Excitotoxicity mediated by non-NMDA receptors causes distal axonopathy in long-term cultured spinal motor neurons
Abstract
Excitotoxicity has been implicated as a potential cause of neuronal degeneration in amyotrophic lateral sclerosis (ALS). It has not been clear how excitotoxic injury leads to the hallmark pathological changes of ALS, such as the abnormal accumulation of filamentous proteins in axons. We have investigated the effects of overactivation of excitatory receptors in rodent neurons maintained in long-term culture. Excitotoxicity, mediated principally via non-N-methyl-d-aspartate (NMDA) receptors, caused axonal swelling and accumulation of cytoskeletal proteins in the distal segments of the axons of cultured spinal, but not cortical, neurons. Axonopathy only occurred in spinal neurons maintained for 3 weeks in vitro, indicating that susceptibility to axonal pathology may be related to relative maturity of the neuron. Excitotoxic axonopathy was associated with the aberrant colocalization of phosphorylated and dephosphorylated neurofilament proteins, indicating that disruption to the regulation of phosphorylation of neurofilaments may lead to their abnormal accumulation. These data provide a strong link between excitotoxicity and the selective pattern of axonopathy of lower motor neurons that underlies neuronal dysfunction in ALS.
Introduction
Amyotrophic lateral sclerosis (ALS), the most common form of motor neuron disease (MND), is a devastating disorder characterised by both lower and upper motor neuron dysfunction and usually resulting in death within 2–5 years of diagnosis. Along with motor neuron loss, the primary pathological feature of the disease is the formation of abnormal proteinaceous inclusions within the motor neuron cell body and axon (Wood et al., 2003). In particular, spheroid structures within the axon contain accumulations of disorganised neurofilament protein, indicating that cytoskeletal disruption plays a significant role in axonal pathology in ALS (Xiao et al., 2006). It has been suggested that this pathology may be caused by a disruption of axonal transport (Williamson & Cleveland, 1999) or, alternatively, changes to the neurofilament proteins themselves, such as phosphorylation, may affect axonal transport with resulting axonal pathology (Miller et al., 2002). Although both upper and lower motor neurons are lost in ALS, this axonal pathology is specific to spinal and bulbar motor neurons. The importance of axonal pathology in MND is demonstrated by mice expressing human familial ALS superoxide dismutase 1 (SOD1) mutations, which develop motor symptoms prior to motor neuron loss (Xu, 2000). Indeed, protecting motor neurons from apoptotic death by Bax deletion failed to prevent peripheral denervation and MND in a transgenic mouse model of ALS (Gould et al., 2006).
ALS is likely to be a multifactorial disease with a final common pathway of degeneration (Bruijn et al., 2004; Goodall & Morrison, 2006; Pasinelli & Brown, 2006). In particular, excitotoxicity has been implicated in ALS (Heath & Shaw, 2002; Van Damme et al., 2005). Excitotoxicity may arise as a result of deficient glutamate transporter activity in affected areas (Rothstein, 1996), and motor neurons are highly susceptible to excitotoxic insult, particularly through α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors (Carriedo et al., 1996; Sun et al., 2006). Riluzole, which may act through multiple routes to dampen excitotoxicity, increases life expectancy by several months (Kiernan, 2005).
Although there have been a number of studies linking excitotoxicity to MND, there have been few mechanistic studies investigating the relationship between disturbed glutamatergic transmission, cytoskeletal disruption and motor neuron axonal pathology. Excitotoxicity has been linked to rapid loss of neurofilament proteins in cortical neurons in both in vitro and in vivo experimental models (Chung et al., 2005; Tekkok et al., 2005), and may disrupt axonal transport (Ackerley et al., 2000), a possible cause of neurofilament aggregation. Interestingly, application of kainic acid to lumbar spinal segments of mice resulted in axonal changes resembling those seen in ALS, including proximal neurofilamentous swellings (Ikonomidou et al., 1996), suggesting a link between the axonal pathology of ALS and excitotoxicity.
We have utilized long-term primary culture models to examine the relationship between excitotoxicity and axonal pathology, particularly cytoskeletal changes, in ALS. These studies have examined the relative effects of excitotoxicity on the axons of cultured cortical and spinal neurons, including an investigation of whether the relative maturity of neurons in vitro may play a role in susceptibility to excitotoxic axonopathy.
Materials and methods
Culture methods
All animal experimentation was approved by the Ethics Committee (Animal Experimentation) of the University of Tasmania.
Cortical astrocyte cultures were prepared as previously described (Chung et al., 2004) from postnatal day four rat pups placed on ice for five minutes prior to decapitation, and grown to confluency on 12-mm poly-l-lysine-coated coverslips in 24-well trays. For neuron cultures time-mated pregnant Hooded-Wistar rats were killed by carbon dioxide exposure. Motor neuron cultures were prepared according to the method of Vandenberghe et al. (1998) from embryonic day 15 rats with some modifications. Briefly, spinal cords were dissected, trypsinised and spun on 1.06 g/mL optiprep™ gradient (Sigma, St Louis, USA) at 400 g for 25 min. Motor neurons were plated at 25 000 cells onto confluent astrocyte feeder layers. Cultures were grown at 37 °C, 5% CO2 for up to 21 days in vitro (DIV). Primary cortical neuron cultures were prepared as previously described (King et al., 2006) from embryonic day 18 rats, and plated onto confluent astrocyte feeder layers or directly onto poly-l-lysine-coated coverslips at an identical concentration to the motor neurons.
Pharmacological manipulations
Kainic acid, N-methyl-d-aspartate (NMDA) or vehicle control (milliQ) was added to the culture in fresh subsequent growth media for 1–24 h prior to fixing. In some experiments 20 µm 6-cyano-2,3-dihydroxy-7-nitro-quinoxaline acid (CNQX) or MK801 were added prior to the addition of the glutamate receptor agonists.
Immunocytochemistry
Cultures were fixed and labelled as previously described (King et al., 2006) using the following primary antibodies: cytochrome c (1 : 1000 BD Biosciences, Franklin Lakes, NJ, USA), alpha-internexin (1 : 5000 Novus Biologicals, Littleton, CO, USA), MAP2 (1 : 1000 Chemicon, Temecula, CA, USA), NFL (1 : 1000 Novus Biologicals), NFM (1 : 1000 Serotec, Oxford, UK), NFH (1 : 1000 Serotec), peripherin (1 : 5000 Chemicon), SMI32 (1 : 2000 Sternberger monoclonals, MD, USA), SMI312 (1 : 10 000, Sternberger monoclonals), synaptophysin (1 : 100 Dako, Denmark), beta-III tubulin (1 : 10 000 Promega, Madison, WI, USA) and tau (1 : 20 000 Dako). All antibodies have been previously characterised by Western blot analysis and are commercially available. Optimum antibody concentrations were individually determined for each antibody, and control experiments, omitting primary antibodies, eliminated all immunoreactivity. Species- and isotype-appropriate fluorescent AlexaFluor secondary antibodies (Molecular Probes, Invitrogen) were applied at a dilution of 1 : 1000. Images were acquired using a Leica (DM LB2) fluorescent microscope fitted with a cooled CCD camera (MagnaFire Optronics), and counting was performed by direct quantification from the entire coverslip (n = 4 coverslips from separate cultures).
Statistical analysis
Statistical analysis was performed with a standard two-way anova and Bonferroni post-tests using Graphpad Prism4® software. All graphical data are presented as mean ± standard error of the mean. For all analyses, multiple observations were taken from four separate cultures.
Results
Characterization of motor neurons in culture
The culture method produced a mixed cell population containing a high proportion of motor neurons. Motor neurons were identified by immunocytochemical and morphological criteria, including labelling with the dephosphorylated neurofilament marker SMI32 (Sternberger & Sternberger, 1983) and a large multipolar cell body (Carriedo et al., 1996; Tsang et al., 2000). SMI32 labelling was present within the cell body and dendrites, and extended into the proximal axon (Fig. 1A). Further immunocytochemical analysis demonstrated that motor neurons had relatively low levels of peripherin throughout the cell body and neurites, as compared with dorsal root ganglion neurons (Troy et al., 1990), and demonstrated immunoreactivity for alpha-internexin in the axon (Ching et al., 1999). Cultures were grown to 21 DIV, at which stage numerous synaptophysin-immunoreactive puncta were present (Fig. 1B), signifying the presence of synaptic connections that we have shown previously to be an indication of mature glutamate receptor expression and vulnerability to excitotoxicity in long-term cultured cortical neurons (King et al., 2006).
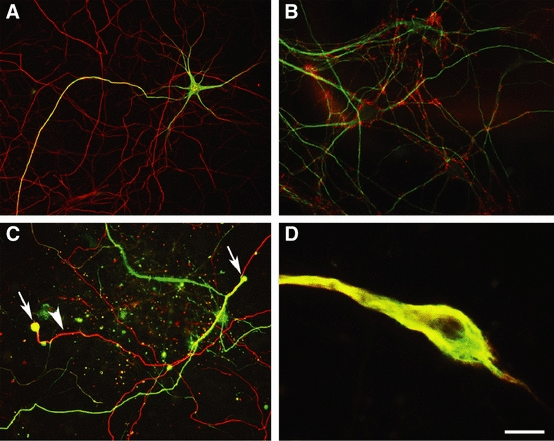
Kainic acid induced the formation of neurofilament-rich swellings in cultured motor neurons. (A) Untreated cultured motor neurons demonstrated dendritic and proximal axonal localization of dephosphorylated neurofilament (green) and an extensive neurofilament-immunopositive (NFM) axonal network, (red), which was not labelled with SMI32. (B) At 21 DIV, the dendrites of cultured neurons (MAP2-immunoreactivity, green) were surrounded by synaptophysin-immunopositive boutons (red), indicating extensive synaptic connections and relative maturity. (C) Following 20 h exposure to kainic acid (100 µm), dephosphorylated neurofilament protein (green) was often present in the axonal network and the distal portions of axonal segments frequently expanded to form large bulbous structures (arrows), which were labelled for dephosphorylated neurofilament protein as well as NFM (red; arrowhead). (D) High-magnification image of distal axonal swelling immunolabelled for dephosphorylated neurofilament (green) and phosphorylation-independent NFM (red). Scale bar: (A) 100 μm, (B) 30 μm, (C) 50 μm, (D) 7 μm.
Kainic acid induces the formation of distal axonal swellings in motor neurons in culture
Immunocytochemical labelling for phosphorylation-independent (NFM) and dephosphorylated (SMI32) neurofilament epitopes were used to examine the neuritic changes in mature (final age 21 DIV) motor neurons in culture following chronic exposure to 25, 50 and 100 µm kainic acid for 20 h. Extensive axonal disruption followed kainic acid toxicity, including neurofilament fragmentation and loss in many axonal branches, similar to previous reports in cultured cortical neurons (Chung et al., 2005). In addition, even at relatively low concentrations of kainic acid, many spinal axons in segments of neurites distant to the cell body developed focal swellings of neurofilaments (Fig. 1C). These dilated and bulbous distal axon swellings often demonstrated abnormal localization of dephosphorylated neurofilament epitopes, which were absent from the distal axonal network prior to excitotoxic stimulation (Fig. 1A and C), with the amount of axonal pathology increasing relative to the concentration of kainic acid. Axon segments were frequently swollen to a width of 12 µm and for up to 50 µm along the axon (Fig. 1D). Whether these distal axonal segments represented presynaptic terminals or an area of axon in which the more distal portion had degenerated could not be determined. Axonal beading was also present along the length of some axons.
Kainic acid-induced distal axonal swelling occurs within 6 h of exposure
To further examine the time course of the formation of distal axonal swellings, mature (final age 21 DIV) motor neurons in culture were exposed to 25, 50, 100 and 250 µm concentrations of kainic acid for 1, 6, 16 and 24 h. Axonal swellings were rarely present in untreated cultures (Fig. 2A). Following 1 h exposure to kainic acid, frequent dendritic beading was observed, which was more prominent in distal segments (Fig. 2B), as has been reported previously for cortical neurons (Hasbani et al., 1998). The extent of dendritic beading increased with kainic acid concentration. At higher concentrations of kainic acid for 1 h exposure, SMI32 labelling of dendrites was frequently lost, although little axonal damage was observed. However, at 6 h of kainic acid exposure, frequent axonal changes were present, including a significant increase in the number of neurofilament-rich swollen axon distal segments at 100 µm and 250 µm kainic acid (P < 0.001 relative to untreated cultures; P < 0.05 relative to 1 h post-exposure; Fig. 2C). The density of distal axonal swelling peaked at 16 h exposure to kainic acid, and was significantly reduced between 16 and 24 h (Fig. 2D), particularly at higher concentrations of kainic acid (P < 0.05 for > 100 µm kainic acid), which were associated with extensive loss of SMI32-immunoreactive neurons, loss of neurofilament proteins and axonal degradation, indicating that this axonopathy precedes the death of the neuron.
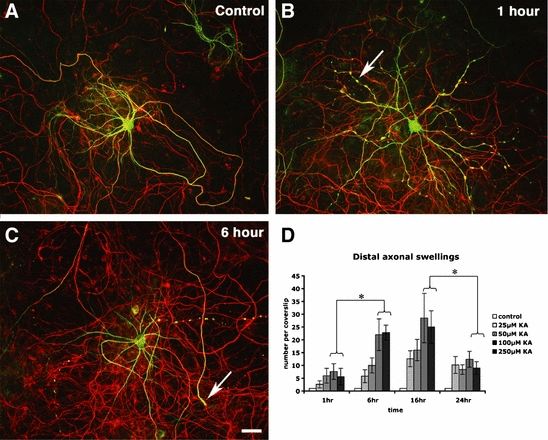
Time course of pathological changes following 100 µm kainic acid exposure in cultured motor neurons. (A) Untreated motor neuron immunolabelled for dephosphorylated neurofilament protein (green) and phosphorylation-independent NFM (red). (B) Following 1 h of kainic acid exposure, beading was present in dendrites particularly at a higher concentration of kainic acid (arrow). (C) Axon swellings (e.g. arrow) developed following 6 h of kainic acid exposure. (D) Axonal swellings were particularly abundant at 6 and 16 h of kainic acid exposure, but were less frequent at 24 h exposure (mean ± standard error of the mean, n = 4 from separate cultures, *P ≤ 0.05). Scale bar: 50 µm. KA, kainic acid.
Distal axonal swellings are immunoreactive for neural intermediate filament proteins and cytochrome c
To further characterise the cytoskeletal composition of distal axonal swelling in motor neurons following excitotoxic injury, we immunolabelled motor neuron cultures following kainic acid exposure with antibodies to cytoskeletal proteins, including neurofilament proteins NFL, NFM and NFH, dephosphorylated neurofilament (SMI32), phosphorylated neurofilament (SMI312), alpha-internexin, peripherin, beta-III tubulin and tau. All three neurofilament triplet proteins were present in these axonal swellings, and this form of axonopathy contained both dephosphorylated (SMI32) and phosphorylated (SMI312) epitopes. Notably, the dephosphorylated neurofilament epitope recognized by the antibody SMI32 was not present in the axons of control preparations for spinal motor neurons, indicating mixed phosphorylation states (phosphorylated/dephosphorylated) for neurofilaments in aberrant axonal swellings. Only a small proportion of axonal swellings were not immunolabelled with SMI32 following excitotoxicity. Alpha-internexin was frequently localized to swollen axonal segments, but relatively few of these neuritic swellings were immunoreactive for peripherin (Fig. 3A and B). This axonal pathology was also immunoreactive for beta-III tubulin and tau, indicating the presence of microtubules (Fig. 3C). To examine the distribution of mitochondria within the motor neuron axon following excitotoxicity, cultures were labelled with an antibody to cytochrome c. Distal axonal swellings typically contained a central mass that was immunoreactive for cytochrome c (Fig. 3D).
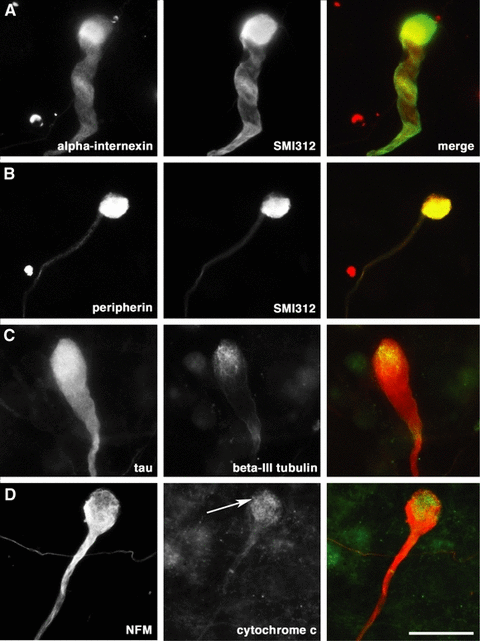
Immunocytochemical characterization of distal axonopathy in cultured motor neurons following exposure to kainic acid. (A and B) Distal axonal swellings demonstrated accumulation of intermediate filament proteins, including phosphorylated neurofilament protein (SMI312), alpha-internexin and peripherin. (C) Components of the microtubule network including beta-III tubulin and tau were present within swellings. (D) Cytochrome c immunoreactivity signified accumulation of mitochondria at the tip of distal axonal swellings (arrow) identified with anti-NFM antibody. Scale bar: 20 µm.
Distal axonal swelling is predominantly induced through non-NMDA receptors
Motor neurons have been previously demonstrated to be particularly vulnerable to kainic acid-induced excitotoxicity, most likely through calcium-permeable AMPA receptors (Carriedo et al., 1996; Sun et al., 2006). To examine the contribution of non-NMDA and NMDA receptors to formation of distal axonal swellings in cultured motor neurons, we exposed neurons to increasing concentrations of kainic acid or NMDA alone or in the presence of the non-NMDA receptor antagonist CNQX, the NMDA receptor antagonist MK801 or a combination of both for 16–20 h. Relative to control preparations, significantly (P < 0.001) increased densities of swollen distal axons occurred with 25, 50 and 100 µm kainic acid exposure, with these axonopathic changes blocked by CNQX, indicating that induction of distal axonal swellings by kainic acid occurs through non-NMDA receptors (Fig. 4A). At low concentrations of kainic acid (25 µm), induction of axonal swelling was also reduced by MK801, although this was not significant, indicating some involvement of NMDA receptors. In contrast, a smaller degree of distal axonopathy was induced by 25, 50 and 100 µm NMDA, even for concentrations of NMDA resulting in a similar amount of loss of SMI32-immunoreactive neurons to kainic acid (Fig. 4B), and axonopathy was not dose related (Fig. 4A). Treatment with CNQX and MK801 attenuated induction of swellings by both NMDA and kainic acid (Fig. 4A). In a similar fashion to axonopathic changes, the loss of SMI32-labelled neurons was most closely related to excitotoxicity mediated through non-NMDA receptors (Fig. 4B).
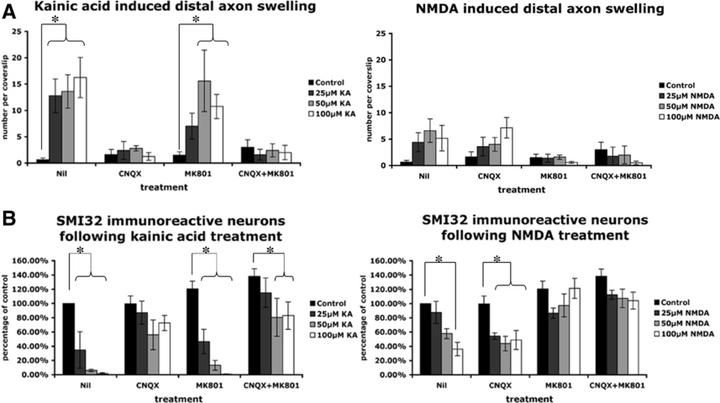
Characterization of distal axonopathy in culture following 16–20 h exposure to excitotoxin. (A) Kainic acid induced significantly more swellings in cultured motor neurons than N-methyl-d-aspartate (NMDA; P < 0.01 at 25 µm). This axonopathy was predominantly mediated through non-NMDA receptor subtypes. (B) Kainic acid induced loss of SMI32-immunolabelled cells predominantly mediated through non-NMDA receptor subtypes. (For all graphs, data are presented as mean ± standard error of the mean, n = 4 from separate cultures, *P ≤ 0.05). CNQX, 6-cyano-2,3-dihydroxy-7-nitro-quinoxaline acid; KA, kainic acid.
Cortical neurons are less susceptible to kainic acid-induced distal axonal swelling
Both cortical and spinal neurons are affected in MND, although the pathological features of these neurons differ, with neurofilamentous swellings occurring in spinal, but not cortical, motor neurons (Delisle & Carpenter, 1984; Nihei et al., 1993). We investigated the relative susceptibility of cortical and motor neurons in culture to develop axonal pathology in response to exposure to kainic acid and NMDA. Cortical neurons were grown with and without an astrocytic feeder layer, and motor neurons were grown solely on an astrocytic feeder layer, due to the critical importance of this cell layer for support and growth of spinal neurons (Vandenberghe et al., 1998; Bar, 2000). Neurons were exposed to 25, 50 and 100 µm kainic acid or NMDA for 6 and 20 h. A similar amount of neurofilament fragmentation and loss of axonal branches was present in cortical and spinal motor neuron cultures following exposure to kainic acid and NMDA (Fig. 5A and B). However, although 100 µm kainic acid induced a small but significant increase in distal axon swellings in cortical neurons grown on astrocytes after 20 h (Fig. 5C), spinal motor neurons were significantly (P < 0.01) and substantially more vulnerable to distal axonopathic alterations for each concentration of kainic acid relative to cortical neurons in culture, both with and without astrocytes (Fig. 5C). Similarly, NMDA did not significantly induce distal axonal swelling in cortical neurons. No increase in numbers of swellings was seen at 72 h post-treatment in spinal motor neurons or cortical neurons (data not shown).
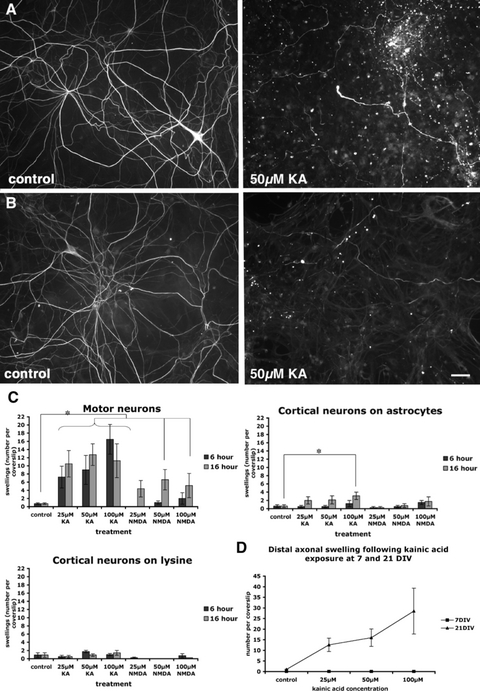
Characterization of axonopathy in spinal motor neurons and cortical neurons in vitro. (A) Sixteen hours exposure to excitotoxin led to neurofilament fragmentation and loss of axonal branches in spinal motor neurons (NFM immunoreactivity). (B) A similar amount of neurofilament fragmentation and loss of axonal branches was present in cortical neurons following excitotoxin exposure for 16 h (NFM immunoreactivity). (C) Following kainic acid exposure, motor neurons were significantly (e.g. P ≤ 0.01 at 25 µm kainic acid) more vulnerable to distal axonal swelling than cortical neurons, the latter grown either with or without an astrocyte feeder layer. (D) Motor neurons were not vulnerable to kainic acid-induced formation of distal axonal swelling at 7 days in vitro (DIV) relative to the dose-dependent sensitivity of older cultured spinal neurons to axonopathy. Scale bar: 50 µm. (For all graphs, data are presented as mean ± standard error of the mean, n = 4 from separate cultures, *P ≤ 0.05). KA, kainic acid; NMDA, N-methyl-d-aspartate.
Immature neurons are not vulnerable to kainic acid toxicity and distal axonal swelling
To examine the susceptibility of motor neurons at varying levels of maturation in vitro to excitotoxicity and formation of distal axon swellings, we exposed cultured motor neurons at 7 DIV and 21 DIV to 25, 50 and 100 µm kainic acid for 16–20 h. At 7 DIV, motor neurons in culture were not immunoreactive for SMI32, suggesting that immunocytochemical detection of this epitope is an indication of maturity, as has been previously reported (Vandenberghe et al., 1998). No axonal degradation or swelling was noted with neurofilament labelling in spinal cortical neurons exposed to kainic acid at 7 DIV (Fig. 5D).
Discussion
These results indicate that excitotoxicity mediated principally through non-NMDA receptors causes an axonopathy in spinal neurons characterized by cytoskeletal disruption and neurofilament accumulation in distal axonal segments. This axonopathy was specific for cultured spinal motor neurons relative to cortical neurons, and was linked to the relative maturity of the spinal neurons in vitro. Distal axonopathy was associated with the abnormal colocalization of phosphorylated and dephosphorylated neurofilament proteins, indicating aberrant post-translational processing in the accumulation of filaments and subsequent localized swelling. The accumulation of neurofilaments within axons following excitotoxicity mimics the pathological cytoskeletal changes of ALS and provides a link between excitotoxicity via non-NMDA receptors implicated in this disorder with a related pathological hallmark feature of motor neuron degeneration.
Motor neurons have been shown to be selectively vulnerable to excitotoxicity particularly through non-NMDA receptors (Rothstein et al., 1993; Sun et al., 2006), and this may be related to the specific compliment of glutamate receptors expressed by neuronal subtypes, particularly calcium-permeable AMPA receptors (Vickers et al., 1993; Van Damme et al., 2002). In the present study, distal axonal swelling and loss of SMI32-immunoreactive neurons was mediated primarily through non-NMDA receptors as indicated by the protective effect of CNQX. However, some loss of SMI32-immunoreactive neurons occurred at higher concentrations of kainic acid in the presence of CNQX, probably due to the competitive nature of the antagonist. NMDA receptor block provided some reduction of distal axonal swelling induced by kainic acid, although this was not significant. In addition, NMDA also induced the formation of some swellings and this was blocked by MK801. It should be noted that occasional swellings were also present in untreated cultures. Increased intracellular calcium influx through NMDA receptors or other calcium channels may contribute to excitotoxic pathology in motor neurons. Additionally, stimulation or blockage of specific glutamate receptor subtypes affects the function and expression of other glutamate receptor types. The contribution of other sources of calcium in axonal degradation and distal axonal swelling, including the contribution of calcium-permeable AMPA receptors, will be the subject of future investigations.
It has been postulated that disruption of axonal transport may make a significant contribution to the development and consequences of axonal dysfunction and degeneration in ALS. Axonal transport has been shown to be impaired in ALS (Sasaki & Iwata, 1996) as well as in transgenic mouse models of MND prior to disease onset (Zhang et al., 1997; Williamson & Cleveland, 1999; Sasaki et al., 2005). Additionally, it has been demonstrated that disrupting axonal transport by overexpression of dynamitin is sufficient to cause a late-onset motor neuron degeneration (LaMonte et al., 2002). Moreover, disruption of dynactin, and retrograde axonal transport, is involved in the pathogenesis of Kennedy's disease, and this is additionally associated with neurofilament accumulation at the neuromuscular junction (Katsuno et al., 2006).
We have shown that kainic acid-induced distal axonal swellings are rich in neurofilament proteins, alpha-internexin, peripherin, microtubules and mitochondria. This indicates a non-specific accumulation of cytoskeletal proteins and organelles indicative of a general disruption of axonal transport. Similarly, in ALS and relevant transgenic models, neurofilament-rich axonal spheroids contain both phosphorylated and dephosphorylated neurofilament proteins, other intermediate filament proteins, cytoplasmic proteins and organelles (Wood et al., 2003). The presence of alpha-internexin and peripherin may be representative of the phenotype of the cell, rather than any changes in expression of intermediate filament proteins.
Our data demonstrate that excitotoxicity via non-NMDA receptors causes swellings at the distal portion of motor neuron axons, and these swellings frequently contain phosphorylated and dephosphorylated neurofilament epitopes, the latter normally not localized to these axonal segments. Neurofilament phosphorylation is likely to play a role in the rate of axonal transport. Increased phosphorylation may result in dissociation of neurofilament from the kinesin motors, resulting in a slowing of axonal transport (Yabe et al., 2000). Interestingly, in cortical neurons in vitro, glutamate toxicity has been shown to slow neurofilament transport, which may be associated with increased sidearm phosphorylation (Ackerley et al., 2000). However, dephosphorylation of neurofilament has also been associated with more irregular and fused cross-bridges between neurofilaments, and resultant slowing of transport (Gotow, 2000). It is therefore possible that both hyper- and hypo-phosphorylation of neurofilament can cause disruption to transport, possibly differentially affecting anterograde and retrograde transport, respectively. Alternatively, increases in the rate of anterograde transport caused by dephosphorylation, without concomitant increases in retrograde transport, could lead to the formation of swellings, particularly at the distal portion of axons. Interestingly, dephosphorylation has been shown to promote calpain-mediated proteolysis of neurofilaments (Goldstein et al., 1987). In this respect dephosphorylation of neurofilaments at the axonal terminal may be a protective response, as this is likely to be involved in degradation of excess neurofilament protein (reviewed in Liu et al., 2004).
It is well known that ALS is characterised by the loss of both upper and lower motor neurons; however, the pathological features of degeneration of these different subsets of neurons differ. Notably, the formation of proteinaceous inclusions including neurofilament-rich swellings, or spheroids, is restricted to the axons of lower motor neurons of the brain stem and spinal cord. Our data indicate that motor neurons are more vulnerable to the formation of excitotoxicity-induced distal axonal swellings. Interestingly, motor neurons and cortical neurons may differ in their neurofilament phosphorylation response to overactivation of non-NMDA receptors, causing dephosphorylation or phosphorylation, respectively (Terro et al., 1996; Vartiainen et al., 1999). Alternatively, phosphorylation and dephosphorylation may occur in different regions of neurons in response to excitotoxicity, with motor neurons being more susceptible to the formation of large swellings, possibly due to the large calibre of their axons. Our previous studies have indicated that excitotoxicity in cortical neurons leads to the rapid loss of specific neurofilament proteins in otherwise intact neurons, mediated particularly through activation of NMDA receptors (Chung et al., 2005). Cumulatively, these data support the proposition that phenotypic differences between neurons will regulate their pathological reaction to a particular insult, such as excitotoxicity. Phenotypic differences may reflect the localization and activity of particular excitatory receptor subunits to particular neurons (Vickers et al., 1993; Bar-Peled et al., 1999; King et al., 2006) or perhaps variability in the constitutive features of cytoskeletal proteins in different subgroups of neurons (Dickson et al., 2005). In this regard, it is interesting that younger cultured spinal neurons in the current study were not vulnerable to excitotoxicity and were not labelled with the SMI32 antibody, and this may be indicative of the immaturity of the neurons in terms of glutamatergic input (Vartiainen et al., 1999), as we have shown previously in cortical cultures (King et al., 2006).
Axonal pathology is a prominent feature of ALS and is likely to be responsible for the clinical symptoms of the disease prior to motor neuron death (Xu, 2000). However, the initial site of motor neuron dysfunction is not yet known. Interestingly, changes in the distal axon of both upper and lower motor neurons have been reported in MND and related transgenic models, including loss of innervation of Betz cells of the motor cortex, motor neurons and motor endplates (Sasaki & Maruyama, 1994; Frey et al., 2000; Fischer et al., 2004; Zang et al., 2005). In mutant SOD1, motor neuron degeneration and progressive motor neuropathy mouse models, changes at the neuromuscular junction predate pathology in ventral root fibres or motor neuron cell bodies, indicating that the disease process may represent a distal dying-back axonopathy (Frey et al., 2000; Fischer et al., 2004). Moreover, in a mouse model of spinal muscular atrophy, a disease caused by mutations in the survival of the motor neuron (SMN) gene, skeletal muscle denervation was accompanied by massive accumulation of neurofilaments, including phosphorylated forms, in the terminal axons of remaining neuromuscular junctions (Cifuentes-Diaz et al., 2002). Autopsies in human patients with ALS have indicated that extensive changes in distal segments of axons can occur in peripheral muscle in the absence of significant motor neuron degeneration centrally (Fischer et al., 2004). The current data indicate that excitotoxicity has its main effect in distal segments of the axons, which is interesting in light of well-described proximal axonal pathology, which can occur in the spine (Hirano, 1991). Whether proximal axonopathy is antecedent or consequent to changes in axons in the periphery remains an important question relative to the staging of pathology in ALS.
Acknowledgements
This study was supported by Rotary Club of Deloraine, Australian Rotary Health Research Fund, Tasmanian Masonic Centenary Research Foundation, Motor Neuron Disease Association of Tasmania, Motor Neuron Disease Research Institute of Australia, Royal Hobart Hospital Research Foundation BG Thomas Bequest, University of Tasmania Brian Frederick Marks Bequest.
Abbreviations
-
- ALS
-
- amyotrophic lateral sclerosis
-
- AMPA
-
- α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
-
- CNQX
-
- 6-cyano-2,3-dihydroxy-7-nitro-quinoxaline acid
-
- DIV
-
- days in vitro
-
- MND
-
- motor neuron disease
-
- NMDA
-
- N-methyl-d-aspartate
-
- SOD1
-
- superoxide dismutase 1.