Dopamine attenuates evoked inhibitory synaptic currents in central amygdala neurons
Abstract
The central nucleus of the amygdala (CeA) plays a critical role in regulating the behavioral, autonomic and endocrine response to stress. Dopamine (DA) participates in mediating the stress response and DA release is enhanced in the CeA during stressful events. However, the electrophysiological effects of DA on CeA neurons have not yet been characterized. Therefore, the purpose of this study was to identify and characterize the effect of DA application on electrophysiological responses of CeA neurons in coronal brain sections of male Sprague–Dawley rats. We used whole-cell patch-clamp electrophysiological techniques to record evoked synaptic responses and to determine basic membrane properties of CeA neurons both before and after DA superfusion. DA (20–250 μm) did not significantly alter membrane conductance over the voltage range tested. However, DA significantly reduced the peak amplitude of evoked inhibitory synaptic currents in CeA neurons. Pretreatment with the D2 receptor antagonist eticlopride failed to significantly block the inhibitory effects of DA. In contrast, pretreatment with the D1 receptor antagonist SCH-23390 significantly reduced the effects of DA on evoked inhibitory neurotransmission in these neurons. Moreover, bath superfusion of the specific D1 receptor agonist SKF-39393, but not the D2 receptor agonist quinpirole, significantly reduced peak amplitude of evoked inhibitory synaptic events. DA reduced the frequency of miniature IPSCs without altering the amplitude, while having no effect on the amplitude of IPSCs elicited by pressure application of GABA. These results suggest that DA may modulate inhibitory synaptic transmission in CeA through D1 receptor activation primarily by a presynaptic mechanism.
Introduction
The extended amygdala is an essential component of the neurocircuitry involved in mediation of the biological response to stress (Inoue et al., 1994; Inglis & Moghaddam, 1999). The central nucleus of the amygdala (CeA) in particular has been implicated in the physiological response to stress and the associated changes in central nervous system dopamine levels. There is strong evidence to support dopaminergic mediation of the amygdala response to stress (Asan, 1997; Guarraci & Kapp, 1999) and although it is well documented that CeA dopamine release is enhanced in response to fear and stress-inducing stimuli, limited research has addressed specific effects of dopamine (DA) within the CeA. While the extended amygdala comprises the bed nucleus of stria terminalis, nucleus accumbens and the CeA, the CeA is the only component whose electrophysiological response to dopamine has not been characterized.
The CeA receives extensive innervation from mesolimbic DA-containing neurons (Freedman & Cassell, 1994). Changes in amygdala DA levels are strongly related to changes in the stress response, as animal studies indicate that DA-releasing afferents are activated during stress conditioning (Coco et al., 1992) and that fear-arousing stimuli induce dopaminergic transmission in the amygdala (Yokoyama et al., 2005). Additionally, rats exposed to inescapable electrical footshock (Yokoyama et al., 2005) and chronic restraint stress (Torres et al., 2002) show elevations in amygdala DA levels for up to 2 h (Yokoyama et al., 2005). Although there is no definitive agreement on specific DA receptor subtype localization (Boyson et al., 1986; Huang et al., 1992; Scibilia et al., 1992), the CeA may contain both D1 and D2 receptor subtypes. To date, there has been only one preliminary report characterizing the physiological responses mediated by the CeA DA receptor (Scheiss et al., 1988); however, several behavioral studies support a role for amygdala D1 receptor activation in the expression of anxiety (de la Mora et al., 2005) and fear in both the potentiated startle (Lamont & Kokkinidis, 1998) and Pavlovian conditioned (Guarraci & Kapp, 1999) paradigms.
As reviewed above, dopaminergic innervation and DA receptor density in the CeA is high. Combined behavioral and physiological evidence support alterations in DA levels during stress conditioning and particular drug states, and this suggests a strong modulatory role for DA within the CeA. Therefore, this study was undertaken to explore the physiological responses to DA receptor activation within the CeA.
Materials and methods
Animals
Male Sprague–Dawley rats, 3–4 weeks old, were housed in communal cages and given access to food and water ad libitum. All experimental procedures were approved and were in compliance with Duke University Medical Center and the Durham Veteran’s Affairs Medical Center (DVAMC) Institutional Animal Care and Use Committees. Duke and the DVAMC are AAALAC-accredited institutions.
Slice preparation
Rats were briefly anesthetized with isoflurane vapor and immediately decapitated. Skulls were opened and brains were removed and transferred to ice-cold artificial cerebral spinal fluid (ACSF) consisting of (in mm) NaCl, 120; KCl, 3.3; NaH2PO4, 1.23; NaHCO3, 25; MgSO4, 1.2; CaCl2, 1.8; and d-Glucose, 10; equilibrated with 95% O2 and 5% CO2; pH = 7.3. Coronal brain slices (350 μm thickness) containing the central amygdala were cut on a vibratome (Vibratome 1000 Plus) and incubated in oxygenated ACSF at room temperature (21–23 °C) for a minimum of 1 h. Individual slices were transferred to the submersion recording chamber and superfused with oxygenated ACSF at a constant rate of 2–4 mL/min.
Whole-cell patch-clamp recording
Whole-cell recordings were obtained using standard whole-cell patch electrophysiological techniques that have been described elsewhere (Li et al., 2002). Briefly, a Zeiss microscope with a fixed stage was used to visualize the tissue. A low power objective (5×) was used to localize the central amygdaloid nucleus, and a high power water-immersion objective (40×) was used to visualize individual neurons. Whole-cell patch pipettes were pulled from thin-walled borosilicate glass capillaries (Model P-97; Sutter Instruments Company) to produce electrodes with a tip resistance of 6–10 MΩ. The internal solution for current-clamp experiments consisted of (in mm) – K-gluconate, 130; KCl, 7; HEPES, 10; Mg-ATP, 4; and Tris-GTP, 0.3. Ten to fifteen depolarizing pulse currents (600 ms) were delivered to the clamped cell through the patch electrode at 10-pA steps. The internal solution for voltage-clamp experiments consisted of (in mm) – CsCl, 135; HEPES, 10; MgATP, 4; GTP, 0.3; MgCl2, 2; EGTA, 0.5; and QX-314, 5; pH 7.23. Inhibitory events were pharmacologically isolated by bath superfusion of both APV and DNQX (20–30 μm). Signals were amplified with an Axopatch-200B amplifier and displayed on an oscilloscope (Nicolet 310). Signals were then digitized using a National Instruments BNC-2100 D/A converter and acquired via Strathclyde Software (Whole Cell program, V3.2.9) or pclamp 10.
Evoked inhibitory postsynaptic currents (IPSCs)
To evoke synaptic events, a tungsten stimulating electrode was placed in the lateral division of the CeA. The stimulating electrode was placed approximately 100–200 μm from the recording electrode and current was applied at 1–50 μA for a duration of 0.2 ms at 20-s intervals.
Miniature IPSCs
Miniature IPSCs (mIPSCs) were recorded from CeA neurons held at −70 mV after adding 1 μm tetrodotoxin (TTX) into the perfused solution to block Na-dependent action potentials. In order to facilitate spontaneous mIPSCs, the temperature in recording chamber was kept at 34 °C throughout the experiments.
Drugs
DA, SKF-38393 and quinpirole were prepared daily immediately before use to minimize oxidation and were diluted in the bath superfusate to known concentrations. Stock solutions of apomorphine and WAY-100635 were aliquoted and stored in the freezer (−20 °C). Stock solutions of the dopaminergic antagonists SCH-23390 and eticlopride were prepared weekly and stored in the refrigerator (4 °C). Stock solutions of (−)-bicuculline methiodide (BMI), 6,7-dinitroquinoxaline-2,3(1H,4H)-dione (DNQX), DL-2-amino-5-phosphonopentanoic acid (APV) and TTX were prepared as needed and stored at room temperature. For measurement of drug effects DA receptor agonists were superfused for at least 10 min, and receptor antagonists were pre-applied for least 15 min. All chemicals were obtained from Sigma (St Louis, MO, USA). As an antioxidant, sodium metabisulfite (Na2S2O5, 50 μm) was added to the bathing solution to prevent dopamine oxidation.
Exogenous gamma-aminobutyric acid (GABA) application
A glass pipette filled with 100 μm GABA was positioned near the soma of the recorded cell held at −70 mV. Whole-cell GABA-induced postsynaptic IPSCs were evoked every 1 min by direct pressure application of GABA (8–20 psi; duration 20 ms) using a pressure injecting apparatus (Picospritzer IID; General Valve, Fairfield, NJ, USA). The applied solution also contained TTX (1 μm), ΑPV (50 μm), DNQX (20 μm) and CGP-55845(1 μm).
Data analysis
All data are expressed as mean and SEM unless otherwise stated. Statistical analyses consist of analysis of variance (anova) and appropriate t-tests. P-values ≤ 0.05 are taken to be statistically significant.
Results
Dopamine inhibited inhibitory currents in CeA neurons
Postsynaptic currents were evoked by direct stimulation of the lateral division of the CeA and recorded from cells in the medial and lateral divisions of the CeA. IPSCs were evoked at a holding potential of −70 mV, and isolated by bath inclusion of the NMDA- and AMPA-specific antagonists D-APV (50 μm) and DNQX (20 μm). Evoked IPSCs were completely blocked by inclusion of the GABAA receptor antagonist bicuculline methiodide. Superfusion of DA (Fig. 1) significantly depressed inhibitory neurotransmission in the majority of CeA cells tested without altering decay kinetics (data not shown). DA (50 μm) reduced total peak IPSC amplitude by 30.58 ± 3.34% (Fig. 1B and C; P < 01, n = 14). There was no significant difference in the effect of DA between the medial and lateral divisions and, therefore, inhibitory postsynaptic currents recorded from the two divisions were pooled for data analysis. In addition, the nonspecific dopaminergic agonist apomorphine also attenuated inhibitory neurotransmission in the CeA by 60.17 ± 13.55% (Fig. 2D; n = 3, P < 0.03).
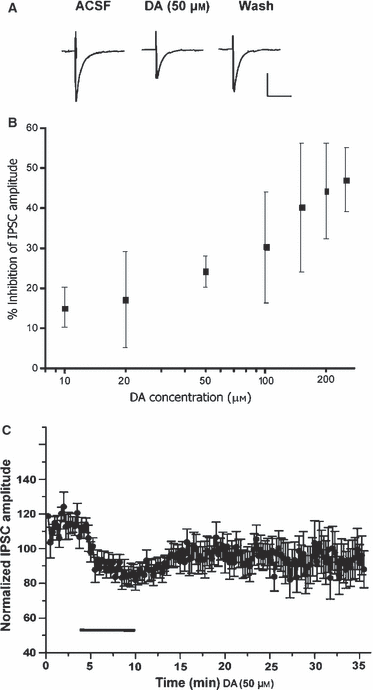
Representative waveforms of evoked inhibitory potentials during application of DA and specific DA antagonists and agonists. (A) IPSC waveforms of baseline, DA (50 μm) and washout. (B) Dose-response curve for DA on evoked IPSCs. (C) Time course of DA (50 μm) effect (applied for the period of bar) on normalized evoked IPSC amplitudes (n = 14). Scale bars in A, 25 pA vertical, 25 ms horizontal.

DA inhibited inhibitory currents through the D1 receptor subtype in CeA neurons. (A) IPSC waveforms after pretreatment with eticlopride (50 μm), DA, and washout. (B) IPSC waveforms after pretreatment with SCH-23390 (20 μm), DA, and washout. (C) Representative waveforms of evoked IPSC activity during baseline, superfusion of SKF-38393 alone, and co-superfusion of SKF-38393 and quinpirole. (D) Bar graph depicting percentage change in IPSC peak amplitude during superfusion of the following chemicals (from left to right) – DA (50 μm; 30.58 ± 3.34%, P < 0.01, n = 14); DA following pretreatment with the D2 receptor antagonist eticlopride (50 μm; 24.44 ± 2.58%, P < 0.001, n = 11); DA following pretreatment with the D1 receptor antagonist SCH-23390 (20 μm; 8.85 ± 5.69%, n = 9); D1 receptor agonist SKF-38393 (80 μm; 21.00 ± 3.33%, P < 0.000, n = 10); D2 receptor agonist quinpirole (80 μm, 3.83 ± 3.85%, n = 6); co-application of 50 μm DA and WAY-100635 (50 nm; 21.21 ± 5.62, P < 0.02, n = 5); apomorphine (50 μm); 60.17 ± 13.55%, P < 0.03, n = 3). Scale bars, 12 pA vertical, 10 ms horizontal; bars in A apply to B.
Dopamine modulated inhibitory currents through the D1 receptor subtype
DA and specific DA receptor antagonists and agonists were bath-applied to slices in order to determine whether specific DA receptor subtypes mediated the inhibitory action of DA on inhibitory transmission in CeA cells. Independent superfusion of DA antagonists resulted in no significant change in baseline responses (data not shown). Pretreatment of the D2 receptor antagonist eticlopride (50 μm) failed to significantly block the inhibitory effects of DA (50 μm) on evoked inhibitory events (Fig. 2A and D). In the presence of eticlopride, DA still reduced peak IPSC amplitude by 24.44 ± 2.58% (P < 0.001, n = 11). In contrast, pretreatment with the D1 antagonist SCH-23390 (20 μm) prevented DA (50 μm) from significantly reducing evoked inhibitory neurotransmission. Pretreatment with SCH-29930 reduced the inhibitory effects of DA on IPSC amplitude, resulting in an 8.85 ± 5.69% reduction in peak amplitude (n = 9; Fig. 2B and D).
To further characterize the D1 receptor-mediated response of CeA neurons, the specific D1-receptor agonist SKF-38393 (80 μm) was bath-applied. SKF-38393 also significantly attenuated evoked inhibitory neurotransmission in the CeA. Specifically, SKF-38393 reduced peak IPSC amplitude by 21.00 ± 3.33% (Fig. 2C; P < 0.0001, n = 10). The specific D2 agonist quinpirole (80 μm) was also bath-applied on another subset of slices containing the CeA. Quinpirole did not significantly change peak amplitude of IPSCs (2.67 ± 1.36%, n = 6; Fig. 2C and D). In order to determine whether full expression of the DA effect required activation of both D1 and D2 receptors, quinpirole (80 μm) was co-applied with SKF-38393 in a separate series of neurons (Fig. 2C and D). Subsequent superfusion of quinpirole did not significantly alter the attenuating effects of the D1 agonist, suggesting that the attenuating effect of DA is primarily mediated via the D1 receptor (SKF-38393, 16.34 ± 4.17% inhibition; SKF-38393 plus quinpirole, 15.25 ± 6.59%; n = 4). In order to rule out a nonspecific effect of DA acting on 5HT1A receptors, Way-100635, a specific 5-HT1A antagonist, was co-applied in the presence of DA. Way-100635 (50 nm) failed to alter the attenuating effects of DA on IPSCs in CeA (Fig. 2D), as application of DA (50 μm) still reduced IPSC amplitude by 21.21 ± 5.62% (P < 0.02, n = 5). Therefore, it is highly unlikely that the inhibitory effects of DA on synaptic activity resulted from a nonspecific action of DA on 5HT1A receptors.
Dopamine did not alter membrane conductance in CeA neurons
Current–voltage relationships (10-pA steps) of CeA neurons were examined both before and after DA application (Fig. 3) At concentrations between 20 and 250 μm DA did not significantly increase steady-state membrane conductance over the voltage range tested (although there was a slight tendency toward an increase in steady-state conductance, these results were not statistically significant). At the highest concentrations of DA used (500 μm) we observed variable membrane effects, although these effects were probably not attributable to specific actions at DA receptors. Apopmorphine (50 μm) did not significantly alter steady-state membrane conductance of CeA neurons (data not shown).
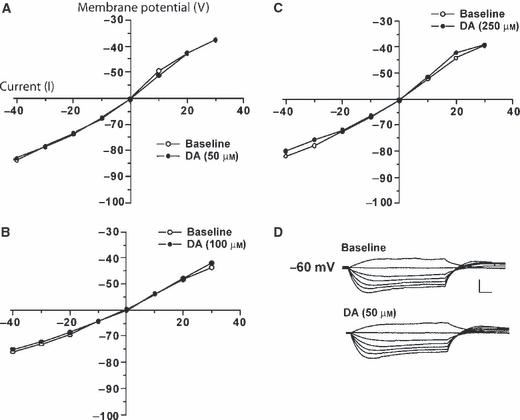
DA had little overall effect on steady state membrane conductance of CeA neurons even at very high concentrations. Current pulses (600 msec) were increased in 10-pA steps. Graphs of current–voltage relationship after application of (A) 50 μm DA (n = 7), (B) 100 μm DA (n = 7) and (C) 250 μm DA (n = 7). (D) Representative traces of current–voltage relationship before (upper) and after (lower) superfusion of 50 μm DA. Scale bars, 50 mV vertical, 50 ms horizontal.
Dopamine reduced GABA release via a presynaptic mechanism
Previous results suggested the depressive effects of DA on IPSCS may involve either presynaptic or postsynaptic mechanisms. We next determined whether DA suppressed the amplitude of IPSCS via a reduction in presynaptic GABA release onto CeA neurons. We isolated mIPSCs from 10 CeA neurons held at −70 mV in the presence of TTX and continuously monitored before and after bath application of DA (75 μm). DA significantly reduced the frequency of mIPSCs but had no effect on their amplitude. Figure 4A shows representative traces of mIPSCs under control conditions and during bath application of DA. In this example, the frequency of mIPSCs was significantly reduced after addition of DA into the bath solution and recovered following washout (Fig. 4B, left panel). However, the amplitude of mIPSCs recorded in this neuron remained unchanged (Fig. 4B, right panel). In 10 CeA neurons tested, DA significantly prolonged inter-event intervals [Fig. 4C; Kolmorogov–Smirnov (K-S) test, P < 0.05] but had no effects on amplitude (Fig. 4C; K-S test P > 0.05).
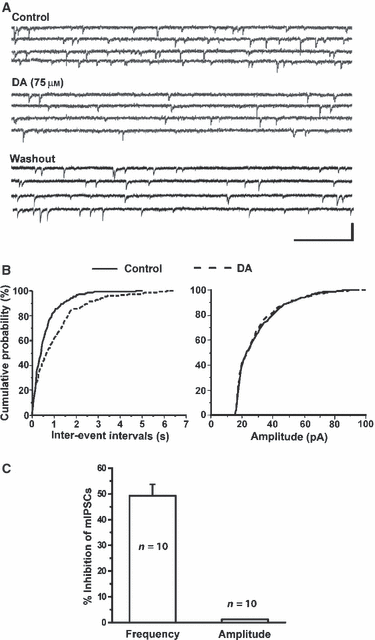
DA reduced the frequency of spontaneous mIPSCs. (A) TTX-insensitive mIPSCs were isolated from CeA neurons held at −70 mV. Bath application of 75 μm DA reduced the frequency. (B) Cumulative probability analysis indicated that DA attenuated the frequency of mIPSCs (K-S test, P < 0.001) but had no effect on the amplitude (K-S test, P > 0.05). (C) Bar graph showing the mean percentage inhibition by 75 μm DA of the frequency (n = 10, paired t-test, P < 0.05) and amplitude (paired t-test, P > 0.05) of mIPSCs.
Dopamine did not suppress postsynaptic response to direct application of GABA
We further designed experiments to rule out postsynaptic GABA responses in the presence of DA. Whole-cell GABA currents were elicited in the presence of TTX (1 μm), ΑPV (50 μm), DNQX (20 μm) and CGP-55845 (1 μm) by puffing GABA (100 μm) onto the soma of the recorded CeA neurons. When ACSF was directly pressure-applied to the soma of the recorded neurons no responses were observed. At a holding potential of −70 mV, application of GABA (100 μm) at intervals of 60 s elicited inward currents. As shown in Fig. 5A, the amplitude of whole-cell currents elicited by GABA were not affected by bath application of DA (75 μm) for 10 min but were blocked by BMI (20 μm) and recovered following washout. A total of 10 cells were examined for their responses to DA and the results from these cells were summarized in Fig. 5B. DA had no effect on the amplitude of GABAergic whole-cell currents (n = 10, P > 0.05). This result suggests that dopamine was not altering postsynaptic responses evoked by pressure application of GABA and dopamine suppression of GABA IPSCs probably occurred at a presynaptic locus.
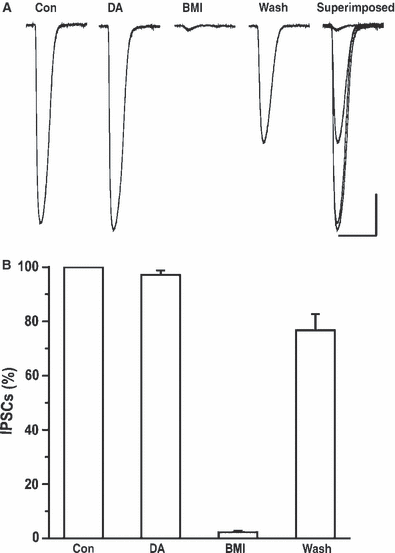
DA did not affect the postsynaptic GABA currents elicited by direct pressure application of GABA. (A) Representative traces of whole-cell currents evoked by puffing 100 nm GABA onto the soma of CeA neurons before and during application of DA are shown. BMI reversibly blocked GABA-evoked whole-cell currents. (B) There was no significant change in mean amplitude of GABA-evoked currents between baseline and DA application (one-way anova, P > 0.05). Scale bars in A, 100 pA vertical, 1 s horizontal.
Discussion
To our knowledge, this study is the first to characterize the effects of DA on inhibitory synaptic responses of neurons within the central nucleus of the amygdala. Our findings indicate that DA reduced evoked inhibitory neurotransmission of CeA neurons, probably through a D1 receptor-mediated mechanism. Furthermore, changes in mIPSC frequency and the absence of the effect on postsynaptic responses to exogenous GABA application suggest a presynaptic mode of action. Similar findings have been previously reported in the lateral (Bissiere et al., 2003; Loretan et al., 2004) and basolateral (Kroner et al., 2005) amygdala nuclei; however, these nuclei are structurally and neurochemically dissimilar to the ‘striatal-like’ CeA (Swanson & Petrovich, 1998).
Delaney and Sah (1999, 2001) reported the presence of a GABAC receptor-mediated response in Ce that is negatively modulated by benzodiazepines and only weakly sensitive to bicuculline. However, under our experimental conditions we were unable to elicit an apparent GABAC-like response. The reason for this discrepancy between these results remains unclear, but may be due to technical differences such as the internal solutions, difference in cell types, differences in stimulation pathways, different slice orientation or different rat strains (Sprague–Dawley vs. Wistar). It seems possible that GABAC-like responses, which might be smaller than GABAA-mediated IPSCs, may require more stringent conditions to be revealed.
The previous research regarding DA receptor localization in CeA is conflicting (Boyson et al., 1986; Dawson et al., 1986; Dubois et al., 1986; Weiner et al., 1991; Huang et al., 1992; Scibilia et al., 1992; Jacobsen et al., 2006); however, our data strongly support a D1 receptor-mediated effect of DA. Pretreatment with the specific D2 receptor antagonist eticlopride failed to attenuate the inhibitory effects of DA on inhibitory responses. In contrast, pretreatment with the specific D1 receptor antagonist SCH-23390 resulted in a substantial reduction in DA-mediated inhibition of inhibitory neurotransmission.
Additional support for a D1-mediated effect is our finding that superfusion of the specific D1 receptor agonist SKF-38393 significantly attenuated inhibitory neurotransmission in the CeA, while independent superfusion of the D2 agonist quinpirole was without significant effect. Specifically, antagonism of the D1 receptor resulted in an almost complete blockade of the DA effect, while antagonism of the D2 receptor failed to significantly block the DA effect. Moreover, the attenuation resulting from superfusion of the D1 receptor agonist accounts for the majority of the inhibition produced by independent application of DA.
Several lines of evidence indicate the presence of both D1 and D2 receptor subtypes in the CeA and, although these receptor subtypes have been classically characterized as having opposing effects on adenylate cyclase activity, the physiological significance of their combined effect is unclear. For example, some studies suggest that simultaneous activation of D1 and D2 receptor subtypes results in a synergistic increase in physiological activity, while other studies suggest that these receptors work in direct opposition (Umemiya & Raymond, 1997; West & Grace, 2002). Moreover, Wachtel et al., (1989) found that activation of the D1 receptor is essential to achieve full postsynaptic expression of D2 effects. Therefore in the present study, as a means to determine whether D1 and D2 receptor subtypes work synergistically to enhance physiological activity or act in opposition, the D2 receptor agonist quinpirole was bath-applied after a stable baseline was achieved in the presence of SKF-38393. Although the results were variable, the additional application of quinpirole did not produce significant changes in evoked inhibitory neurotransmission. Based on these results alone, we cannot rule out the possibility that D2 receptor subtypes are involved in the attenuation of inhibitory events in the CeA, although it appears that the effect is primarily mediated via the D1 receptor subtype. The most likely reason that the the D1 receptor agonist SKF-38393 had a somewhat smaller effect that DA is that SKF-388393 is a partial agonist at D1 receptors, at least with regard to stimulation of adenyl cyclase (Andersen & Jansen, 1990).
We also considered the possibility that DA may have actions at sites other than DA receptors. 5HT1A receptors are also widely expressed throughout the amygdala (Ohuoha et al., 1993) and are presumably located both pre- and postsynaptically. Moreover, although DA has a low affinity for 5HT1A receptors, DA nonselectively binds at these receptors at high concentrations. However, it seems unlikely that the attenuating effects of DA resulted from activation of 5HT1A receptors as the specific 5HT1A antagonist WAY-100653 failed to block the inhibitory effects of DA on IPSCs. Moreover, the lower DA concentrations utilized in this experiment would be highly unlikely to activate 5-HT1A receptors. Higher concentrations of DA (500 μm) did begin to affect membrane properties, resulting in slight shifts in membrane conductance and resting potential, although at this concentration DA would probably not act specifically on dopamine receptors.
Although this study supports a D1 receptor-mediated effect of DA in the CeA, it is possible that activation of the D1 receptor in the CeA fails to stimulate adenylate cyclase activity. In contrast to the classic adenylate cyclase-coupled D1 receptor responses characterized in several other brain areas, Leonard et al. (2003) found that the effects of D1 receptor activation in the amygdala are not mediated by cAMP and further reported that D1 receptor activation in the CeA sometimes produced a decrease in adenylate cyclase activity (Leonard et al., 2003). Moreover, the specific D1A receptor subtype appears to activate the adenylate cyclase pathway; it is distinct from other D1 receptor subtypes that activate inositol phosphate formation (Friedman et al., 1997). Dopaminergic stimulation of inositol phosphate formation is high in the amygdala (Undie & Friedman, 1990) and, taken together, these findings suggest that the inhibitory effects of DA on CeA neurons could result from D1 receptor-activated inositol phosphate formation, rather than from adenylate cyclase.
Our findings that DA attenuates evoked inhibitory neurotransmission in the CeA are consistent with a preliminary study of dopaminergic responses in the central amygdala (Scheiss et al., 1988) and the DA response reported in several other brain areas. DA has been shown to decrease IPSC peak amplitudes in the striatum by both pre- and postsynaptic mechanisms (Mercuri et al., 1985), and presynaptic D1 activation reduces inhibitory inputs to the nucleus accumbens shell (Pennartz et al., 1992). Additional reports indicate that the dopaminergic depression of inhibitory neurotransmission in the nucleus results from a reduction in presynaptic calcium influx (Nicola & Malenka, 1997).
Our approximate ED50 concentration was 50 μm, which appears to correspond with the effects of DA in diverse brain areas. In nucleus accumbens, DA depressed both excitatory and inhibitory synaptic transmission in the range 60–100 μm (Nicola & Malenka, 1997). In prefrontal cortex, DA reduced evoked IPSCs at an ED50 of approximately 30 μm (Gonzalez-Islas & Hablitz, 2001). DA inhibits GABAergic responses in midbrain dopaminergic neurons with an IC50 of 54 μm (Federici et al., 2002).
The CeA has a well-established role in conditioned fear responses (Davis, 1992; LeDoux, 2000). Dopamine may have a critical role in fear conditioning (Fadok et al., 2009), but the role of dopaminergic input specifically to the CeA is unclear in this regard. Although DA D1 receptor antagonists reportedly produce anxiolytic effects when injected into the amygdala (de la Mora et al., 2005), DA D1 receptor activation in CeA may mediate anxiolytic effects of opiates (Rezayof et al., 2009).
The current study is the first to investigate the effects of DA on inhibitory synaptic responses of neurons within the CeA. Results indicate that DA functions to reduce evoked inhibitory neurotransmission primarily through the D1 receptor. The CeA is strongly implicated in mediating the body’s response to stress and drug use, and is heavily innervated by DA-releasing afferents sensitive to such stimuli. It is important that the dopaminergic response in CeA becomes better understood in order to further determine the CeA’s involvement in response and mediation of stress. Additional research is necessary to confirm whether DA attenuates evoked synaptic activity through a pre- and/or postsynaptic mechanism of action, and to determine the particular membrane channels involved.
Acknowledgements
This project was funded by VA Merit Reviews (S.D.M. and W.A.W.), NIH-NIDA grant #DA09079 (C.K.), the Institute for Medical Research (J.C.N.), and the VISN 6 MIRECC.
Abbreviations
-
- ACSF
-
- Artificial cerebrospinal fluid
-
- APV
-
- DL-2-amino-5-phosphonopentanoic acid
-
- BMI
-
- (-)-bicuculline methiodide
-
- CeA
-
- central nucleus of the amygdala
-
- DA
-
- dopamine
-
- DNQX
-
- 6,7-dinitroquinoxaline-2,3(1H,4H)-dione
-
- GABA
-
- gamma-aminobutyric acid
-
- IPSC
-
- inhibitory postsynaptic current
-
- K-S
-
- Kolmogorov–Smirnov
-
- mIPSC
-
- miniature inhibitory postsynaptic current
-
- TTX
-
- tetrodotoxin




