of MS (Compston & Coles, 2008). It is well accepted that MS prevalence increases with latitude (Alonso & Hernan, 2008) and that its incidence is higher in people with low levels of vitamin D and low sun exposure (Ascherio & Munger, 2007). Its onset has also been associated with Epstein–Barr virus (EBV) infections and possibly also to infections resulting from additional strains of herpesviruses (Kakalacheva et al., 2011). However, there has been no conclusive evidence that any of these factors, per se, is capable of inducing the disease. Rather, viral infections, low vitamin D levels, stress, diet and other lifestyle changes have been proposed to act as co-factors to trigger disease onset in genetically susceptible individuals.
Identifying susceptibility genes for MS has been the subject of intense research since the early 1970s when genes encoding for class II alleles of the major histocompatibility complex (MHC) were first identified (Jersild et al., 1973; Compston et al., 1976; Terasaki et al., 1976). Consequent gene linkage studies in multiplex families and genetic association studies mapped this region in detail to the HLA-DR15 haplotype (Fogdell et al., 1995) and suggested the existence of a gradient on susceptibility to MS depending on the specific combination of allelic variants within this locus (Rasmussen et al., 2001; Dyment et al., 2005). In addition, specific MHC class I alleles showed an independent association and modulation of the risk on HLA-DR15 haplotype carriers, with the HLA-A3 allele as risk-increasing and the HLA-A2 allele as risk-decreasing (Fogdell-Hahn et al., 2000; Harbo et al., 2004; Friese et al., 2008). Further genome-wide association (GWA) studies, analysing > 100 000 single nucleotide polymorphism (SNP) markers, identified additional non-MHC polymorphisms related to MS risk and highlighted the importance of the MHC class II region on disease susceptibility (Lincoln et al., 2005; Sawcer et al., 2005; Burton et al., 2007; Hafler et al., 2007; Friese et al., 2008; Baranzini et al., 2009; De Jager et al., 2009a; The Australian and New Zealand Multiple Sclerosis Genetics Consortium, 2009). Most of the non-MHC genes identified by these studies related to the immune system and had been previously associated with increased susceptibility to autoimmune disorders. Among them, interleukin-7 receptor alpha chain (IL7RA) and other genes in the IL7/IL7R axis showed a close association to MS (Hafler et al., 2007; De Jager et al., 2009b; Zuvich et al., 2010), and therefore were identified as potential therapeutic targets (Sasson et al., 2006). Similarly, the association of the interleukin-2 receptor alpha chain (IL2RA) to MS (Hafler et al., 2007; De Jager et al., 2009b; The Australian and New Zealand Multiple Sclerosis Genetics Consortium, 2009) led to the development of monoclonal antibodies against this protein which are currently being assessed in small clinical trials (Schippling & Martin, 2008). An additional polymorphism associated with MS includes the SNP rs2300747 in the CD58 gene (Hafler et al., 2007; De Jager et al., 2009b; The Australian and New Zealand Multiple Sclerosis Genetics Consortium, 2009), which is critical for regulation of T-cell receptor signaling. Higher levels of CD58 transcripts were detected in remitting MS patients and this allele was associated with a protective effect on MS progression (De Jager et al., 2009a).
Although a thorough review of the genetic studies is beyond the scope of this article, we note that a recent collaborative study including 9772 cases recruited by 23 centers worldwide, including the USA, Australia and several European countries (Sawcer et al., 2011) has confirmed the critical importance of HLA-DRB1 as risk alleles and validated several genes involved in cytokine signaling, including IL2, interferon (IFN) gamma, IL12, tumor necrosis factor (TNF) alpha and IL6 pathways as genes associated with the disease.
Together, the vast number GWA studies have identified the immune system as the primary system modulated by susceptibility genes. We believe that an integrated review of the distinct loci might lead to the development of panels of candidate susceptibility genes that may contribute to a better diagnostic classification of patients and potentially identify individuals that are more likely to benefit from therapies targeting a specific pathway, such as the IL7 (Sasson et al., 2006) or IL2 (Schippling & Martin, 2008) pathway.
Immune-based animal models of demyelination to test therapeutic approaches in MS
The rodent model for MS, experimental autoimmune encephalomyelitis (EAE), is one of the most extensively studied animal models of immune disease to identify the molecular mechanisms involved in the inflammatory response and to assess the validity of new therapies for MS. The origins of this model go back to 1885 when Louis Pasteur’s rabies vaccine was first used clinically and some cases of paralysis were reported subsequently (reviewed by Baxter, 2007). Since the first studies, this has evolved with current immunizations with whole-brain emulsions and myelin proteins or peptides. Immunization of the animals with these compounds leads to a disease that shares clinical and neuropathological changes with MS (Steinman, 1999). Most therapies tested in MS patients are based on concepts derived from studies in EAE, but unfortunately an important number of potential therapies that showed effectiveness in this model failed to demonstrate a positive effect in patients. This can be explained by the fact that the artificial immunization of the animals may not necessarily reproduce all the pathogenic mechanisms in human disease so animal models should be adapted to account for the genetic variability shown in MS. It has been noted that the genetic background of different mouse strains influences the disease course in the EAE model (Miller et al., 2010). Based on these considerations, the development of partially humanized mouse models represents a promising tool to address the influence of the genetic component in disease onset and progression (Gregersen et al., 2004). Transgenic mice expressing MHC-class II molecules and T-cell receptor variants from MS patients may better represent many of the various clinical manifestations and corresponding central nervous system (CNS) lesions of MS (Madsen et al., 1999; Quandt et al., 2004). This represents a very powerful and promising strategy for testing and propose that it could be fully exploited to test proof-of-principle concepts for the involvement of pathways identified by the many GWA studies.
Pharmacogenomic approaches to ‘personalized’ medicine
Growing evidence shows that the clinical response to MS therapies varies widely among patients (Miller et al., 2008) and thus the paradigm of ‘one drug fits all’ has been shifted towards the search for a more ‘personalized’ therapy. Pharmacogenomic studies focus on the identification of genetic variations that have an influence on drug response, in the end aiming at the identification of the patients who will show a more effective response to therapy and will present fewer adverse side effects. This area has already gained great importance as many FDA-approved drugs currently show pharmacogenomic information in its labels (http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm). Some of these drugs are used in the treatment of cancer, viral infections, neurologic and cardiovascular diseases. In many cases the genetic markers identified belong to the cytochrome P450 family, related to the pharmacokinetics (metabolism and clearance) of the drugs, and thus individual variations could affect the effectiveness, adverse events and drug interactions of these treatments. Other examples of markers are different receptors whose expression in several types of tumors is assessed clinically to prescribe antagonists that will be effective in the treatment of the disease. Among them are the detection of estrogen receptors (ER) in breast cancer and epidermal growth factor receptor (EGFR) for colorectal cancer. In other cases, the expression of certain variants of genes will confer significant response to certain drugs, such as the allele rs12979860 of the interferon-lambda-3 gene (IL28B) for the treatment of hepatitis C. Regarding MS treatment, azathioprine (AZA) is an immunosuppressive drug used as an alternative to interferon (IFN) beta and also indicated in other neuroimmunological diseases. The most severe adverse effect is hematological toxicity, which can be fatal in about 0.3% of cases (Connell et al., 1993). Methylation by thiopurine-methyl transferase (TPMT) is the limiting step for the inactivation of AZA. Therefore, the FDA recommends TPMT genotyping and/or phenotyping prior to prescribing AZA treatment, being contraindicated in patients with low or absent TPMT activity (0.3% of the cases). Dose escalation is recommended for intermediate metabolizing enzymes (10%). Several pharmacogenomic studies have identified diverse genetic variants for other disease-modifying therapies for MS but further investigations need to be addressed to provide strong evidence for these observations. Among them, several polymorphisms in the interferon (IFN) pathway and IFN-beta responsive genes (e.g. IL17) have been associated with response to this drug, as well as other neurogenesis- (GPC5, glypican 5; NPAS3, neuronal PAS domain protein 3) and neurotransmission-related genes (AMPA type glutamate receptor GRIA3) (Cunningham et al., 2005; Martinez et al., 2006; Byun et al., 2008; Comabella et al., 2009; Axtell et al., 2010). In addition, responsiveness to glatiramer acetate (GA) treatment has been linked to HLA-DRB1*1501, rs71878 for T-cell receptor-beta and rs2275235 for cathepsin S (CTSS) (Fusco et al., 2001; Grossman et al., 2007). Finally, several polymorphisms in the ATP-binding cassette (ABC)-transporter genes have also been identified as potential predictors for clinical response and toxicity of the immunosuppressant mitoxantrone (Cotte et al., 2009; Dorr et al., 2009).
In conclusion, pharmacogenomic studies are contributing to providing ‘personalized’ therapy for different disorders. The hope is that more data will be available for MS and lead to the creation of platforms that could be used to better stratify the patient population and possibly inform therapeutic decisions.
Environmental effects on individuals with genetic traits induce disease onset and related animal models
Although the genetic evidence implying an effect of the immune system in disease onset is irrefutable, it is undoubtedly significant to note that not all the individuals with a given risk allele develop the disease. In this respect, it is worth noting that even identical twins are only 30% concordant for development of the disease (Hawkes & Macgregor, 2009). What are the factors that allow individuals with the same genetic risk factor to develop or not develop the disease? One of the potential explanations, based on epidemiological data, is that additional environmental components are needed to develop the disease. The concept of genetic susceptibility was described by Poser (2006) as ‘the MS trait’ that would manifest itself only if additional events were to take place (Poser, 2006). This is an attractive hypothesis that is quite reminiscent of the two-hits cancer model proposed by Knudson (2001). Low sun exposure, low vitamin D levels, stressful situations and viral infections could all contribute to trigger the disease in predisposed individuals (Lauer, 2010). An interesting correlation between MS and diet has also been proposed (Ghadirian et al., 1998), related to the consumption of dairy products in countries with a high incidence of MS (Guggenmos et al., 2004). The effect of diet on disease onset in a group of genetically susceptible individuals has been reported in children with type I diabetes, where those who were fed an omega-6-rich diet developed the disease, while those who were fed an omega-3-rich diet were protected (Norris et al., 2007).
An interesting model to test the hypothesis of the link between diet and demyelination is provided by cuprizone. Feeding mice with a diet containing 0.2% of the copper chelator cuprizone results in oligodendrogliopathy followed by myelin loss, microglial infiltration and astrogliosis (Matsushima & Morell, 2001). Furthermore, removal of the toxicant from the diet results in recovery of the lesions due to spontaneous remyelination. Aged mice are more prone to axonal degeneration upon cuprizone-induced demyelination (Irvine & Blakemore, 2006). Previous studies in our laboratory have used this model to test the effect of age in modulating myelin repair (Shen et al., 2008). Some studies have reported a lower extent of cuprizone-induced demyelination in SJL mice compared with C57BL/6 mice (Taylor et al., 2009), highlighting the importance of this model in studying the role of genetic susceptibility in the development of a pathology induced by dietary intake of the copper chelator. To further address the interplay between genetic traits and external dietary factors, we fed eight distinct mouse strains with the same diet for the same period of time and then assessed the levels of demyelination and microglial activation. Quantitative RT-PCR and immunohistochemistry analyses of corpus callosum showed that two mouse strains (i.e. DBA and A/J mice) were highly sensitive to cuprizone, as shown by decreased levels of the myelin protein CNPase in the corpus callosum (Fig. 1A and B). Consistent with the specific effect of cuprizone in the corpus callosum, the effect was only observed in this area and not in the spinal cord (Fig. 1C). In contrast, NOD mice did not respond to the cuprizone diet shown by no decrease in CNPase, indicating that different genetic backgrounds affect the susceptibility to develop a demyelinating pathology in response to dietary manipulations. Previous studies also reported differences in microglia infiltration in the corpus callosum of BALB and C57BL/6 mice, even in the presence of similar demyelination patterns in this specific region (Skripuletz et al., 2008). To determine if microglial activation could account for the differences observed in our experiments, we examined mRNA levels of microglial activators, including chemokine L3 (Chem3), lysozyme, beta2-microglobulin, TNFalpha and cathepsin Z in the strains showing greater differences in CNPase levels (Fig. 1D–H). In general, a greater activation was observed in A/J mice, which could partially explain the higher sensitivity of this strain to cuprizone action. Strikingly, however, DBA mice showed a milder microglial activation pattern, thereby revealing a pattern of extreme sensitivity to cuprizone diet in this strain. In support of a dissociation between microglial activation and demyelination, NOD mice (the most resistant strain to cuprizone diet) also had a significant microglial activation (Fig. 1D and E). Together, these experiments reveal a highly heterogeneous response to dietary cuprizone that depends on the individual genetic background. In connection with the findings mentioned previously in this section, these data support the importance of the interplay between intrinsic (i.e. genetic) and extrinsic (i.e. diet) factors in the onset of demyelinating diseases.

Different susceptibility of mouse strains to cuprizone-induced demyelination. Eight different mouse strains were fed a diet containing 0.2% cuprizone over 4 weeks. Total RNA from freshly dissected corpus callosum (A) and spinal cord (C) was isolated and CNP levels analysed by quantitative (q)RT-PCR. Values obtained were normalized to GAPDH transcript levels and the bar graphs represent the normalized values relative to those measured in the corpora callosum of C57BL/6 mice. (B) Immunofluorescence micrograph of coronal brain sections of DBA, A/J and NOD mice, immunostained with the antibody against the myelin protein CNPase (red) and the nuclear stain DAPI (blue). Images show the corpus callosum. V, ventricle; scale bar = 100 μm. (D–H) Quantitative PCR analyses of the same three mouse strains for different microglial activators normalized to GAPDH transcript levels and expressed relative to the values measured in DBA controls. Statistical differences for all qRT-PCR analyses were determined using two-tailed independent t-tests with Bonferroni correction (*P < 0.05, **P < 0.01, ***P < 0.001).
Importance of early intervention to prevent disease onset
The incidence and prevalence of MS increase at higher latitudes (Alonso & Hernan, 2008). Interestingly, adults migrating from a high- to low-risk area are thought to carry their former high risk with them, whereas people who migrate during childhood seem to have the risk associated with the new area to which they migrated (Dean, 1971; Alter et al., 1978; Elian et al., 1990; Cabre et al., 2005). These observations implicate an environmental event involved in MS pathogenesis which acts sometime between birth and young adulthood but does not act thereafter. It is also believed that even environmental factors during preborn life can influence MS risk. There is evidence of a month-of-birth effect on MS reported in Canada and northern Europe, with more patients born in May and fewer in November (Templer et al., 1992; Willer et al., 2005; Sadovnick et al., 2007). Many environmental factors have been proposed to be linked to MS pathogenesis, including EBV and other virus infection, vitamin D deficiency, low sunlight, occupational hazards, living with domesticated animals, dietary habits, trauma, stress and toxic exposure (Compston et al., 2005). Among them, however, vitamin D deficiency and EBV infection have been increasingly implicated in having a potential role in MS pathogenesis. Careful control of these variables in childhood, for instance by providing adequate vitamin D3 supplementation and monitoring its levels in individuals at high genetic risk, is crucial in determination of the disease course (Fig. 2).
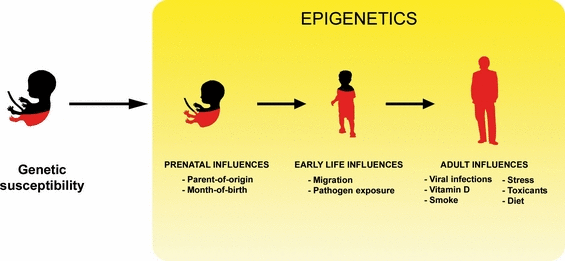
Multiple sclerosis is a complex disease with cumulative risk factors leading to its onset. In addition to genetic predisposition, one or more environmental events need to occur for MS to develop. The time sequence and age of incidence of each factor appears to play a role in disease development. There is growing evidence of an epigenetic mechanism driven by the different environmental risk factors, so it is conceivable that epigenetic modulators could be used during early stages of life to prevent MS onset or during the early stages of the pathology to decrease the severity of the symptoms.
Epigenetic changes detected during neural development may inform therapies later in life
Our laboratory has previously characterized important mechanisms of regulation of developmental myelination that depend on the modification of chromatin components. We have shown that the activity of chromatin-modifying enzymes – histone deacetylases (HDACs) – is essential for oligodendrocyte differentiation in vitro (Marin-Husstege et al., 2002) and in vivo (Shen et al., 2005). We extended this work to the analysis of mechanisms of remyelination in the adult brain (Shen et al., 2008). We previously demonstrated that HDAC enzyme activity is necessary for oligodendrocyte differentiation because it decreases the levels of inhibitors of oligodendrocyte process outgrowth (i.e. stathmin) (Liu et al., 2003, 2005) and of myelin gene expression (i.e. Id4, Hes5) (Gokhan et al., 2005; Marin-Husstege et al., 2006). The use of HDAC inhibitors (HDACi) for MS treatment has been proposed based on their approved use as anti-cancer agents (Marks et al., 2001). However, the use of HDACi for treatment of MS is more controversial as studies on the animal EAE model of demyelination have shown contrasting results (Natarajan & Bright, 2002; Camelo et al., 2005). We also reported the negative effects of treatment with pharmacological blockers of HDAC on oligodendrocyte progenitor differentiation in vitro (Marin-Husstege et al., 2002) and on developmental myelination in vivo (Shen et al., 2005) and during myelin repair after cuprizone-induced demyelination (Shen et al., 2008). We have also described the occurrence of similar mechanisms in the adult MS human brain (Pedre et al., 2011) and therefore caution against the use of HDACi during the early stages of oligodendrocyte differentiation and myelin repair. The involvement of epigenetic changes, particularly in terms of chromatin modifications, is exciting for two reasons. First, it sheds light on the etiology of the early aspects of the disease process. Second, and perhaps more importantly, it may provide insight into how environmental factors can influence disease development and acquisition even in genetically identical patients. This insight comes from the fact that epigenetic changes in the brain have been documented to change significantly over the lifetime of individuals (Hernandez et al., 2011), and to diverge significantly in identical twins (Fraga et al., 2005).
In summary, we have reviewed the different factors that contribute to MS susceptibility, including genetic variants and environmental factors, and have noted that populations which build up several of these risk factors could be eligible for early therapeutic interventions to prevent the onset or lessen the severity of the disease.
We propose integrating available information into the development of a two-stage treatment platform. The first stage would include a careful stratification of patients, based on vitamin D3 levels and on the results of genetic screens, designed on the basis of the currently available GWA study data sets. The second stage would include pharmacological and environmental intervention, aimed at promoting repair. This would be best achieved by taking into account genotypes associated with greater responsiveness or resistance to specific treatments, while awaiting for the development of targeted epigenomic approaches.
Acknowledgements
This work was supported by grants from the National Institute of Health (NINDS-1R01NS069835-01; R01 NS42925-10) and from the National Multiple Sclerosis Society (RG 4134A9/1) to P.C. and by a postdoctoral fellowship from the National Multiple Sclerosis Society to J.L. (FG1874-A-1) and from the Multiple Sclerosis Society of Canada and the Fonds de la Recherche en Santé du Québec to J.H.
Abbreviations
-
- ABC
-
- ATP-binding cassette
-
- EAE
-
- experimental autoimmune encephalomyelitis
-
- EBV
-
- Epstein–Barr virus
-
- EGFR
-
- epidermal growth factor receptor
-
- ER
-
- estrogen receptors
-
- GA
-
- glatiramer acetate
-
- GWA
-
- genome-wide association
-
- HDACi
-
- HDAC inhibitors
-
- HDACs
-
- histone deacetylases
-
- IFN
-
- interferon
-
- IL
-
- interleukin
-
- MHC
-
- major histocompatibility complex
-
- RRMS
-
- relapsing-remitting multiple sclerosis
-
- SNP
-
- single nucleotide polymorphism
-
- SPMS
-
- secondary progressive multiple sclerosis
-
- TNF
-
- tumor necrosis factor
-
- TPMT
-
- thiopurine-methyl transferase




