Neurotrophin receptors TrkB.T1 and p75NTR cooperate in modulating both functional and structural plasticity in mature hippocampal neurons
Abstract
Tropomyosin-related kinase (Trk) receptors modulate neuronal structure and function both during development and in the mature nervous system. Interestingly, TrkB and TrkC are expressed as full-length and as truncated splice variants. The cellular function of the kinase-lacking isoforms remains so far unclear. We investigated the role of the truncated receptor TrkB.T1 in the hippocampus of transgenic mice overexpressing this splice variant by analyzing both neuronal structure and function. We observed an impairment in activity-dependent synaptic plasticity as indicated by deficits in long-term potentiation and long-term depression in acute hippocampal slices of transgenic TrkB.T1 mice. In addition, dendritic complexity and spine density were significantly altered in TrkB.T1-overexpressing CA1 neurons. We found that the effect of TrkB.T1 overexpression differs between subgroups of CA1 neurons. Remarkably, overexpression of p75NTR and its activation by chemical induction of long-term depression in slice cultures rescued the TrkB.T1-dependent morphological alterations specifically in one of the two subgroups observed. These findings suggest that the TrkB.T1 and p75NTR receptor signaling systems might be cross-linked. Our findings demonstrate that TrkB.T1 regulates the function and the structure of mature pyramidal neurons. In addition, we showed that the ratio of expression levels of p75NTR and TrkB.T1 plays an important role in modulating dendritic architecture and synaptic plasticity in the adult rodent hippocampus, and, indeed, that the endogenous expression patterns of both receptors change reciprocally over time. We therefore propose a new function of TrkB.T1 as being dominant-negative to p75NTR.
Introduction
Ligand binding of the neurotrophin tropomyosin-related kinase (Trk) receptors activates a series of different, well-described signaling cascades, and thereby controls neuronal survival, differentiation, axonal outgrowth, and synaptic plasticity (Huang & Reichardt, 2003) [for reviews, see Patapoutian & Reichardt (2001)]. Interestingly, alternative splicing generates truncated, kinase-lacking forms of both the TrkB and TrkC receptors [TrkB.T1 (T1), TrkB.T2 and TrkC.T1] (Klein et al., 1990; Middlemas et al., 1991; Tsoulfas et al., 1993). Since the discovery of the truncated Trk receptors, their physiological function in vivo has been a matter of debate. Interestingly, the ratio between the expression levels of full-length and truncated receptors depends both on the developmental stage and on the neuronal cell type investigated (Allendoerfer et al., 1994; Escandon et al., 1994; Fryer et al., 1996). Overexpression studies in ferret cortical slices have shown that the ratio of T1 to full-length TrkB (TrkB.TK+) can act as a switch between two different modes of dendritic growth –de novo growth and elongation of already existing dendrites (Yacoubian & Lo, 2000).
The role of the truncated Trk receptors was initially identified as restricting the action of the full-length receptors through the formation of heterodimers. Indeed, T1 overexpression studies suggest a role of the truncated receptor as a dominant-negative inhibitor of TrkB.TK+ signaling (Eide et al., 1996; Drake et al., 1999; Haapasalo et al., 2001, 2002; Ohira et al., 2001; Lahteinen et al., 2002). Furthermore, T1 has been shown to act as a brain-derived neurotrophic factor (BDNF)-scavenging receptor (Klein et al., 1990; Middlemas et al., 1991; Biffo et al., 1995; Eide et al., 1996; Saarelainen et al., 2000a), thereby indirectly regulating the action of TrkB.TK+. Deletion of T1 partially rescues BDNF haploinsufficiency, suggesting that it may, in fact, negatively modulate BDNF–TrkB.TK+ signaling in vivo (Carim-Todd et al., 2009).
A possible signaling role has been proposed independently of TrkB.TK+ (Baxter et al., 1997; Rose et al., 2003; Ohira et al., 2005; Cheng et al., 2007). In addition, Rose et al. (2003) showed that T1 is the predominant variant expressed in astrocytes, and leads to calcium release via protein kinase A and inositol trisphosphate.
Furthermore, there is ample evidence that T1 is able to interact with another neurotrophin receptor – p75NTR (Hartmann et al., 2004). In developing hippocampal neurons, overexpression of T1 has been shown to induce the formation of dendritic filopodia. Remarkably, this outgrowth of protrusions seems to require the action of p75NTR (Hartmann et al., 2004).
In this study, we aimed at characterizing the functional role of T1 in the structure and function of mature hippocampal neurons, and to clarify its active (signaling) or passive (dominant-negative) mechanism of action.
Materials and methods
Mouse strains
Transgenic mice expressing T1 under the Thy1.2 promoter were generated by the group of Eero Castren (University of Helsinki), and all procedures were approved by the Experimental Animal Ethics Committee of the National Laboratory Animal Center, University of Kuopio, Finland (Saarelainen et al., 2000c; Koponen et al., 2004a,b). Mice were housed in metal cages under standard animal room conditions (12 : 12-h light/dark cycle, ambient temperature of 23 °C, and free access to food and water).
Briefly, cDNA of T1 was tagged N-terminally with an eight amino acid FLAG peptide and inserted into the murine Thy1.2 expression cassette to direct expression to postnatal neurons. The constructs were transferred by pronucleus injection into embryos from CD2F1 females (BALB/c × DBA/2) mated with CD2F1 males. Male and female mice were used for the experiments. BALB/c × DBA/2 mice were used as wild-type (WT) controls.
Expression of the transgene starts at postnatal day (P)10, increases until P18, and remains stable thereafter (Saarelainen et al. 2000b). Strong expression of the transgene can be detected in hippocampal pyramidal neurons, dentate granule cells and pyramidal neurons of the cerebral cortex (Saarelainen et al. 2000b).
For all experiments, the mice were genotyped by PCR with genomic DNA extracted from tail pieces. PCR was performed with thy1.2 and trkB specific primers (thy1.2, CTCCCACTTCCTTGGCTT; TrkB, GCCCCACGTAAGCTTCGA (Koponen et al., 2004b). All other experiments were performed with cultures of C57BL/6 mice.
Cell culture techniques
Organotypic hippocampal slice cultures were prepared as previously described (Stoppini et al., 1991). P5/6 mice were decapitated, the skulls were removed, and the dorsal halves of the brain were transferred to ice-cold GBSS (Gey's Balanced Salt Solution). Four-hundred-micrometer transversal hippocampal slices were cut with a McIllwain tissue chopper, and kept at 4 °C for 30 min in GBSS. Subsequent cultivation was performed on tissue culture inserts [Millicell; 0.4-μm pore size, hydrophilic poly(tetrafluoroethylene) membrane], four slices each insert, at 37 °C, 5% CO2 and 99% humidity. To reduce the number of non-neuronal cells, antimitotic drugs (uridine, cytosine-β-d-arabinofuranoside hydrochloride, and 5-fluoro-2′-deoxyuridine) were applied for 24 h 3 days after preparation. Subsequently, 50% of the medium was changed every 3 days.
Primary hippocampal cultures were prepared from C57Bl/6 mice [embryonic day (E)18]. The tissue was incubated for 30 min in trypsin–EDTA at 37 °C, and then mechanically dissociated with a Pasteur pipette. Cells were plated at high density (105 per coverslip) on poly (l-lysine)-coated coverslips (13 mm) and kept in Neurobasal medium (Gibco) supplemented with 2% B27 (Gibco) and 0.5 mm Glutamax at 37 °C, 5% CO2, and 99% humidity. The cell culture medium was not changed.
Transfection of hippocampal neurons
Organotypic hippocampal slice cultures were transfected at 14 days in vitro (DIV) with the Helios Gene Gun system of Bio-Rad. Gold microcarriers 600 nm in diameter were shot onto the slice with helium at a pressure of 100 lb/in2. To avoid damage to the tissue, culture inserts with a pore diameter of 3 μm were used as filters.
Bullets for transfection were prepared according to the manufacturer’s instructions (BioRad). Briefly, 2 μg/mg plasmid DNA was coated onto 0.6-μm gold microcarriers. To visualize neuronal morphology in detail, a membrane targeted form of enhanced green fluorescent protein (EGFP) [farnesylated form of EGFP (FGFP)] was transfected at a 1 : 2 ratio. For coating of DNA onto the gold microcarriers, Ca2Cl precipitation was performed (Wellmann et al., 1999; O’Brien & Lummis, 2006). Primary cultures of hippocampal neurons made at E18 were transfected at 17 DIV with Lipofectamine2000, following the manufacturer’s instructions.
Electrophysiological recordings
Four-hundred-micrometer transverse hippocampal slices of TrkB.T1 transgenic or WT CD2F1 mice (P14–P19) were prepared and maintained by standard procedures, as described previously (Gartner et al., 2006) – (in mM) 124 NaCl; 2.95 KCl; 1.25 KH2PO4; 2 MgSO4; 26 NaHCO3; 2.5 CaCl2; 10 d-glucose; 95% O2; 5% CO2. Monopolar tungsten electrodes were used for stimulation in the CA3 Schaffer-collateral region. Synaptic field potentials were elicited with a frequency of 0.1 Hz, and responses were recorded with borosilicate glass electrodes, with a resistance between 5 and 15 MΩ, filled with 3 m NaCl in the dendritic region (stratum radiatum) of the CA1 pyramidal neurons. The slope of the excitatory postsynaptic potential was calculated and used to measure synaptic strength. Long-term depression (LTD) was induced by low-frequency stimulation (LFS) with 900 1-Hz pulses for 15 min. Long-term potentiation (LTP) was induced with a tetanus of 3 × 30 pulses (100 Hz, 60-μs duration, 5-s interstimulus interval). Data were collected with a program written in labview (National Instruments, Austin, TX, USA). All measurements were carried out in a strictly blind fashion with regard to the genotype of the mice used. Statistical analysis was performed with a paired Student’s t-test (two-tailed and two-sample unequal variance). Results were considered to be significantly different if the average response 55–60 min after the induction of LTD or LTP showed P-values below 0.05.
Immunocytochemistry
Organotypic and primary hippocampal cultures were fixed overnight at 4 °C with 4% paraformaldehyde in phosphate buffer. Washing was performed at room temperature in phosphate-buffered saline (PBS) for 1 h, and this was followed by blocking and permeabilization at room temperature in PBS containing 1% bovine serum albumin, 10% goat serum and 0.2% Triton X-100 for 1 h. All primary antibodies were incubated at 4 °C in PBS containing 10% goat serum. Rabbit polyclonal anti-human p75NTR [anti-human p75 pAb G3231 (Promega, Mannheim, Germany), directed against the cytoplasmic domain of p75NTR] was used at a dilution of 1 : 500 (3 days) for organotypic cultures and at a dilution of 1 : 4000 on primary hippocampal neurons (overnight). Polyclonal rabbit anti-T1 antibody [rabbit anti-TrkB(TK−), C-terminus, gp95, C13/sc-119 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), directed against the intracellular domain specific for T1] was diluted 1 : 1000 (overnight) for primary neurons. Secondary anti-mouse or anti-rabbit antibodies conjugated with Cy2, Cy3 or Cy5 (Jackson ImmunoResearch, PA, USA) were incubated at a dilution of 1 : 500 in PBS for 2 h at room temperature, and this was followed by washing in PBS for 1 h. Subsequently, the cultures were mounted in aqueous medium containing anti-fading agents (Biomeda, CA, USA). Golgi staining was performed with the FD Rapid GolgiStain kit (FD NeuroTechnologies, MD, USA). Briefly, 2-month-old T1 transgenic mice or WT control mice were rapidly anesthetized (CO2) and decapitated. The brains were removed, briefly rinsed with distilled water to remove the remaining blood, and processed according to the manufacturer’s instructions. One hundred and fifty-micrometer transversal hippocampal slices were cut with a vibratome (Leica VT 1000S). Slices were mounted on gelatine-coated slides, stained according to the manufacturer’s instructions, and imaged by light microscopy as described below for neurons derived from organotypic cultures.
Image acquisition and analysis
Neurons were imaged using an Axioplan 2 microscope (Zeiss) equipped with an ApoTome module (Zeiss) controlled by the axiovision software. For imaging of the entire CA1 neurons in the organotypic cultures, several z-stacks of 1 μm were acquired with a × 20 0.8 NA Plan-APO objective (Zeiss). In primary hippocampal cultures, plain fluorescence images of hippocampal neurons were acquired. For analysis of spine density in organotypic cultures, parts of basal and both proximal and distal apical dendrites were imaged at a higher magnification with a × 63 1.4 NA Plan-APO oil immersion objective (Zeiss) and a z-stack thickness of 0.5 μm. In primary neurons, spines of secondary and tertiary dendrites in the mid-part of the dendritic tree were imaged with the same settings as above. For the expression time-course of T1 and p75NTR, images were taken with a Zeiss 510 META confocal microscope and a × 40 1.3 NA oil immersion objective.
Morphological analysis was performed with neurolucida and neurolucida explorer software (Microbrightfield).
Statistical analysis
Obtained values for Sholl analysis (Sholl, 1953), spine density or dendrite number and length were exported to Excel and Graphpad prism for statistical analysis with a paired Student’s t-test (two-tailed and two-sample unequal variance); significance was set at P < 0.05. For the Sholl analysis, data significance was tested point by point, and statistical significance was only considered if more than two adjacent points showed P-values below 0.05. All data are shown as mean ± standard error of the mean.
Results
Overexpression of T1 alters the morphology of mature pyramidal neurons
For study of the role of T1 in regulating the architecture of mature CA1 pyramidal neurons, organotypic hippocampal slice cultures of transgenic mice expressing T1 under the Thy1.2 promoter were used (Saarelainen et al., 2000a; Koponen et al., 2004b). Strong expression of the transgene has been detected in hippocampal pyramidal neurons, dentate granule cells and pyramidal neurons of the cerebral cortex (Koponen et al., 2004b).
For detailed morphological analysis, hippocampal slice cultures of transgenic T1 mice were transfected with FGFP, by the use of particle-mediated gene transfer. Mature CA1 pyramidal neurons overexpressing T1 showed marked simplification of the mid-apical dendrite (Fig. 1A, arrows). Detailed Sholl analysis of the apical dendrites of T1-overexpressing neurons confirmed this impression. Dendritic complexity was significantly reduced at a distance between 330 and 430 μm from the soma (Fig. 1A and B). Interestingly, morphological alterations were restricted to the distal half of the apical dendrite – a decrease in complexity of the mid-apical compartment and an elongation of the apical tufts. In addition, the apical dendrite displayed striking elongation (Fig. 1A).
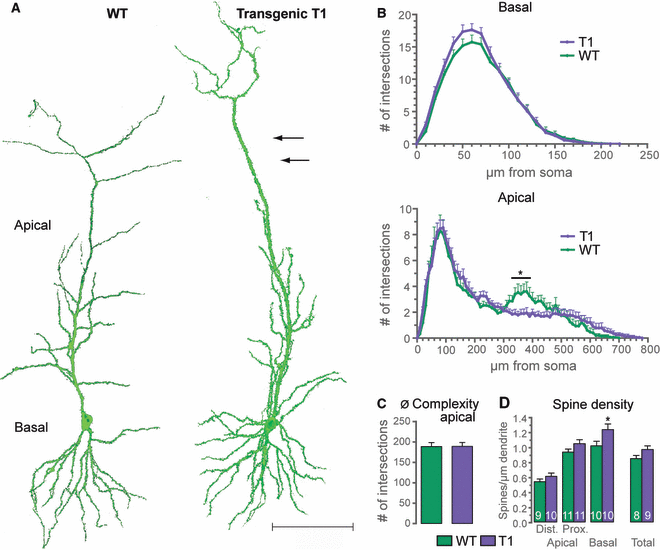
Overexpression of T1 alters neuronal morphology. (A) CA1 neurons (17 DIV) of transgenic mice overexpressing T1 as compared with WT neurons. T1-overexpressing neurons show a reduction in dendritic complexity of the mid-apical dendrite (arrows) as compared with control cells. Images were captured from maximum intensity projections. Scale bar – 100 μm. (B) Sholl analysis (basal and apical dendrites) of T1-overexpressing CA1 neurons (n = 17) as compared with control cells (n = 15). Overexpression of T1 significantly decreases the dendritic complexity of the apical dendrite at a distance between 330 and 430 μm from the soma; in addition, T1-overexpressing neurons are longer than WT cells. (C) Total dendritic complexity and (D) spine density of CA1 pyramidal neurons overexpressing T1; overexpression of T1 significantly increases the spine number of the basal compartment, whereas the total dendritic complexity is unaltered in T1-overexpressing neurons as compared with control cells; *P < 0.05.
As it is known that even pyramidal neurons of the same subgroup (e.g. CA1) can display morphological differences from each other, we investigated whether certain subpopulations of CA1 neurons would react differently to T1 overexpression. Among 17 neurons analyzed, seven displayed Sholl values between 600 and 700 μm from the soma for the apical dendrite, whereas only two of 15 neurons among the control cells exceeded this length. Therefore, we divided the T1-overexpressing pyramidal neurons into two subgroups – those showing Sholl values for the region above 600 μm from the soma (Fig. 2, group A); and those that did not exceed this length (Fig. 2, group B). Interestingly, both groups displayed significant differences when compared to control cells that were, in each group, restricted to different compartments. Specifically, group A neurons showed significant elongation of the distal apical tuft, whereas group B neurons were significantly more complex at approximately 200 μm from the soma (Fig. 2).
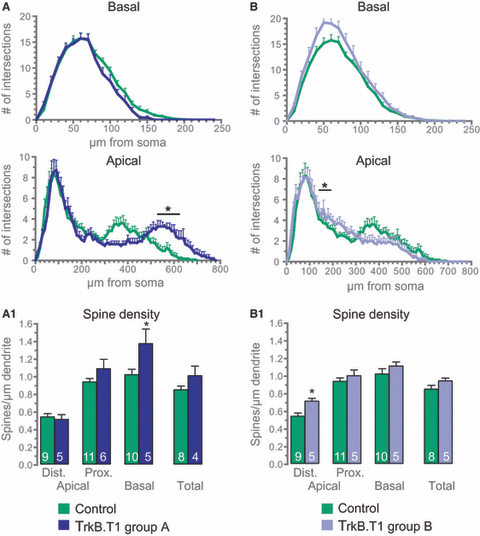
Overexpression of T1 has different effects on subgroups of CA1 pyramidal neurons. (A) Neurons overexpressing T1 that displayed Sholl values for a distance of 600–700 μm from the soma were considered as group A and analyzed separately (n = 7) from control neurons (n = 15). (B) Neurons that displayed no Sholl values exceeding a distance of 600 μm from the soma were considered as group B (n = 10). Sholl analysis reveals that the two subgroups show significant differences in the different dendritic compartments when compared to control cells. (A1) Spine density of group A CA1 neurons. (B1) Spine density of group B neurons. Spine numbers are also differentially affected by overexpression of T1 in the two subgroups; *P < 0.05.
As a next step, spine density counts were performed on T1-overexpressing CA1 neurons. Total spine density was increased in T1-overexpressing neurons (Fig. 1D). However, only the basal dendritic compartment showed a significantly increased number of dendritic protrusions (Fig. 1D; Table S1). Again, as with the analysis of dendritic structure, we divided the neurons overexpressing T1 into two groups, A and B, and analyzed the spines separately. Interestingly, both groups showed significant changes in spine number when compared to control cells that were again restricted to different compartments, as was observed for dendritic complexity (Fig. 2A1 and B1; Table S1). Spine analysis of group A neurons revealed a significantly increased spine number in the basal dendritic compartment, whereas group B neurons showed a significant increase in the number of dendritic protrusions only in the distal apical tuft.
These data and a previous report showing an increase in filopodia-like protrusions following T1 overexpression in developing primary hippocampal neurons (Hartmann et al., 2004) prompted us to investigate the spine subtype composition in T1 transgenic slice cultures. By means of morphological analysis, three different spine subtypes can be classified: stubby, thin and mushroom spines (Fig. 3A) (Chicurel & Harris, 1992; Koh et al., 2002). This classification is based on measurements of the total spine length as well as the ratio between the spine head and the spine neck diameters, and provides an objective, observer-independent classification of spines (Fig. 3A). In line with previous reports (Zagrebelsky et al., 2005; Chakravarthy et al., 2006), mushroom spines comprised the predominant spine type in mature pyramidal neurons analyzed in this study (Fig. 3). T1-overexpressing neurons showed no alterations in spine subtype composition (Fig. 3) in the basal and in the proximal apical compartments as compared with control cells.
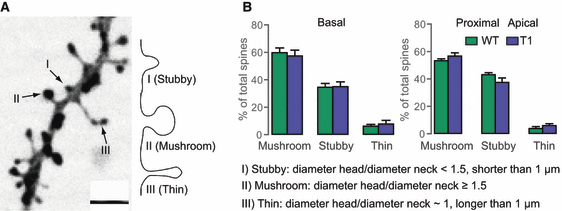
Spine subtype composition is not altered in CA1 neurons overexpressing T1. (A) Representative image of a dendrite from a WT cells showing the three spine types. Scale bar – 2 μm. Spine types can also be seen in the illustration at the right (the criteria used for classification are indicated in B, and are based on the length of the spine and the ratio of the diameters of the head and neck) – type I, stubby spine; type II, mushroom spine; and type III, thin spine. (B) Proportions of the three spine types (basal and proximal apical dendrites) of T1-overexpressing CA1 neurons (n = 5) as compared with control cells (n = 5); spine subtype composition is not affected by overexpression of T1.
We chose organotypic slice cultures for the analysis of T1 function because of the superior properties of these cultures in terms of spine type composition and analysis of dendritic complexity. As a next step, we addressed the question of whether the phenotype of T1 overexpression can also be detected in vivo. Therefore, we stained the whole brains of 8-week-old mice with the Golgi method, and analyzed dendritic complexity and spine density in the hippocampal CA1 subfield (Fig. S1). The phenotype of a significant increase in spine density of the basal compartment was also found in the intact brains of transgenic T1 mice in comparison with control cells (P < 0.05). Because of the high density of labeled cells, we were only able to analyze the dendritic structure of the apical dendrite for a distance of up to approximately 300–400 μm from the soma. However, the dendritic phenotype of reduced dendritic complexity in the mid-apical dendrite and an increase in the complexity of the basal dendrites was confirmed by in vivo experiments.
Overexpression of T1 impairs synaptic plasticity in CA1 pyramidal neurons
The observation of an altered dendritic architecture in T1 transgenic slice cultures led us to consider whether overexpression of T1 would also affect synaptic plasticity in the hippocampal CA1 region. This is of special importance, because although the BDNF–TrkB system has been shown to regulate positive synaptic plasticity (Gottmann et al., 2009) [for reviews, see Poo (2001)], the p75NTR receptor has been implicated in negative synaptic plasticity (Rosch et al., 2005; Woo et al., 2005), and T1 overexpression might interfere with both signaling pathways (Biffo et al., 1995; Eide et al., 1996; Haapasalo et al., 2001; Hartmann et al., 2004; Carim-Todd et al., 2009). To this end, LTP and LTD were induced in the Schaffer-collateral pathway by the use of high-frequency stimulation or LFS protocols, respectively. LTP could be evoked in slices of both WT and T1-overexpressing mice. However, in the latter, we found LTP maintenance to be significantly impaired (Fig. 4A). Sixty minutes after high-frequency stimulation, the mean field excitatory postsynaptic potential (fEPSP) slope (as a percentage of the baseline) of transgenic T1 mice was 139.0 ± 4.6% as compared with 160.7 ± 6.2% in WT mice (P < 0.001, t-test).
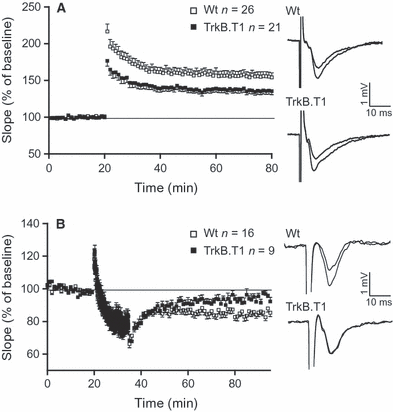
Synaptic plasticity is impaired in mice overexpressing T1. (A) LTP (induced by stimulation with 100-Hz tetanus) is significantly impaired in mice overexpressing T1 (WT, 26 slices/13 mice; T1, 21 slices/nine mice). Single fEPSPs from both genotypes before and 60 min after the tetanus are shown at the right. (B) LTD (induced by LFS, 15 min, 1 Hz) is significantly impaired in mice overexpressing T1 (WT, 16 slices/six mice; T1, nine slices/six mice), Single fEPSPs from both genotypes during baseline and 60 min after the application of LFS are shown at the right.
The overall capacity to induce changes in synaptic strength seems to be compromised in T1-overexpressing mice, as we found that LTD was also impaired. Again, as was the case for LTP, the maintenance but not the induction of LTD was significantly impaired in transgenic mice overexpressing T1 (Fig. 4B). In T1 mice, the mean slope 60 min after LFS was 93.8 ± 2.1% (as a percentage of baseline) as compared with 82.8 ± 2.1% in WT mice (P < 0.01, t-test).
T1-mediated changes involve p75NTR
Neurotrophins modulate synaptic transmission. BDNF and its receptor TrkB have been shown to be crucial for the long-lasting enhancement of synaptic efficacy (LTP) (Korte et al., 1995; Lu, 2003) [for reviews, see Poo (2001)]. In contrast, studies in p75NTR knockout (KO) mice suggest an important role of this receptor in maintaining LTD (Rosch et al., 2005; Woo et al., 2005). In our study, the observed impairment of LTP in T1-overexpressing mice might be induced either by its dominant-negative effect on TrkB.TK+ or by its ligand-scavenging function for BDNF. However, the additional impairment in LTD indicated a possible role of T1 beyond its function as the dominant-negative form of TrkB.TK+, and our next experiments were therefore aimed at further characterizing the possible mechanisms involved. In this context, it has been reported before that T1 might interact with p75NTR to modulate neuronal morphology (Hartmann et al., 2004). In addition, T1 has been shown to functionally interact with p75NTR to induce neuronal differentiation (Hapner et al., 1998). Another indication that p75NTR is involved in modulating the phenotype of T1 transgenic mice was provided by our analysis of dendritic structure. Specifically, the grouping of T1-overexpressing neurons revealed that the dendritic morphology of group A neurons resembled that of p75NTR KO cells, as described previously (Zagrebelsky et al., 2005), with an increase in complexity in the distal apical dendrite. Therefore, we explored whether the structural changes that we observed following overexpression of T1 were indeed linked to p75NTR. We addressed this question by comparing the morphological changes occurring in WT and T1-expressing neurons biolistically transfected with p75NTR in organotypic slice cultures. Neurons overexpressing p75NTR or both transgenic T1 and p75NTR showed no signs of degeneration in the form of swellings or retraction bulbs (Fig. 6A). Overexpression of p75NTR alone led to a reduction in both dendritic complexity and spine density of CA1 hippocampal pyramidal cells (Fig. S2A and B; Table S1). This phenotype is in line with the role of p75NTR as a negative modulator of dendritic architecture (Zagrebelsky et al., 2005).

Induction of LTD can compensate for T1-induced morphological alterations. (A) Sholl analysis (basal and apical dendrites) and (B) spine density of CA1 pyramidal neurons of transgenic mice overexpressing T1 as well as neurons overexpressing T1 48 h after the chemical induction of LTD by a 10-min application of 20 μm NMDA. The induction of LTD reverses morphological changes caused by the transgenic expression of T1 both at the level of dendrites and at the level of spines; *P < 0.05.
Remarkably, the phenotype of the T1-overexpressing neurons of the group A subtype could no longer be detected in cells overexpressing both T1 and p75NTR, as none of the 11 neurons analyzed displayed Sholl values for the region above 600 μm from the soma (Fig. 5). Indeed, a detailed Sholl analysis revealed that the dendritic complexity of neurons overexpressing both T1 and p75NTR was no longer significantly different from that of WT cells (Fig. 5A and B, apical). Similarly, the analysis of spine density in CA1 neurons coexpressing T1 and p75NTR revealed that the increase in spine number of the basal dendrites in group A T1-overexpressing neurons (1, 2) could no longer be detected in neurons overexpressing T1 and p75NTR (Fig. 5C; Table S1). Interestingly, the significant increase in spine density of the distal apical tuft found in T1-overexpressing cells of the group B type (Fig. 2B1) (Fig. 5C; Table S1) was also observed in neurons overexpressing both T1 and p75NTR.

Expression of p75NTR in CA1 neurons overexpressing T1 compensates for morphological alterations mediated by both receptor types. (A) Maximum intensity projections of a CA1 pyramidal neuron overexpressing transgenic T1 (organotypic cultures, DIV 17) and a cell overexpressing T1 and p75NTR (anti-p75NTR labeled in red). Both neurons were transfected with FGFP to allow detailed morphological analysis. Overexpression of both receptors compensates for the T1-mediated reduction in dendritic complexity in the mid-apical dendrite. Scale bar – 100 μm. (B) Sholl analysis (basal and apical dendrites) of CA1 neurons overexpressing transgenic T1 or transgenic T1 together with p75NTR. (C) Spine density of control cells, neurons overexpressing T1 and neurons overexpressing both T1 and p75NTR. Note that the significant changes in spine density caused by overexpression of T1 are reversed by concomitant overexpression of p75NTR; *P < 0.05.
To determine whether an induction of activity known to involve the action of endogenous p75NTR would have a comparable effect, we bath applied 20 μmN-methyl-d-aspartic acid (NMDA) for 10 min in order to induce LTD, and fixed the slices 2 days later. This protocol has been reported before to induce a p75NTR-dependent form of LTD (Woo et al., 2005), and we confirmed the reliability of the approach in our system with fEPSP recordings (data not shown). The overall dendritic structure of NMDA-treated neurons was normal as compared with control cells, suggesting that the stimulation did not lead to any degeneration of CA1 cells. Sholl analysis of basal and apical dendrites revealed only a slight (not significant) decrease in the dendritic complexity of NMDA-treated neurons as compared with control cells, which was restricted to the basal dendrites (Fig. S2C). A detailed analysis showed that the chemical induction of LTD resulted in the loss of spines both in the basal dendrites and in the proximal apical dendrites, as spine density counts revealed a significant reduction for both compartments as compared with control cells (Fig. S2D; Table S1).
Remarkably, Sholl analysis of T1-overexpressing neurons treated with 20 μm NMDA showed no difference as compared with WT cells (Fig. 6A). Again, as was the case for neurons overexpressing p75NTR and T1, cells of the group A type were not detected, as only one of 14 neurons displayed Sholl values for the region above 600 μm from the soma. This observation indicates that the changes in dendritic morphology observed in T1-overexpressing neurons of the group A type (1, 2 and A1) could be completely restored by the induction of LTD (Fig. 6A). Interestingly, the phenotype of a significant increase in spine number of the distal apical dendrite that could be observed in T1-overexpressing neurons of the group B type was also found in T1-overexpressing neurons treated with NMDA (Fig. 5B; Table S1), indicating that this phenotype could not be restored.
Comparable treatment with 60 mm KCl instead of NMDA to increase overall synaptic activity, while not inducing synaptic plasticity, in slice cultures showed that neurons of the group A type did not react to this stimulus, in striking contrast to the plasticity-inducing NMDA treatment (Fig. S3).
Taken together, these observations show that the overexpression of T1 has different effects in subpopulations of CA1 pyramidal neurons, with a group of cells showing a comparable phenotype to that of p75NTR KO neurons. This indicates a dominant-negative role for T1 for p75NTR. Further support of this hypothesis is the fact that LTD is negatively affected in slices from T1 transgenic mice. This is also the case in p75NTR KO mice. Moreover, overexpression of p75NTR or activation of the endogenous receptor in transgenic T1 slice cultures leads to a restoration of the dendritic structure, making it indistinguishable from that of WT cells.
Mutual inhibition of T1 and p75NTR depends on the extracellular domain of T1 but not on ligand binding
Trk receptors and p75NTR are coexpressed in many neuronal populations. In this study, we performed immunohistochemistry on primary hippocampal cultures to follow the developmentally regulated endogenous expression levels of both T1 and p75NTR (Figs S4 and S5). Indeed, both receptors are coexpressed in these neurons; however, their expression levels vary in an age-dependent manner. p75NTR immunostaining reveals high expression levels in developing neurons at two DIV and seven DIV that decrease during maturation (Fig. S4). On the other hand, T1 levels were found to be regulated in the opposite direction. T1 expression was low at early developmental stages and increased after 14 DIV (Fig. S5).
To further characterize the presumably inhibitory effect of T1 on p75NTR, T1 deletion mutants (Haapasalo et al., 1999) were transfected in primary hippocampal neurons. In a first step, overexpression of T1 and p75NTR in primary hippocampal neurons confirmed the results obtained in organotypic slice cultures shown in Fig. 6. Indeed, overexpression of T1 reduced the dendritic complexity of primary hippocampal neurons, as indicated by a reduction in the number of dendritic endings as compared with control cells (Fig. 7B; 71 ± 3 dendrites in the control group; 58 ± 3 dendrites in T1-overexpressing neurons; P = 0.004). Overexpression of p75NTR alone also led to a reduction in dendritic complexity (55 ± 4 dendrites in p75NTR-overexpressing cells; P = 0.003). The dendritic complexity of neurons overexpressing both T1 and p75NTR was indistinguishable from that of control cells (Fig. 7B; 63 ± 5 dendrites). A similar effect was observed after the activation of endogenous p75NTR via the chemical induction of LTD (20 μm NMDA, 10 min) in primary hippocampal neurons overexpressing T1. Remarkably, dendritic complexity in these cells was not significantly different from that in control cells (Fig. 7C; 61 ± 5 dendrites in neurons treated with NMDA; 70 ± 4 dendrites in T1-overexpressing cells treated with NMDA).
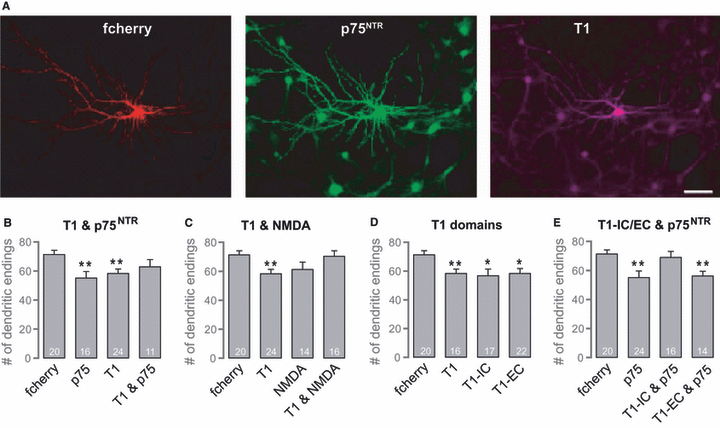
The compensatory effects of coexpression of T1 and p75NTR on neuronal morphology are dependent on the extracellular domain of T1. (A) Fluorescence images of a primary hippocampal neuron transfected with fcherry (control), p75NTR and T1. Scale bar – 50 μm. (B) Histogram showing the number of dendritic endings of primary hippocampal neurons transfected with fcherry, p75NTR, T1, or p75NTR and T1. In primary hippocampal cultures, overexpression of T1 or p75NTR significantly reduces dendritic complexity; coexpression of both neurotrophin receptors prevents this reduction. (C) Histogram showing the number of dendritic endings of neurons transfected with fcherry or T1, and/or treated with 20 μm NMDA for 10 min and fixed 48 h later. As in organotypic cultures, the chemical induction of LTD prevents the T1-dependent reduction in dendritic complexity. (D) Histogram showing the number of dendritic endings of neurons transfected with fcherry, T1 or T1 deletion mutants lacking the extracellular domain (T1-EC) or the intracellular domain (T1-IC). The T1-mediated reduction in dendritic complexity is dependent on the extracellular as well as the intracellular domain of T1. (E) Histogram showing the number of dendritic endings of cells transfected with fcherry, p75NTR or T1 deletion mutants and p75NTR. The extracellular domain of T1 is necessary and sufficient to rescue the p75NTR-dependent reduction in dendritic complexity; *P < 0.05, **P < 0.005.
The expression of T1 lacking either the extracellular domain (T1-EC) or the intracellular domain (T1-IC) resulted in a significant simplification of the dendritic tree (Fig. 7D; 57 ± 4 dendrites in neurons overexpressing T1-IC, as compared with control cells, P = 0.008; 58 ± 3 dendrites in neurons overexpressing T1-EC, as compared with control cells, P = 0.006). This is especially interesting because the intracellular domain of T1 comprises only 23 amino acids. However, coexpression of T1 deletion mutants and p75NTR revealed that the mutual compensation effect of the two receptors observed before (Fig. 5B) depends on the extracellular domain of T1. Specifically, neurons coexpressing T1-EC and p75NTR showed a significantly reduced dendritic tree and therefore no compensation. However, the concomitant expression of T1-IC and p75NTR induced no morphological alterations (Fig. 7E; 56 ± 3 dendrites in neurons overexpressing T1-EC and p75NTR, P = 0.001; 69 ± 4 dendrites in neurons overexpressing T1-IC and p75NTR).
In a last set of experiments, we invstigated whether the inhibitory effect depends on the presence of the TrkB ligand BDNF. BDNF-scavenging antibodies (provided by M. Sendtner, Würzburg, Germany) (Wiese et al., 2007) were used to block BDNF in primary hippocampal neurons. Remarkably, blocking of BDNF had no effect on the inhibitory effect of T1 on p75NTR (Fig. S6). Neurons overexpressing T1 and p75NTR treated with function-blocking anti-BDNF antibody were not significantly different from WT cells (Fig. S6; 56.6 ± 3 dendrites in control neurons vs. 56.6 ± 4 dendrites in neurons overexpressing T1 and p75NTR and treated with anti-BDNF antibody).
In summary, experiments in primary hippocampal neurons confirmed an inhibitory effect of T1 on p75NTR with regard to morphological alterations. Furthermore, we showed that this effect depends on the extracellular domain of T1 but not on binding of the ligand BDNF.
Discussion
The fact that T1 is upregulated during late developmental stages suggests a particular role for this receptor in the adult nervous system. Interestingly, we found both synaptic plasticity – LTP and LTD – and dendritic architecture to be altered in the hippocampus of transgenic T1-overexpressing mice. Further experiments aimed at revealing a mechanism responsible for the T1-mediated structural changes identified a functional inhibitory effect of T1 on p75NTR. We showed that T1 seems to play different roles in subsets of CA1 pyramidal neurons, with a group of T1-overexpressing neurons morphologically resembling p75NTR KO cells. Indeed, when p75NTR was overexpressed in T1 transgenic CA1 neurons, the phenotype of this group of cells was abolished. Interestingly, this inhibitory effect depends on the extracellular domain of T1 but not on the neurotrophin BDNF. The expression of T1 and the ratio of expression levels to those of the other neurotrophin receptors – TrkB and p75NTR– might therefore be particularly used in the adult central nervous system to tightly control neuronal function and structure.
T1 as a modulator of both neuronal architecture and function in the adult nervous system
Whereas TrkB.TK+ constitutes the predominant isoform early in development, the expression level of T1 rises slowly to exceed the amount of TrkB.TK+ in the adult brain (Fryer et al., 1996). It is still a matter of debate whether this rise in T1 expression is predominantly attributable to expression in glial cells; however, we and others have previously shown that T1 is indeed expressed in neurons (Allendoerfer et al., 1994; Escandon et al., 1994; Armanini et al., 1995; Fryer et al., 1996; Kryl et al., 1999; Ohira et al., 1999, 2004; Silhol et al., 2005, 2007).
The overexpression of T1 in this study was restricted to neurons. In mature CA1 pyramidal neurons, an increase in the expression level of T1 resulted in different morphological alterations in distinct subsets of the cells analyzed. Remarkably, both subgroups of T1-overexpressing neurons showed significant differences, indicating that T1 plays different roles in neurons. The factors that might account for these differences are not yet clear; however, the experiments performed in this study give a first hint that the endogenous expression levels of all three types of neurotrophin receptor might be responsible for the differences in the phenotypes of transgenic T1-overexpressing neurons. It is known that pyramidal neurons of the hippocampus can vary in the expression levels of the different neurotrophin receptor types – p75NTR vs. TrkB receptors (Friedman, 2000; Zagrebelsky et al., 2005). Friedman et al. (2000) reported that 40% of the hippocampal principal neurons show no expression of TrkB.TK+. In this neuronal subpopulation, overexpression of T1 might, in fact, have different consequences than in neurons expressing both TrkB and p75NTR or only TrkB. Indeed, approximately 40% of our T1 transgenic neurons showed a phenotype comparable to that of p75NTR KO cells, indicating that T1 might indeed block p75NTR signaling in these cells. Two observations made in this study further underline this functional model. First, we observed a deficit in NMDA receptor-dependent LTD in transgenic T1 mice; this is a form of synaptic plasticity that depends on p75NTR. Second, overexpression of p75NTR and activation of the endogenous p75NTR receptor abolished this phenotype in organotypic slice cultures, whereas a stimulation paradigm that simply increased neuronal activity (60 mm KCl) had no effect on the morphology of these cells. Interestingly, the phenotype of the second subset of cells overexpressing T1 (group B) was not restored by the overexpression of p75NTR. This finding supports the notion that this could be a different neuronal subpopulation with distinct expression levels of neurotrophin receptors. Therefore, the phenotype of the overexpression of T1 might have different underlying mechanisms in this population, for example blockade of TrkB.TK+. Interestingly, this would confirm the findings of previous studies (Haapasalo et al., 2001, 2002). Indeed, this second phenotype of an increase in the spine density of the distal apical tuft (group B) was restored in T1 slices stimulated with 60 mm KCl, a treatment that is known to increase basal neuronal activity. This treatment might lead to an activity-dependent increase in the expression or the membrane targeting of TrkB.TK+. It has been shown previously that different depolarizing stimuli can increase the expression of TrkB or p75NTR mRNA [for a review, see Nagappan & Lu (2005)].
The question of whether the structural changes observed in T1 transgenic animals are directly linked to functional alterations in the ability to undergo synaptic plasticity remains unanswered. The fact that structural changes could be restored either by overexpression of p75NTR or by chemical induction of LTD, which is known to involve the action of p75NTR, is in favor of two independent phenomena – functional and structural changes – that could, however, be eventually connected by converging signaling cascades. In this respect, it is of special interest that both T1 and p75NTR can signal to the actin cytoskeleton, for instance via modulating the activity of the small GTPase RhoA (Yamashita et al., 1999; Ohira et al., 2005), and we have also shown that there is a link between p75NTR and the actin-binding protein profilin (Michaelsen et al., 2010). For BDNF and its receptor TrkB, accumulating evidence indicates that this receptor system could indeed translate functional changes in synaptic efficacy (LTP) (Korte et al., 1995) into more persistent structural changes (e.g. spine growth) (Tanaka et al., 2008). Whether p75NTR could perform a similar function for the other – negative – side of synaptic plasticity still needs to be clarified. p75NTR has indeed been shown to mediate opposite functional changes – LTD (Rosch et al., 2005) – and, in a separate study, to be involved in dendritic spine retraction (Zagrebelsky et al., 2005). It is intriguing to speculate that p75NTR is actually involved in translating negative functional changes into structural ones, because this would suggest that the T1 could indeed emerge as a central mediator by tightly restricting/balancing the action of both neurotrophin receptors.
In summary, we provide evidence for a role of T1 in modulating both functional and structural plasticity in mature neurons. This goes far beyond what has been shown earlier, such as that the truncated splice variant can alter dendritic complexity (Yacoubian & Lo, 2000) and spine shape (Hartmann et al., 2004; Chakravarthy et al., 2006) in neurons. We can now show that the precise action of T1 depends on the subset of neurons and on the T1/p75NTR expression ratio. This might also explain differences between studies, as the outgrowth of filopodia in hippocampal neurons was reported in one case (Hartmann et al., 2004), whereas Chakravarthy et al. (2006) detected changes in spine number in cortical neurons but not in hippocampal cells. In this study, the authors used sparse expression of T1 that might have been restricted to only one subset of neurons.
T1 as an inhibitor of p75NTR?
Trk receptors – both full-length and truncated TrkB – have been shown to physically interact with p75NTR (Bibel et al., 1999). In this study, overexpression of p75NTR and chemical induction of LTD in organotypic slice cultures of T1 animals inhibited some of the morphological alterations induced by overexpression of T1. One can argue, indeed, that one subset of T1-overexpressing neurons might have reacted with apoptosis to overexpression of p75NTR or chemical induction of LTD, and therefore could not be analyzed. However, we found no increase in the number of apoptotic cells in slice cultures transfected with both neurotrophin receptors.
Our findings do indeed suggest that T1 and p75NTR functions may be linked to each other, resulting in mutual inhibition of the two receptors, thus suggesting the possibilities of either a physical protein–protein interaction or of partly converging signaling pathways. The expression of T1 deletion mutants in primary hippocampal neurons revealed that the intracellular domain of T1 is dispensable for the rescuing effect of T1 on p75NTR-mediated structural changes. This result does not support the hypothesis that the observed mutual inhibition of both receptors is attributable to an overlap in signaling pathways, for example at the level of activation of different members of the Rho GTPase family.
Another important finding of the current study is that the mutual inhibition of the two receptors occurred independently of the ligand BDNF. Remarkably, this is in line with earlier studies showing that the T1-mediated phenotype of an increase in the number of filopodia was not dependent on neurotrophin binding (Haapasalo et al., 1999; Hartmann et al., 2004). Taken together, these results point to a function of T1 and p75NTR as modulators of neuronal morphology without the actual involvement of neurotrophins, and thereby broaden our picture of their range of action.
The results of the current study shed new light on the function of T1. We provide evidence for a role of T1 not only as the dominant-negative form of TrkB.TK+ but also as a receptor that can inhibit p75NTR signaling. T1 expression and transport to the membrane could be used to tightly regulate the action of neurotrophin receptors, especially as T1 is predominantly expressed in the adult brain, where changes in neuronal structure should be restricted to regions of neuronal plasticity. Although heterodimers of T1 and TrkB.TK+ have been previously reported (Ohira et al., 2001), the nature of the interaction between p75NTR and T1 remains elusive. T1 and p75NTR might interact – and block each other’s action – directly or through adaptor molecules clustering neurotrophin receptors (Chang et al., 2004). What kind of interaction occurs between these two receptors – T1 and p75NTR– and what could account for their complex crosstalk still need to be clarified. However, different models exist, such as receptor clustering in lipid rafts or interactions via different intracellular adaptor molecules (Barker, 2007; Wehrman et al., 2007). One candidate could be the ankyrin-rich membrane-spanning molecule (ARMS), which has been shown to interact both with p75NTR and with Trk receptors (Chang et al., 2004). Moreover, it has been shown that ARMS is involved in the regulation of dendritic branching and spine stability in mature neurons of the cortex and hippocampus (Wu et al., 2009). ARMS could therefore provide a link between p75NTR and T1, mediating the inhibitory effect of T1 on p75NTR.
Overall, we demonstrate here that T1 regulates the function and the structure of mature pyramidal neurons, and that the expression levels of p75NTR and T1 play an important role in modulating dendritic architecture and synaptic plasticity in the adult rodent hippocampus. In line with this, the endogenous expression patterns of both receptors do indeed change reciprocally over time. We therefore propose a new function of T1 as being dominant-negative to p75NTR.
Acknowledgements
We kindly thank Eero Castren for providing us with T1 transgenic mice and T1 deletion mutants and Anita Remus for experimental help. We would like to thank Diane Mundil and Jasmin Will for their outstanding technical assistance. This work was supported by the DFG (M. Korte).
Abbreviations
-
- ARMS
-
- ankyrin-rich membrane-spanning molecule
-
- BDNF
-
- brain-derived neurotrophic factor
-
- DIV
-
- days in vitro
-
- E
-
- embryonic day
-
- EGFP
-
- enhanced green fluorescent protein
-
- fEPSP
-
- field excitatory postsynaptic potential
-
- FGFP
-
- farnesylated form of enhanced green fluorescent protein
-
- KO
-
- knockout
-
- LFS
-
- low-frequency stimulation
-
- LTD
-
- long-term depression
-
- LTP
-
- long-term potentiation
-
- NMDA
-
- N-methyl-d-aspartic acid
-
- P
-
- postnatal day
-
- PBS
-
- phosphate-buffered saline
-
- T1
-
- TrkB.T1
-
- T1-EC
-
- TrkB.T1 lacking the extracellular domain
-
- T1-IC
-
- TrkB.T1 lacking the intracellular domain
-
- Trk
-
- tropomyosin-related kinase
-
- TrkB.TK+
-
- full-length TrkB
-
- WT
-
- wild-type




