Pronociceptive changes in response properties of rostroventromedial medullary neurons in a rat model of peripheral neuropathy
Abstract
The spared nerve injury (SNI) model of peripheral neuropathy produces a robust and long-lasting hypersensitivity. Previous behavioural studies suggest that brainstem–spinal pathways originating in or relaying through the rostroventromedial medulla (RVM) contribute to neuropathic hypersensitivity. We determined whether SNI induces changes in response properties of RVM neurons that might influence descending modulation of nociception. RVM neurons included in the study were classified into presumably pronociceptive ON-cells and antinociceptive OFF-cells (giving excitatory or inhibitory responses to noxious stimulation, respectively). Spontaneous activity and the response to cold, pinch and colorectal distension were assessed under light anaesthesia in the rat, 1 week and 8 weeks following nerve injury or sham operation. Spontaneous activity was increased 1 week but not 8 weeks after nerve injury in ON-cells but decreased in OFF-cells at both time points. In the SNI group, cold-evoked responses were enhanced particularly in ON-cells, independent of the postoperative time point. Responses of ON-cells to pinch and visceral stimulation were enhanced 8 weeks but not 1 week following nerve injury, whereas OFF-cell responses to pinch or colorectal distension were not changed. The results indicate that SNI induces pronociceptive changes in spontaneous activities of ON-cells and OFF-cells and peripherally evoked responses of ON-cells that vary with the postoperative time point. Increased ON-cell activity and decreased OFF-cell activity in the RVM are likely to enhance spinal nociception in a tonic fashion, whereas increased responses of ON-cells to peripheral stimulation are likely to enhance ascending nociceptive signals by a positive feedback following peripheral noxious stimulation.
Introduction
Peripheral nerve injuries may produce long-lasting neuropathic pain and hypersensitivity that is particularly prominent with mechanical and cold stimulation of the skin (Scadding & Koltzenburg, 2006). The maintenance of these nerve injury-induced symptoms depends on abnormal discharge from peripheral nerves (Devor, 2006) and pronociceptive changes in the spinal segmental mechanisms mediating and modulating pain-related signals (Woolf & Salter, 2006). Additionally, changes in descending pain modulation contribute to neuropathic symptoms (Pertovaara, 2000; Porreca et al., 2002; Ossipov et al., 2006). The rostroventromedial medulla (RVM), consisting of the raphe magnus and its adjacent reticular nuclei, is a final common pathway for many descending pathways and is involved in descending facilitation as well as inhibition of pain-related spinal responses (Gebhart, 2004; Vanegas & Schaible, 2004). Among the various cell types of the RVM, one giving an excitatory response to noxious stimulation just prior to nociceptive withdrawal reflex (ON-cell) is considered to have a pronociceptive role, whereas one giving an inhibitory response to noxious stimulation (OFF-cell) is considered to have an antinociceptive role (Fields et al., 2006). A third cell type of the RVM giving no response to noxious stimulation (NEUTRAL-cell) has a less clear role, although it has been proposed to contribute to tonic modulation of various spinal processes (Mason, 2006), possibly including nociception. Whereas there is accumulating behavioural evidence indicating that the RVM contributes to neuropathic hypersensitivity through descending pathways, the contribution of various cell types of the RVM to hypersensitivity during an early vs. a later phase of neuropathy is only partly known (see Discussion for references).
In this study, we assessed the contribution of presumably pro- and antinociceptive RVM cells to acute and prolonged neuropathy by determining their response properties at two different postoperative time points in sham-operated controls vs. in animals with a spared nerve injury (SNI) model of peripheral neuropathy (Decosterd & Woolf, 2000). This model of neuropathy provides stable and long-lasting symptoms mimicking those observed in clinical conditions.
Materials and methods
The experiments were performed in adult, male Hannover-Wistar rats weighing 180–190 g at the beginning of the experiment (Harlan, Horst, The Netherlands). The experimental protocol was accepted by the Institutional Ethics Committee and the experiments were performed according to the guidelines of European Communities Council Directive of 24 November 1986 (86/609/EEC).
Techniques for producing neuropathy
The unilateral axotomy and ligation of the tibial and common peroneal nerves was performed under pentobarbitone anaesthesia (50 mg/kg i.p.) as described in detail earlier (Decosterd & Woolf, 2000). Briefly, the skin of the lateral surface of the thigh was incised and a section made directly through the biceps femoris muscle exposing the sciatic nerve and its three terminal branches. Following ligation and removal of 2–4 mm of the distal nerve stumps of the tibial and common peroneal nerves, muscle and skin were closed in two layers. In sham-operated animals, the surgical procedure was identical, except that the tibial and common peroneal nerves were not ligated or sectioned.
Behavioural verification of neuropathy
Development of hypersensitivity was verified behaviourally in animals habituated to the experimental conditions 1–2 h daily for 2–3 days. For assessment of tactile allodynia, the hind limb withdrawal threshold was determined by stimulating the sural nerve area in the hind paw of the operated limb with monofilaments. The calibrated series of monofilaments used in this study produced forces ranging from 0.16 to 15 g (North Coast Medical, Inc. Morgan Hill, CA, USA). The monofilaments were applied to the foot pad with increasing force until the rat withdrew its hind limb. The lowest force producing a withdrawal response was considered the threshold. The threshold for each hind paw of each rat was based on three separate measurements and the median of these values was considered to represent the threshold. Threshold values ≤1 g were considered to represent hypersensitivity. It should be noted that the currently used strain of rats delivered by Harlan (Horst, The Netherlands) has an exceptionally low withdrawal threshold to monofilament stimulation in the baseline (unoperated) condition: in 10 unoperated control animals, the lowest withdrawal threshold was only 4 g, and therefore the criterion for hypersensitivity was set to as low as ≤1 g in this study.
Electrophysiological recordings
For electrophysiological recordings, the anaesthesia was induced by pentobarbitone at a dose of 50 mg/kg i.p. and the animal was placed in a standard stereotaxic frame according to the atlas of Paxinos & Watson (1998). Anaesthesia was maintained by infusing pentobarbitone (15–20 mg/kg/h). The level of anaesthesia was frequently monitored by observing the size of the pupils and by assessing withdrawal responses to noxious stimulation. When necessary, the infusion rate of pentobarbitone was increased. The rats were spontaneously breathing. A warming blanket was used to maintain body temperature within the physiological range. Peripheral perfusion was checked by evaluating the colour of ears and extremities. The skull was exposed and a hole drilled for placement of a recording electrode in the RVM. The desired recording site in the RVM was 1.8–2.3 mm posterior from the ear bar, 0.0–0.9 mm lateral from the midline, and 8.9–10.7 mm ventral from the dura mater.
Single neuron activity was recorded extracellularly with lacquer-coated tungsten electrodes (tip impedance 3–10 MΩ at 1 kHz) and then amplified and filtered using standard techniques. Data sampling was performed with a computer connected to a CED Micro 1401 interface and using Spike 2 software (Cambridge Electronic Design, Cambridge, UK).
Actual recordings did not start until the animal was under light anaesthesia; that is, the animals gave a brief withdrawal response to a noxious pinch, but the pinch did not produce any longer-lasting motor activity, and nor did the animals have spontaneous limb movements. Neurons were classified on the basis of their response to noxious pinch of the tail with a haemostatic clamp. This stimulus was painful when applied to the finger of the experimenters. Neurons giving excitatory responses to pinch were considered ON-cells, those giving inhibitory responses were considered OFF-cells and neurons showing no or only a negligible (<10%) change in their discharge rates as a response to pinch were considered NEUTRAL-cells. This classification scheme of medullary neurons was modified from that described by Fields et al. (2006). A noteworthy difference is that we did not verify whether pinch-evoked responses of RVM neurons were associated with spinal reflex responses as in the original classification scheme (Fields et al., 2006). Therefore, the populations of ON- and OFF-cells in this study may not be identical with those in a study in which cells are classified strictly according to the classification scheme of Fields et al. (2006). Our previous results suggest, however, that there is only little difference in the classification of RVM neurons whether or not spinal reflex responses are concurrently measured in lightly anaesthetized animals (Pertovaara et al., 2001).
Peripheral test stimuli
In electrophysiological experiments, cold stimuli (peak stimulus temperature, 4 °C; baseline temperature, 35 °C; rate of stimulus temperature decrease, 4 °C/s; duration of the peak temperature of 4 °C, 10 s) were applied with a feedback-controlled Peltier device (LTS-3 Stimulator; Thermal Devices Inc., Golden Valley, MN, USA) to the lateral side of the hind paw innervated by the sural nerve. Whereas some models of neuropathy may be associated with significant skin temperature changes that provide a confounding factor to assessment of thermal responses, particularly when using radiant heat for stimulation (Luukko et al., 1994), applying thermal stimuli with a contact thermostimulator and starting from a standard adapting temperature reduces the possibility that a neuropathy-associated change in skin temperature will influence the results.
ON- and OFF-neurons in the RVM typically have very large receptive fields covering almost the whole body and allowing testing of not only responses evoked by stimulating the operated limb but also responses evoked by the nonoperated region within the same neurons. In this study, responses evoked by stimulating the tail and the viscera were assessed to observe potential nerve injury-induced changes outside of the injured region. Mechanical stimulation of the skin consisted of applying a haemostatic clamp to the tail for 5 s. When applied to the experimenter's hand, this stimulus produced a painful pinch sensation. Noxious visceral stimulation consisted of colorectal distension (CRD) at a noxious intensity (80 mmHg) (Ness et al., 1991). CRD was applied for 10 s by inflating with air a 7–8-cm flexible latex balloon inserted transanally into the descending colon and rectum. The pressure in the balloon was controlled by an electronic device (Anderson et al., 1987).
When the stimulus-evoked responses were analysed, the baseline discharge frequency recorded during a corresponding period just before the stimulation was subtracted from the discharge frequencies determined during stimulation; that is, positive values represent excitatory responses evoked by peripheral stimulation and negative ones inhibitory responses.
Course of the study
Four groups of animals were included in the electrophysiological study: (i) sham group tested 1 week after operation; (ii) sham group tested 8 weeks after operation; (iii) SNI group tested 1 week after operation; and (iv) SNI group tested 8 weeks after operation. In each of these groups, behavioural assessment of sensitivity to monofilament stimulation was performed before the start of the electrophysiological experiment.
After induction of anaesthesia, the microelectrode was lowered to the RVM. After a single cell had been found, its spontaneous activity was recorded for 2–3 min. Then, cold stimulation was applied to the sural nerve area in the operated limb followed by pinch of the tail and CRD at 1-min intervals. The testing procedure, including the order of testing different submodalities of nociception, was the same in all experimental groups. To minimize serial effects, every other animal tested in this series belonged to the sham group and every other to the SNI group.
At the completion of the study, an electrolytic lesion was made in the recording site, the animals were given a lethal dose of pentobarbitone and the brains were removed for verification of recording and microinjection sites.
Statistics
Data are presented as mean ± SEM. For assessment of differences in incidence of various types of neurons in different experimental conditions, the chi-squared test was used. Two-way anova followed by the Student–Newman–Keuls test was used for assessing differences in neuronal responses between the experimental conditions. P < 0.05 was considered to represent a significant difference.
Results
All animals with SNI showed mechanical hypersensitivity both 1 week and 8 weeks following surgery, as indicated by hind limb withdrawal thresholds that were ≤1.0 g ipsilateral to the nerve injury. In contrast, sham-operated animals did not develop mechanical hypersensitivity and their hind limb withdrawal thresholds were significantly higher than those in the SNI group (F1,28 = 40, P < 0.0001), independent of the postoperative time point (1 or 8 weeks, Fig. 1).
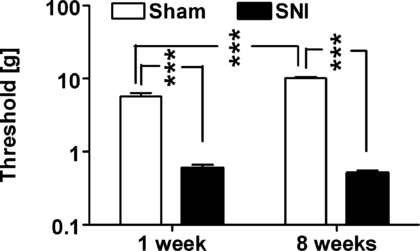
Hind limb withdrawal thresholds evoked by monofilament stimulation of the operated limb determined in nerve-injured (SNI) and sham-operated (Sham) animals 1 week and 8 weeks following the operation. Thresholds were determined prior to induction of anaesthesia. The error bars represent SEM (n = 7–9). ***P < 0.005 (Student–Newman–Keuls test).
In general, ON-cells gave excitatory and OFF-cells inhibitory responses to noxious stimulation. Their receptive fields covered bilaterally wide areas of the body and all extremities. NEUTRAL-cells, in contrast, gave no marked responses to noxious stimulation of the extremities, and they were not further studied here. Distributions of RVM cells with different types of response properties were not significantly different between SNI and sham groups at either 1 week or 8 weeks following surgery (chi-square test; Table 1).
| ON-cells | OFF-cells | Neutral-cells | |
|---|---|---|---|
| Sham | |||
| 1 week | 17 | 11 | 6 |
| 8 weeks | 27 | 18 | 10 |
| SNI | |||
| 1 week | 18 | 12 | 5 |
| 8 weeks | 29 | 16 | 8 |
- SNI, spared nerve injury; Sham, sham operation; 1 week and 8 weeks refer to the postoperative time point of the study.
Spontaneous activity
The spontaneous discharge rate of ON-cells was significantly changed from 1 week to 8 weeks postoperatively (F1,69 = 8.9, P < 0.005; Fig. 2A), and the change in the discharge rate varied with the experimental group (Sham vs. SNI animals; F1,69 = 4.8, P < 0.04). One week following the operation, the spontaneous discharge rate of ON-cells was significantly higher in the SNI than in the Sham group. By the 8-week postoperative time point, the spontaneous discharge rate of ON-cells was significantly decreased in the SNI group only. Consequently, the spontaneous discharge rate of ON-cells 8 weeks following the operation was no longer higher in the SNI than in the Sham group, but was, if anything, the opposite.
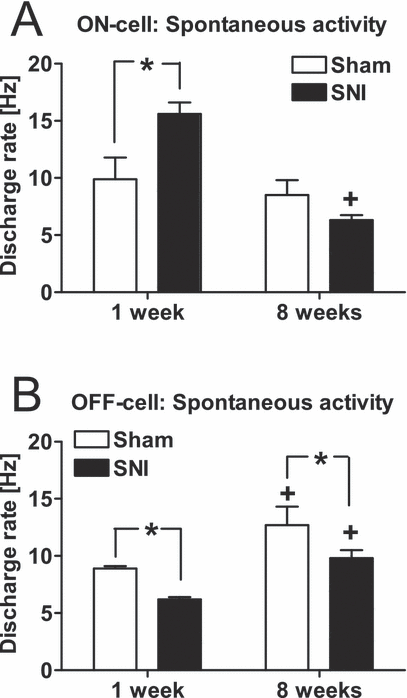
Mean spontaneous activities of ON-cells (A) and OFF-cells (B) inthe RVM of sham-operated (Sham) and nerve-injured (SNI) animals 1 week and 8 weeks following sham operation or nerve injury. The error bars represent SEM (n = 11–29). *P < 0.05 (Student–Newman–Keuls test; reference, the corresponding value in the Sham group), +P < 0.05 (Student–Newman–Keuls test; reference, the corresponding value in the 1-week group).
The spontaneous discharge of the OFF-cells tended to increase from the 1-week to the 8-week postoperative time point (F1,69 = 14.8, P < 0.0005; Fig. 2B). The spontaneous discharge rate of OFF-cells was significantly lower in the SNI group than in the Sham group (F1,69 = 8.5, P < 0.005; Fig. 2B), independent of the postoperative time point (1 week vs. 8 weeks; F1,69 = 14.8, P < 0.0005).
Peripherally evoked responses
Application of cold (4 °C) to the sural nerve area in the operated hind limb evoked markedly stronger excitatory responses in ON-cells of the SNI than of the Sham group (F1,87 = 116, P < 0.0001; 3, 4), independent of the postoperative time point (F1,87 = 0.67). Cold-evoked responses of ON-cells did not vary with elapsed time from the surgery (1 week vs. 8 weeks; F1,87 = 0.9). Cold stimulation induced stronger inhibitory responses in the OFF-cells of the SNI than in the Sham group (F1,53 = 64.6, P < 0.0001; Fig. 4B). Postoperative time (1 week vs. 8 weeks) did not influence the magnitude of the cold-evoked inhibitory response in OFF-cells (F1,53 = 0.8).
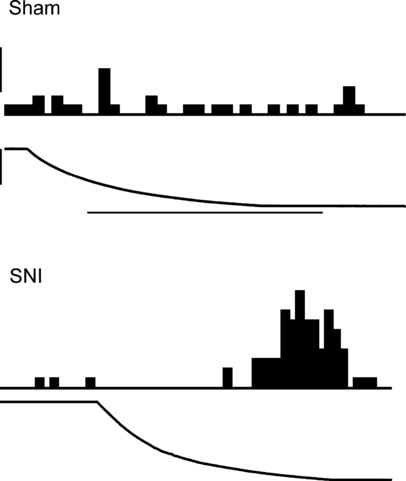
Examples of original recordings of cold-evoked responses of ON-cells in a sham-operated animal and a nerve-injured (SNI) animal 8 weeks following the operation. Cold stimulation was applied to the skin of the sural nerve area in the operated limb. The peristimulus time histogram of the neuronal response (above) and the change of stimulus temperature from 35 °C to 4 °C (below) are shown for each neuron. The calibration bar for the peristimulus time histogram represents 10 impulses/s and the horizontal calibration bar represents 25 s for the Sham condition and 20 s for the SNI condition.
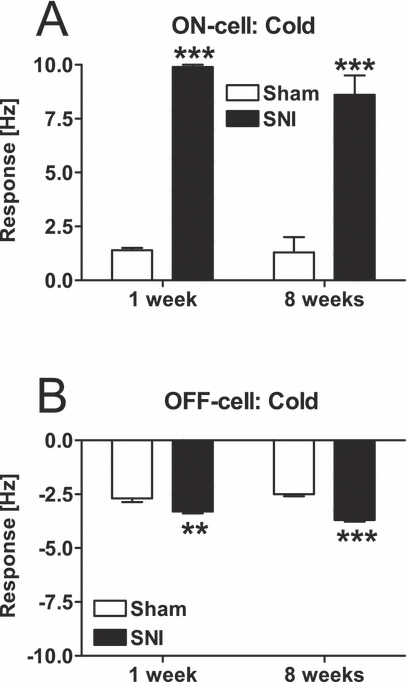
Mean changes of ON-cell (A) and OFF-cell (B) discharge rates induced by cold applied to the sural nerve area in the operated limb 1 week and 89 weeks following the operation. SNI, spared nerve-injury group; Sham, sham-operated group. **P < 0.01, ***P < 0.005 (Student–Newman–Keuls test; reference, the corresponding Sham group). The error bars represent SEM (n = 11–29).
Noxious tail pinch produced significantly stronger excitatory responses in ON-cells of the SNI than of the sham group (F1,87 = 54, P < 0.0001; 5, 6), and this difference in the response magnitude was significantly larger at 8 weeks than at 1 week after the operation (F1,87 = 17.4, P < 0.0001). There were no significant differences in pinch-evoked inhibitory responses of OFF-cells between the SNI and the Sham groups (F1,53 = 3.2; Fig. 6B). Pinch-evoked inhibitory responses in OFF-cells, however, were stronger at 8 weeks than at 1 week after the operation (F1,53 = 6.7, P < 0.02), independent of nerve injury (F1,53 = 0.4).
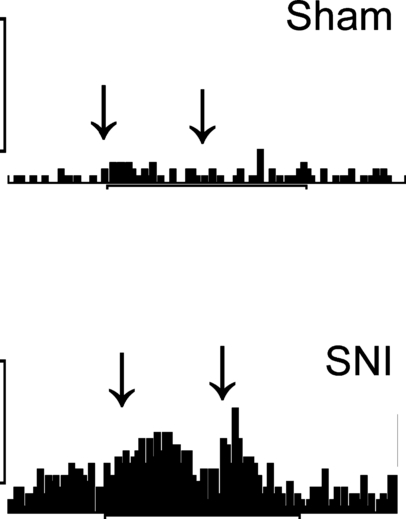
Examples of original recordings of tail pinch-evoked responses of ON-cells in a sham-operated animal and a nerve-injured (SNI) animal 8 weeks following the operation. The arrows show the duration of stimulation (5 s). The vertical calibration bars represent 20 impulses/s.
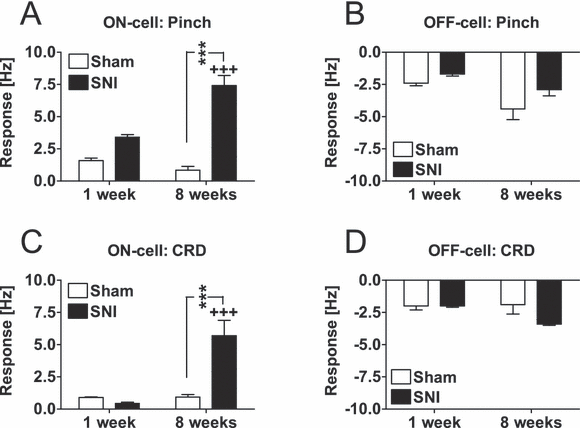
Mean changes of ON-cell (A and C) and OFF-cell (B and D) discharge rates induced by noxious pinch of the tail or CRD at a noxious intensity (80 mmHg) 1 week and 8 weeks following sham operation or nerve injury. SNI, spared nerve-injury group; Sham, sham-operated group. ***P < 0.005 (Student–Newman–Keuls test; reference, the corresponding Sham group). +++P < 0.005 (Student–Newman–Keuls test; reference, the corresponding group 1 week following operation). The error bars represent SEM (n = 11–29).
CRD at a noxious intensity (80 mmHg) evoked significantly stronger excitatory responses in ON-cells of the SNI than of the Sham group (F1,87 = 7.2, P < 0.01; 6, 7), and this difference in the response magnitudes was significantly larger at 8 weeks than at 1 week following the operation (F1,87 = 10.7, P < 0.005). One week following the operation, ON-cell responses evoked by CRD were minor ones and not different between the experimental groups, whereas at 8 weeks following operation, the CRD-evoked ON-cell response was significantly increased in the SNI group only (see Fig. 6C for detailed results of post hoc tests). In OFF-cells, the inhibitory response evoked by CRD was not significantly different between the SNI and Sham groups (F1,53 = 2.4; Fig. 6D), and the magnitude of the response evoked by CRD in OFF-cells was not significantly different when assessed 1 week vs. 8 weeks postoperatively (F1,53 = 1.8).
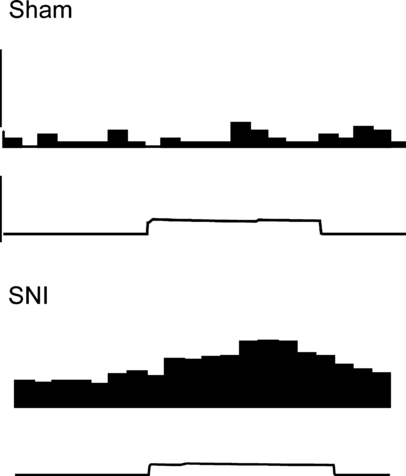
Examples of original recordings of CRD-induced responses of ON-cells in a sham-operated animal and a nerve-injured (SNI) animal 8 weeks following the operation. The peristimulus time histogram of the neuronal response (above) and the change of stimulus intensity from 0 mmHg to 80 mmHg and back (below) are shown for each neuron. The calibration bar for the peristimulus time histogram represents 30 impulses/s. The duration of the CRD is 10 s.
Recording sites
Figure 8 shows the recording sites in the medulla.
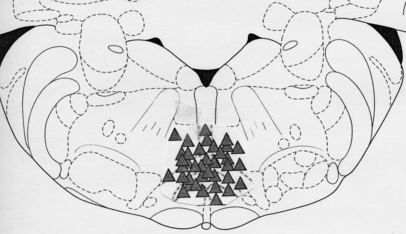
Schematic diagram showing recording sites in the RVM.
Discussion
The results indicate that the SNI model of peripheral neuropathy induces marked pronociceptive changes in response properties of cells in the RVM, a nucleus with an important role in descending modulation of pain (Pertovaara & Almeida, 2006). These plastic changes vary with elapsed postoperative time and they are likely to contribute to maintenance of neuropathic hypersensitivity.
Spontaneous activity and peripherally evoked responses
Spontaneous discharge rate of RVM ON-cells, which are known to have an excitatory effect on nociceptive transmission (Fields et al., 2006), was significantly increased 1 week but not 8 weeks after nerve injury. The spontaneous discharge rate of RVM OFF-cells, which have an inhibitory effect on nociceptive transmission (Fields et al., 2006), was decreased both 1 week and 8 weeks following nerve injury. Taken together, these findings suggest that both ON- and OFF-cell activities in the RVM promote neuropathic hypersensitivity in a tonic fashion during the first week following nerve injury. At a later phase of neuropathy, however, only the OFF-cell activity has a tonic pronociceptive effect, as a result of its decreased activity.
When assessing responses of RVM cells to peripheral stimulation, we focused on mechanical and cold stimulation, because clinical studies indicate that hypersensitivity to mechanical stimulation and cold are frequent and prominent symptoms after traumatic nerve injuries, whereas hyperalgesia to heat occurs only occasionally under neuropathic conditions (Scadding & Koltzenburg, 2006). Importantly, hypersensitivity to cold and mechanical stimulation is also a prominent finding in rats with the SNI model of neuropathy (Decosterd & Woolf, 2000). Because very little is known about the possible influence of somatic neuropathy on visceral sensations, we also determined responses of RVM cells to CRD.
The results indicate that SNI produced a significant hypersensitivity to cold in both ON- and OFF-cells throughout the observation period of 8 weeks. It should be noted, however, that the increase of excitatory responses to cold was considerably stronger in ON-cells than the increase of inhibitory ones in OFF-cells. Possibly, the SNI-induced reduction in the spontaneous activity of OFF-cells limited the amount of a further stimulus-evoked inhibition of OFF-cells. Responses to cutaneous pinch and visceral stimulation were markedly enhanced in ON-cells 8 weeks but not 1 week following nerve injury, whereas OFF-cell responses to pinch or visceral stimulation were not influenced by SNI. The present finding that hypersensitivity to peripheral stimulation was observed predominantly in presumably pronociceptive ON-cells of the RVM is in line with the hypothesis that a positive feedback loop involving ON-cells in the RVM is involved in promoting neuropathic hypersensitivity to peripheral stimulation. This hypothesis is further supported by the earlier behavioural findings indicating that nerve injury-induced hypersensitivity to mechanical stimulation (Pertovaara, 2000; Porreca et al., 2002) and cold (Urban et al., 2003) is dependent on descending facilitatory influence from the RVM. Moreover, it is noteworthy that although behaviourally assessed mechanical hypersensitivity develops within 24 h in the SNI model of neuropathy, it may not be fully developed until the second postoperative week (Decosterd & Woolf, 2000), whereas hypersensitivity to cold is fully developed within a week (Allchorne et al., 2005). These behavioural findings parallel the present neurophysiological results with ON-cells. However, when considering the potential behavioural significance of SNI-induced changes in stimulus-evoked responses of RVM neurons in the present study, one should note that assessments of neuronal responses to noxious peripheral stimulation were not accompanied by simultaneous assessments of corresponding behavioural responses. Moreover, behavioural responses of SNI animals to two of the currently used stimuli (pinch of an uninjured tail and CRD) have not been assessed in any previous study.
Comparison with previous studies
In line with the present findings, it has been shown earlier that ON-cells in the RVM have a pronociceptive role in acute inflammation as indicated by the association of their discharge rate with heat hypersensitivity induced by mustard oil (Kincaid et al., 2006; Xu et al., 2007). Previous studies addressing the contribution of RVM cells to neuropathic hypersensitivity have given partly contradictory results. It has been reported that chemical ablation of RVM cells expressing the µ-opioid receptor both prevents and reverses spinal nerve ligation-induced experimental neuropathy (Porreca et al., 2001). Because ON-cells are the only RVM neurons responding directly to µ-opioid agonists (Heinricher et al., 1992), the selective reversal of neuropathic hypersensitivity by ablation of medullary cells expressing µ-opioid receptors suggests, in line with the present results, that ON-cells promote neuropathic hypersensitivity. On the other hand, the results of our previous electrophysiological study (Pertovaara et al., 2001) suggested that spinal nerve ligation-induced neuropathy of 2–3 weeks' duration may not produce as marked pronociceptive changes in response properties of RVM cells as the SNI model of neuropathy in the present study or acute inflammation in other studies (Kincaid et al., 2006; Xu et al., 2007). Among possible explanations of the difference between the results of our present and earlier study (Pertovaara et al., 2001) are the differences in the genetic background of the animals, model of neuropathy, and postoperative time point of study. Interestingly, the sciatic constriction-induced model of neuropathy had no significant influence on response properties of RVM cells giving excitatory responses to noxious stimulation (Luukko & Pertovaara, 1993), whereas complete denervation of the sciatic area, as opposed to incomplete denervation in the present study, produced an enhancement of excitatory responses of RVM cells to peripheral stimulation of the adjacent intact skin area (Pertovaara & Kauppila, 1989). Together, these findings support the hypothesis that the magnitude or time course of the change in the response properties of RVM cells may vary with the model of peripheral neuropathy, with models involving sectioning of the peripheral nervous system resulting in strong neuropathic changes of the supraspinal pain control system. As anatomical feedback loops implicated in the crosstalk between the brainstem and spinal cord are essential for pain modulation (Almeida et al., 2006), the drastic changes associated with the afferent barrage of nociceptive messages after nerve fibre sectioning may have a role in the differences observed between models. In the specific case of the RVM, a clear anatomical basis for reciprocal interaction exists, as it receives direct projections from the spinal cord (Chaouch et al., 1983) and ON- and OFF-cells are known to project directly to dorsal horn laminae I–II and V (Fields et al., 1995) and modulate spinal nociceptive transmission (Fields et al., 2006).
Various models of peripheral neuropathy may increase spontaneous discharge rates and responses to somatic stimulation in nociceptive spinal dorsal horn neurons (e.g. Palecek et al., 1992; Laird & Bennett, 1993; Takaishi et al., 1996; Pertovaara et al., 1997), whereas their responses to visceral stimulation were not influenced 2–3 weeks after spinal nerve injury (Kalmari et al., 2001). It remains to be studied whether the SNI-induced changes in response properties of RVM cells observed in the present study reflect corresponding changes in response properties of spinal dorsal horn neurons, changes in supraspinal processing of nociceptive signals, or both.
Conclusions
The SNI model of peripheral neuropathy induced pronociceptive changes in response properties of RVM neurons that are considered to have an important role in descending regulation of pain. These pronociceptive changes included an increased baseline discharge rate in presumably pronociceptive ON-cells and a decreased baseline discharge rate in presumably antinociceptive OFF-cells. Moreover, responses to mechanical stimulation and cold were markedly enhanced in the pronociceptive cell type. It is proposed that the SNI-induced changes in spontaneous discharge rates of ON- and OFF-neurons of the RVM make a tonic contribution to maintenance of neuropathic hypersensitivity, whereas the enhanced responses of pronociceptive ON-cells to peripheral stimulation promote hypersensitivity via a positive feedback loop.
Acknowledgements
This study was supported by the Academy of Finland, and the Sigrid Jusélius Foundation, Helsinki, Finland, and the Portuguese Foundation for Science and Technology and the Gulbenkian Foundation, Lisbon, Portugal.
Abbreviations
-
- CRD
-
- colorectal distension
-
- RVM
-
- rostroventromedial medulla
-
- SNI
-
- spared nerve injury.