Fatigue-induced increase in intracortical communication between mid/anterior insular and motor cortex during cycling exercise
Abstract
In the present study, intracortical communication between mid/anterior insular and motor cortex was investigated during a fatiguing cycling exercise. From 16 healthy male subjects performing a constant-load test at 60% peak oxygen consumption (VO2peak) until volitional exhaustion, electroencephalography data were analysed during repetitive, artefact-free periods of 1-min duration. To quantify fatigue-induced intracortical communication, mean intra-hemispheric lagged phase synchronization between mid/anterior insular and motor cortex was calculated: (i) at the beginning of cycling; (ii) at the end of cycling; and (iii) during recovery cycling. Results revealed significantly increased lagged phase synchronization at the end of cycling, which returned to baseline during recovery cycling after subjects’ cessation of exercise. Following previous imaging studies reporting the mid/anterior insular cortex as an essential instance processing a variety of sensory stimuli and signalling forthcoming physiological threat, our results provide further evidence that during a fatiguing exercise this structure might not only integrate and evaluate sensory information from the periphery, but also act in communication with the motor cortex. To the best of our knowledge, this is the first study to empirically demonstrate that muscle fatigue leads to changes in interaction between structures of a brain’s neural network.
Introduction
Exercise-induced muscle fatigue is defined as a reversible reduction in the neuromuscular system’s capacity to generate force or power (Fitts & Holloszy, 1976; Bigland-Ritchie et al., 1983). Underlying causes of muscle fatigue are manifold, and have been addressed not only to peripheral changes within the working muscle but also to central processes residing in the sensorimotor pathway of the CNS. As a component of central fatigue, supraspinal fatigue was identified and defined as a loss of force due to an insufficient output from the motor cortex (Gandevia et al., 1996; Taylor et al., 2000).
Muscle afferents type III/IV sensitive to mechanical, chemical or noxious stimuli of other origin have been assumed to inhibit motor cortical output cells (Martin et al., 2008) and/or sites driving the motor cortex (Gandevia et al., 1996; Taylor et al., 1996, 2000), thus exerting potential influence on supraspinal fatigue. Indeed, a causal relationship between spinal μ-opioid receptor-sensitive muscle afferents and motor cortical inhibition was demonstrated by a temporary afferent blockade attenuating a fatigue-related increase in intracortical inhibition (Hilty et al., 2011). However, a comprehensive understanding of a brain’s fatigue-related neural network comprising assumed relay stations communicating with motor cortex cells remains elusive.
In a functional magnetic resonance imaging (fMRI) study investigating neural activity during a maximal 2-min handgrip contraction, brain structures such as the insular and cingulate cortex have been reported to play an important role in integrating inhibitory influence arising from group III and IV muscle afferents (Liu et al., 2002, 2003). In studies of experimentally induced muscle pain, an increase in neural activity within widespread regions of both the insular and cingulate cortex was proven, besides other regions found to be activated also by other forms of pain (Kupers et al., 2004; Henderson et al., 2006). Furthermore, the anterior insular cortex has consistently been shown to be involved not only in pain processing but also in the evaluation of other homeostatic processes, such as air hunger and hunger for food (Tataranni et al., 1999; Brannan et al., 2001; Liotti et al., 2001; Evans et al., 2002; Craig, 2003b). In accordance, prominent mid/anterior insular activation was revealed directly before task failure in an isometric muscle fatiguing handgrip contraction potentially alerting the organism to urgent homeostatic imbalance (Hilty et al., 2010).
In consideration of these findings, we assume that during fatiguing exercise the mid/anterior insular cortex is of essential relevance not only by signalling forthcoming physiological threat (Reiman, 1997), but also by acting in communication with the motor cortex. On the basis of surface electroencephalography (EEG) signals transformed into 3D spatial current density distributions, neurophysiological inter- and intrahemispheric communication between time series corresponding to different spatial locations can be assessed by computing lagged phase synchronization of oscillatory neural activity (Pascual-Marqui, 2007a). We hypothesized that at the end of an exhaustive cycling exercise signalling between mid/anterior insular and motor cortex is increased, and thus lagged phase synchronization is higher than at the beginning.
Materials and methods
Subjects
Seventeen male subjects [age: 25.9 ± 3.5 years (mean ± SD); height: 184.4 ± 5.8 cm; mass: 80.7 ± 8.0 kg; peak oxygen consumption, VO2peak: 4.7 ± 0.5 L/min] participated in this study. Due to technical difficulties, data from one subject had to be excluded, resulting in a final sample of 16 subjects considered for data analyses. All subjects were regularly performing a minimum of 3 h weekly endurance training. They did not suffer from any neurological/respiratory disorders, and did not take any medication/nutritional supplements. Subjects were instructed to refrain from stressful activities and to adhere to usual caffeine consumption, 24 and 12 h, respectively, prior to exercise participation. Each subject was precisely informed about any risks and inconveniences associated with the experiment before written informed consent was obtained. All procedures were conducted in accordance to the standards set by the Declaration of Helsinki.
Procedure
In a pre-test, participants had to perform an all-out incremental exercise test on a cycling ergometer (Ergoline 800; Ergonometriesysteme, Bitz, Germany) in order to assess VO2peak using a calibrated metabolic cart (OxyconPro, Jaeger, Hoechberg, Germany). This exercise test, throughout which a freely chosen pedalling frequency of > 70 revolutions/min had to be kept constant (Kohler & Boutellier, 2005), consisted of a 4-min warm-up cycling at 100 W followed by subsequent, iterative workload increases of 50 W every 2 min.
Separated by at least 4 days from this pre-test, an experimental exercise test had to be performed. Subjects exercised on a racing bicycle attached to an electromagnetic cycle trainer (Tacx cycleforce basic T1601, Wassenaar, the Netherlands) by providing the same pre-assessed pedalling frequency at a workload of 60% VO2peak (245.6 ± 28.6 W) until volitional exhaustion. Instead of using the electromagnetic power control of the cycle trainer, its resistance was mechanically adjusted until appropriate constant workload was generated at the individual’s chosen pedalling frequency. Both power output and pedalling frequency were continuously assessed by means of an SRM Training System (PowerMeter IV; SRM, Jülich, Germany). However, only pedalling frequency was visualized to the subjects throughout exercise. An EEG cap was mounted on the subjects’ scalp and two electrodes were placed on the subjects’ neck muscles bilaterally (M. trapezius, pars descendens) in order to check for muscle artefacts. Cables of the cap were stuck on the subjects’ back in order to minimize shifting during exercise. After a 4-min warm-up cycling inside an air-conditioned Faraday cage (temperature: 19.6 ± 0.3 °C, humidity: 31.2 ± 5.6%), subjects rested on the bicycle and EEG electrodes were checked for required impedances (< 30 kΩ; see EEG recordings). In addition, three 30-s sessions of eyes closed (EC) alternating with three 30-s sessions of eyes open (EO) were recorded. During EO, subjects had to fixate a cross marked on a white sheet, which was stuck on a wall in front of them. Throughout the following constant-load exercise test, EEG analysis periods of 1 min duration were given, during which the subjects were instructed to keep their upper body as still as possible while keeping up cycling. These periods were repeated every 5 min, with the first period assessed 1 min after exercise beginning. Onsets and time progression of each 1-min period were verbally announced in order to sensitize and remind the subjects to fixate the cross, relax their jaw muscles and move their upper body as little as possible. Directly after each period, the subjects had to verbally rate their perceived exertion (RPE) on the commonly used Borg scale ranging from 6 (‘no exertion at all’) to 20 (‘maximal exertion’; Borg, 1975). Heart rate was continuously recorded with a Polar Sports Tester (Polar Electro, Kempele, Finland). When subjects were exhausted and gave up cycling, they were instructed to rest calmly on the bicycle. During this resting period, the same alternating sessions of EO and EC were carried out again. Thereafter, the resistance of the cycle trainer was released and subjects cycled at the same cadence as before providing 50–60 W for another 15 min. During the last minute of recovery cycling, a final EEG analysis period was assessed with the same instructions mentioned above.
EEG recordings
Dense array EEG System from Geodesics (128-channel HydroCel Geodesics Sensor Net, Net Station software 4.3.1; Electrical Geodesics, Eugene, OR, USA) was used for EEG data acquisition. An appropriate elastic tension 128-Ag/AgCl-Electrode Sensor Net cap was individually selected from three different sizes, and impedances were improved by soaking synthetic sponges with KCl-solution for 10 min prior to the experiment. Data were sampled at 500 Hz, filtered using an infinite impulse filter (0.3–100 Hz), digitized with a 16-bit A/D converter and re-referenced to the vertex (Cz).
Data analysis
Brain Vision Analyzer 2.0 software (Brain Products, München, Germany) was used for off-line processing of the EEG raw data. A rather narrow band-pass filter from 3.75 to 30 Hz (time constant: 0.0424, 24 db/octave) was used in order to remove movement and muscle artefacts, which were to be expected at about 1–1.5 Hz [pedalling frequency (movement artefacts)] and > 30 Hz (muscle artefacts). Independent Component Analysis was subsequently calculated in order to effectively detect, separate and remove activity in EEG records arising from various artefactual sources such as eye blinks (Jung et al., 1998). In each participant, 20 channels located marginally on the EEG cap were excluded from further analysis as they frequently suffered from bad skin contact. From the remaining 108 electrodes intervals of EEG signals were skipped if any of the following criteria defined in a semi-automatic raw data inspection were not met: low activity, > 0.3 μV; amplitude, −200 μV to 200 μV; difference (Max−Min), < 200 μV; gradient, < 50 μV/ms. Moreover, if a channel’s signal comprised > 10% artefact contaminated intervals (occurring on average in 2.9 ± 1.7 electrodes per subject), its signal was interpolated with data of its four nearest neighbours. Then, EEG artefact-free data were fragmented into data sets of 1-s duration providing on average 55 ± 6 and 54 ± 5 segments per subject from the first and last period of the fatiguing cycling exercise, as well as 56 ± 2 from the last period of recovering cycling. Finally, 1-s segments of the four conditions were exported into standardized low-resolution brain electromagnetic tomography (sLORETA) software (http://www.unizh.ch/keyinst/loreta.htm; Fuchs et al., 2002; Pascual-Marqui, 2002; Jurcak et al., 2007).
In order to eliminate potential remaining risks of electromyographic activity contaminating EEG data in the β (12.5–35 Hz) band (O’Donnell et al., 1974; Davidson, 1988), sLORETA analysis of EEG data during exercise cycling was restricted to investigations of EEG signals in the α/μ (7.5–12.5 Hz) band. This frequency band has been shown to be particularly sensitive to various aspects of motor activity (Pfurtscheller et al., 2000). In order to judge possible remaining artefacts, we inspected spectral plots at the scalp locations C3, C4, T9, T10, O1, O2, AF3, AF4. These plots are provided in Supporting Information Fig. S1.
Two anatomical regions of interest (ROI) were constructed on each hemisphere by defining all voxels located within a radius of 5 mm around designated seed points. For the first ROI on each hemisphere, seed points were located at Montreal Neurological Institute coordinates (Evans et al., 1993) [−10, −40, 65 (x y z) and 10, −40, 65 (x, y, z), respectively], as they cover left and right representations of the legs within the motor cortex. In terms of the second ROI covering left and right mid/anterior insular region, Montreal Neurological Institute coordinates [−40, 5, 10 (x, y, z) and 40, 5, 10 (x, y, z), respectively] were determined with respect to a previously conducted study, showing that this part of the insular cortex is involved in limiting physical performance in a muscle-fatiguing exercise (Hilty et al., 2010).
Dynamic changes of fatigue-related functional coupling in the α/μ band between the defined ROI have been assessed by calculating mean lagged phase synchronization (Pascual-Marqui, 2007a) of intra-hemispheric connections (left insular cortex with left motor cortex and right insular cortex with right motor cortex) from both the first and the last period of fatiguing cycling as well as during the last minute of recovery cycling. Because lateralization was not the focus of this study, lagged phase synchronization values within each hemisphere were averaged over hemispheres. Lagged phase synchronization computation is based on a collection of single epoch data (cross-spectra calculated for each condition and subject) and a corresponding discrete Fourier transform for signal within the ROI. Unlike other coherence measures, phase synchronization is insensitive to amplitude information. Because we merely test for increasing communication but would like to include activating as well as inhibiting signalling, we do not necessarily assume a positive correlation of amplitudes and thus expect phase synchronization to be the most suitable measure. In fact, inhibitory influences from mid/anterior insular to motor cortex would be most likely, for which phase synchronization is likely to be most sensitive. We computed lagged measures rather than regarding instantaneous synchronization for several reasons. First, this approach has the advantage over non-lagged calculations that it is insensitive towards volume conduction (Nolte et al., 2004; Stam et al., 2007). Second, sLORETA searches for the smoothest possible current density distribution explaining the measured EEG topography and thereby maximizes similarity of the assumed signal over a spatially distributed number of voxels. This artificially inflates instantaneous coherence measures, which therefore should be avoided. And third, neuronal signal conduction requires time, which means that only delayed (lagged) coherence between brain regions can result from neuronal communication.
Calculations of lagged phase synchronization used in this study are performed using sLORETA, and are more explicitly described by Pascual-Marqui (2007b). Briefly, phase synchronization PS is based on the absolute value of the covariance s between the normalized Fourier transforms of two signal time series X and Y at frequency ω.

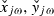 denoting the normalized discrete Fourier transform at frequency ω of the time series X and Y segmented into NR segments of equal duration. The value of this measure is determined to a large degree by its instantaneous component, which receives its largest contribution from non-physiological artefacts such as volume conduction (Nolte et al., 2004) and by spatial smoothing. This problem was addressed early on by Nolte et al. (2004) for the classical coherence measure, where they proposed the imaginary part as a measure of physiological connectivity that is not influenced by volume conduction. Related methods were proposed by Stam et al. (2007) for phase synchronization. Partly motivated by these previous results, an improved measure of physiological connectivity was developed (Pascual-Marqui, 2007b; Pascual-Marqui et al., 2011) that explicitly models and appropriately separates the instantaneous (non-lagged) connectivity. Instead of the imaginary part of the phase synchronization:
denoting the normalized discrete Fourier transform at frequency ω of the time series X and Y segmented into NR segments of equal duration. The value of this measure is determined to a large degree by its instantaneous component, which receives its largest contribution from non-physiological artefacts such as volume conduction (Nolte et al., 2004) and by spatial smoothing. This problem was addressed early on by Nolte et al. (2004) for the classical coherence measure, where they proposed the imaginary part as a measure of physiological connectivity that is not influenced by volume conduction. Related methods were proposed by Stam et al. (2007) for phase synchronization. Partly motivated by these previous results, an improved measure of physiological connectivity was developed (Pascual-Marqui, 2007b; Pascual-Marqui et al., 2011) that explicitly models and appropriately separates the instantaneous (non-lagged) connectivity. Instead of the imaginary part of the phase synchronization:

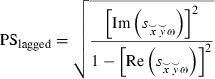
It is shown in Pascual-Marqui et al. (2011) that when the instantaneous connectivity component is large, the Nolte et al. (2004) imaginary part tends to zero, whereas the lagged measure retains non-zero physiologically relevant information on connectivity.
In order to ensure that modulations in lagged phase synchronization did not arise due to changes in EEG power (Florian et al., 1998), mean α/μ power was assessed at the same pre-defined ROI (left and right insular cortex, left and right motor cortex) from the first and the last period of fatiguing cycling as well as during the last minute of recovery cycling. Additionally, μ power serves as an indicator of overall neuronal activity in the motor cortex (Laufs et al., 2003; Pfurtscheller et al., 2006).
Another parameter having been presumed to confound EEG activity is hyperthermia (Nielsen et al., 2001; Rasmussen et al., 2004; Ftaiti et al., 2010). Therefore, body temperature was assessed by using an electronic thermometer (Welch Allyn 690 SureTemp Plus, Welch Allyn, NSW, Australia; ± 0.1 °C accuracy) and by averaging values from three repetitive oral measurements just prior to exercise, and from eight measurements performed within 4 min after exhaustion, respectively. During temperature measurements, subjects were instructed to keep their mouth permanently closed with the probe maintained in the right posterior sublingual pocket at the base of their tongue. Subjects were allowed to drink from a water-filled CamelBak® as much as preferred, though, only between periods of EEG measurements and until a RPE of 18 was reached. Furthermore, after subjects’ cessation of exercise they were instructed to close their mouth and breathe through their nose. These restrictions were met, firstly, in order to avoid movement artefacts due to drinking and, secondly, to ensure proper post-exercise measurements of oral temperature unaffected by water intake or increased respiration.
With particular regard to a pioneering study of Schneider et al. (2009), who recorded brain cortical activity in the α and β band with EC before and after different kinds of fatiguing exercises, in the present study both the α and β band were analysed during resting conditions with EC before and after fatiguing cycling.
Statistics
Electrophysiological data were subjected to repeated-measures anovas with the factor Time with three levels: beginning of cycling; end of cycling; and recovery cycling. To guard against effects of heteroscedasticity, Greenhouse–Geisser correction was used. Where anova revealed significant effects, post hoc t-tests were applied. Results are expressed as mean ± SD.
To assess differences in α and β band power at resting conditions during EC between measurements before and after exercise, non-parametric randomization tests were used. Reported are voxels that survived significance thresholding at P = 0.05, defined by 5000 randomizations, corrected for multiple comparisons.
Results
Behavioural data, heart rate and body temperature
The mean exercise time of the experimental cycling exercise was 35.5 ± 9.9 min (mean ± SD), with 6.4 ± 1.7 periods of EEG analysis included. After subjects’ access to water was quit (RPE 18), mean exercise time until exhaustion (RPE 20) was 10.3 ± 4.7 min. At the end of the fatiguing protocol, mean heat rate was significantly higher than at the beginning of cycling (150.5 ± 10.1 vs. 181.7 ± 8.6 beats/min, respectively, t15 = −18.5, P < 0.001). A significant increase was also found in body temperature after fatiguing cycling compared with pre-exercise values (36.9 ± 0.2 °C vs. 37.0 ± 0.2 °C, respectively, t15 = −2.375, P = 0.031).
Electrophysiological data
anova of lagged phase synchronization between intra-hemispheric mid/anterior insular and motor cortex connection revealed a significant effect of Time within the α/μ band (F1.965,15 = 10.049, P = 0.001). Subsequent post hoc t-tests showed a significantly higher lagged phase synchronization at the end of cycling than at the beginning of cycling (t15 = −3.383, P = 0.004; Fig. 1). A significantly lower lagged phase synchronization was found during recovery cycling than at the end of cycling (t15 = 4.158, P = 0.001). No significant change in lagged phase synchronization was observed from the beginning of cycling to recovery cycling (t15 = 0.571, P = 0.576).
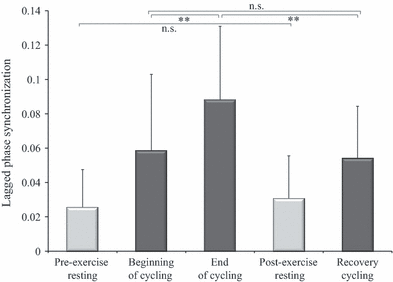
Mean lagged phase synchronization between the mid/anterior insular and the motor cortex within α/μ frequency band during resting (pre-/post-exercise) and cycling conditions (beginning/end/recovery) of EO. Error bars represent inter-individual SD. Within the cycling conditions a main effect of Time was revealed. Significant changes from post hoc t-tests are depicted by **P < 0.01; n.s., not significant.
Analysis of mean α/μ power within the mid/anterior insular cortex revealed a significant effect of Time (F1.101,15 = 5.470, P = 0.03; Fig. 2A). During recovery cycling, α/μ power was significantly lower than at both the beginning (t15 = 2.787, P = 0.014) and the end of cycling (t15 = 6.480, P < 0.001). No significant change in α/μ power could be detected from the beginning to the end of cycling (t15 = 0.995, P = 0.336).
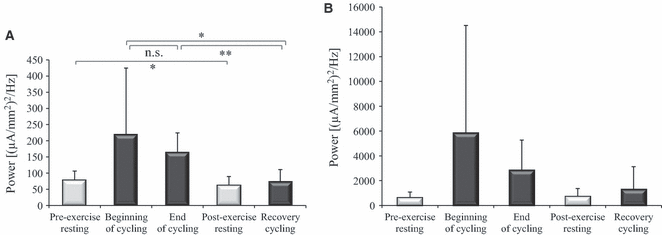
Mean α/μ power in the mid/anterior insular (A) and the motor cortex (B) during resting (pre-/post-exercise) and cycling conditions (beginning/end/recovery) of EO. Error bars represent inter-individual SD. For the mid/anterior insular cortex but not for the motor cortex, a main effect of Time was revealed within the cycling conditions. Significant changes from post hoc t-tests are depicted by **P < 0.01; *P < 0.05; n.s., not significant.
Only a trend was found for Time to influence α/μ power within the motor cortex (F1.039,15 = 3.838, P = 0.067; Fig. 2B).
Whole-brain analysis during resting conditions of EC revealed a widespread increase within both the α and β power, which reached significance (t15 = 4.229, Max t = 9.04, Extreme P < 0.001 for α, and t15 = 4.08, Max t = 4.95, Extreme P = 0.005 for β) only in Brodmann area 11 after exhaustive cycling exercise (Fig. 3).
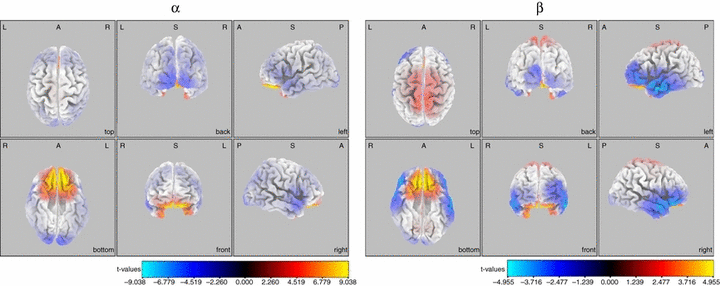
Statistical parametric maps of sLORETA showing regional changes within the α and β frequency bands after fatiguing cycling exercise compared with prefatigue baseline during EC. Areas coloured in yellow/red and blue represent power increases and decreases, respectively, within the specific frequency band. Orthogonal views from different perspectives are displayed. A, anterior; L, left hemisphere; P, posterior; R, right hemisphere. For interpretation of color references in figure legend, please refer to the Web version of this article.
Discussion
In the present EEG study, fatigue-related neural communication between mid/anterior insular and motor cortex was investigated during a constant-load cycling exercise by calculating lagged phase synchronization within α/μ rhythm at the beginning vs. the end of exercise as well as vs. recovery cycling. As hypothesized, data revealed a significant increase in lagged phase synchronization at the end of cycling, which levelled off and returned to baseline during recovery cycling after subjects’ cessation of exercise.
Lagged phase synchronization unaffected by power and temperature
This increase in lagged phase synchronization cannot be explained by changes in power of the respective frequency band (Jung et al., 1998), because regarding mean α power in the mid/anterior insular and the motor cortex at the end of cycling, no significant increase was found. Rather, α power tended to decrease over time, which – considering negative correlation of α power with cortical activity (Laufs et al., 2003) – confirms previous findings from fMRI studies showing an increased cortical activity within the mid/anterior insular (Hilty et al., 2010) and the sensorimotor cortex (Liu et al., 2003) towards the end of a fatiguing exercise.
As a consequence of prolonged exercise in the heat, high body temperature has been shown to increase α/β power index, which is defined as the area of α power spectrum divided by the area of β power spectrum (Nielsen et al., 2001; Nybo & Nielsen, 2001; Rasmussen et al., 2004; Ftaiti et al., 2010). In order not to misinterpret EEG data potentially contaminated by movement and muscle artefacts, β power was not assessed during cycling exercise and, thus, α/β power index could not be calculated. However, controlled ambient temperature of 19.6 ± 0.3 °C and an observed increase in body temperature of < 1 °C at the end of exercise do not allow the assumption of an influence on EEG, as similar conditions in control trials of hyperthermia-related studies did not lead to changes in α/β power index (Nielsen et al., 2001; Nybo & Nielsen, 2001). Moreover, by positioning an electrode 1 cm in front of Cz chosen to represent motor areas of the legs, hyperthermic-induced increase in α/β power index was demonstrated to arise from decreased β power (Nybo & Nielsen, 2001) rather than an increase in α power. Thus, we conclude that our result of decreased α/μ power at the end of fatiguing exercise is not influenced by thermic effects.
Role of mid/anterior insular cortex in homeostasis considering anatomic connections
Over the past few years, a concept of a central governor has been elaborated, which posits a brain’s regulative network both integrating afferent information from the periphery and determining work rate as well as exercise termination, all to ensure that homeostasis is maintained (St Clair Gibson & Noakes, 2004; Noakes et al., 2005). Using neuroimaging methods, the anterior insula has been elucidated as a structure being involved in the evaluation of various distressing stimuli threatening homeostasis (Cannon, 1935), such as visceral and cutaneous pain (Binkofski et al., 1998; Baciu et al., 1999; Casey, 1999), air hunger (Brannan et al., 2001; Liotti et al., 2001; Evans et al., 2002) and hunger for food (Tataranni et al., 1999). Furthermore, prominent mid/anterior insular activity was also demonstrated just before cessation of a fatiguing handgrip exercise, suggesting this structure may be of essential relevance for mediating the termination of a muscle-fatiguing exercise in order to protect the integrity of the organism (Hilty et al., 2010). With the current data, previous findings of a mid/anterior insular cortex signalling potential physiological threat (Reiman, 1997; Craig, 2003a) are extended by demonstration of a fatigue-related increase in communication between the insular and the motor cortex in the context of an exhaustive bodily exercise. However, it remains speculative whether this increased intracortical communication arises from processes driven bottom up, for example by muscle afferents type III/IV and/or from influences interacting from top down. From anatomical observations, evidence exists for an afferent processing via lamina I spino-thalamic pathway involved in homeostatic regulation in humans: small-diameter afferents relating information on the physiological status of various tissues – for example pain (nociceptors), heat/cold (thermoreceptors), acidic pH (metaboreceptors) and hypo-osmolarity (osmoreceptors), monosynaptically activate neurons, which originate in the lamina I of the superficial spinal dorsal horn (Craig et al., 2000). In primates, lamina I neurons project not only to various brainstem homeostatic integration sites, but also to two sides of the contralateral thalamus, namely, the posterior part of the ventral medial nucleus and the ventral caudal part of the medial dorsal nucleus, with the former region being assumed to further project topographically to the mid/anterior insular cortex (Craig et al., 1994). Lamina I neurons have been shown to provide an ascending pathway for automatic cardiorespiratory adjustments to muscular work (Wilson et al., 2002), and may be of essential relevance also during prolonged cycling (Rauch et al., 2005).
From the mid/anterior insular cortex efferents were found to project to the primary somatosensory cortex (Flynn et al., 1999), which in turn has been shown to be directly connected with the motor cortex in cats (Porter & Sakamoto, 1988). Alternatively, intracortical communication between the mid/anterior insular and the motor cortex may occur via relay stations such as motor control areas, for example the supplementary motor area, which has been shown both to receive input from mid insula (Flynn et al., 1999) and to project directly to the motor cortex (Picard & Strick, 1996). Thus, anatomical knowledge gives reason to assume that afferent information about the physiological status of the periphery might reach the motor cortex by motor and/or sensory efferents from the mid/anterior insular cortex, which in turn receives input from the lamina I pathway via thalamic relay.
Fatigue-induced changes in brain cortical activity
Indications that other regions are also involved in the cortical phenomenon related to muscle fatigue come from α and β power analyses before and after cessation of exhaustive exercise (Schneider et al., 2009). These authors found significant increases in α and β power within parietal as well as limbic regions after an incremental cycling exercise. In part, we can replicate their findings revealing an increased β power over parietal regions in our data but, moreover, widespread increases in α and β power reaching significance were found in Brodmann area 11 (medial orbitofrontal cortex, mOFC).
Termination of an unpleasant or painful event can be rewarding (Seymour et al., 2005) – an emotional experience the mOFC has consistently been shown to play a prominent role in (McClure et al., 2004; Xu et al., 2009; Sellitto et al., 2010). Moreover, the mOFC has been demonstrated to be involved in processing mental fatigue (Tajima et al., 2010), which has been associated with physical fatigue not only in various disorders such as Parkinson’s disease (Lou et al., 2001) or multiple sclerosis (Schreurs et al., 2002), but also in exercising healthy humans (Marcora et al., 2009). Furthermore, the mOFC is associated with a ‘quasi-hyperbolic’ time-discounting function (Laibson, 1997), reflecting a trade-off between short-term and long-term reward-related decision outcomes, and has been shown to be preferentially activated for choices involving an immediate reward (McClure et al., 2004). In the context of an exhaustive exercise, reward has to be postponed, but instead an increasing perception of discomfort has to be endured. A prominent increase in power within the mOFC immediately after termination of cycling might be interpreted as a consequence of a prolonged deactivation of this structure.
Limitations
Fatigue-induced lagged phase synchrony does not give evidence about any directional properties of functional interconnectivity, i.e. whether mid/anterior insular activity influences motor cortex activity or vice versa. Based on previous anatomical findings showing efferent pathways from the mid/anterior insular cortex projecting onto the motor cortex, an increasing influence from the mid/anterior insular cortex to the motor cortex seems more likely than vice versa. Moreover, we assume that active inhibitory influence of the mid/anterior insular cortex has been exerted on motor cortex activity for the following reason: α/μ power, which has been shown to inversely relate to cortical activity, significantly decreased within the mid/anterior insular cortex, whereas only a trend of decrease within the motor cortex was detected at the end of fatiguing exercise with respect to its beginning. Thus, neuronal activity in the insular cortex may have increased, but no proportional increase could be observed in M1.
Conclusion
Data from the present study revealed enhanced lagged phase synchronization between the mid/anterior insular and the motor cortex at the end of a fatiguing cycling exercise, demonstrating a fatigue-induced increase in communication between those regions. These new insights provide a valuable basis for further studies investigating cortical mechanisms of supraspinal fatigue. Applying causal analysis methods, the motor cortex could be tested on inhibitory/excitatory influences exerted from the mid/anterior insular cortex. Moreover, it remains to be established whether the motor cortex is further influenced directly or indirectly by other regions, for example the prefrontal cortex involved in motivational processes.
Acknowledgement
The present work was in part supported by the Swiss National Science Foundation (SNF, Grant Nr. 320030-777111).
Abbreviations
-
- EC
-
- eyes closed
-
- EEG
-
- electroencephalography
-
- EO
-
- eyes open
-
- fMRI
-
- functional magnetic resonance imaging
-
- mOFC
-
- medial orbitofrontal cortex
-
- ROI
-
- region of interest
-
- RPE
-
- ratings of perceived exertion
-
- sLORETA
-
- standardized low-resolution brain electromagnetic tomography
-
- VO2peak
-
- peak oxygen consumption