Calbindin D28K protein cells in a primate suprachiasmatic nucleus: localization, daily rhythm and age-related changes
Abstract
In mammals, the suprachiasmatic nucleus (SCN) of the hypothalamus is the master circadian pacemaker. The SCN controls daily rhythms and synchronizes the organism to its environment and especially to photic signals. Photic signals via the retinohypothalamic tract reach the ventral part of the SCN, where the majority of calbindin-containing neurons are located. Calbindin cells seem important for the control of circadian rhythmicity. As ageing leads to marked changes in the expression of circadian rhythms, we investigated in the mouse lemur, a nocturnal primate, age-related changes in the oscillation of calbindin protein expression in SCN neurons. We used immunohistochemistry and quantitative analysis of calbindin expression in the SCN of adult and aged mouse lemurs. In this primate, a dense cluster of calbindin-positive neurons was found in the ventral part of the SCN. In adult animals, calbindin-positive SCN neurons did not exhibit daily rhythms in their number or intensity, but exhibited significant daily variations in the percentage of cells with a calbindin-positive nucleus, characterized by high values during the daytime and low values during the night. Immunoreactive intensity peaked in the middle of the daytime. Calbindin expression in the nuclei of calbindin cells in the SCN tends to be modified by ageing. The amplitude of daily variation in calbindin expression was damped, with a lower immunointensity during the daytime and a delayed decrease during the night. These changes may affect the ability of the SCN to transmit rhythmic information to other SCN cells and thereby modify the synchronization of the different cell populations in the SCN.
Introduction
In mammals, the master circadian pacemaker is the suprachiasmatic nucleus (SCN) of the hypothalamus (review in Antle & Silver, 2005). This endogenous circadian clock controls the temporal organization of many physiological and behavioural processes, and coordinates and adjusts circadian rhythms according to daily and seasonal changes in light conditions. With age, many endogenous biological rhythms become blunted, and their synchronization to the light–dark (LD) cycle changes with age (Turek et al., 1995; Huang et al., 2002; Cayetanot et al., 2005b). Because SCN-controlled behavioural rhythms are affected by ageing, it has been suggested that such alterations would mainly result from changes in SCN function.
The SCN responds to timing signals received from afferent pathways and drives overt circadian rhythms through its efferent connections. Within the nucleus itself, the phenotype of neurons is characterized by the expression of a variety of neuropeptides. The SCN is classically divided in two parts, the ventrolateral part with cells that express vasointestinal polypeptide (VIP) and the dorsomedial part predominantly contains arginine vasopressin (AVP) neurons (Moore, 1996; Moore et al., 2002). Modifications with age of the daily or circadian rhythms of these two neuropeptides of the SCN have been reported in different species including human (Hofman & Swaab, 1994; Zhou et al., 1995; Krajnak et al., 1998).
Silver et al. (1996) described a compact subnucleus of calcium-binding protein calbindin-D28K (CalB) cells in the mid-caudal region of the hamster SCN. A majority of the CalB cells in the SCN express Fos protein in response to a light pulse (Silver et al., 1996). CalB cells receive a retinal innervation (Bryant et al., 2000) and send intra-SCN projections, in particular onto AVP and VIP cells (LeSauter et al., 2002). Lesions of the CalB subnucleus in the SCN lead to a disturbance of the locomotor activity rhythm (LeSauter & Silver, 1999). This has led to the hypothesis that the normal pattern of locomotor activity rhythm would be dependent upon the SCN-CalB cell group. Very few data are available, however, on the effect of ageing on calbindin protein expression in the SCN. Because the locomotor activity rhythm is one of the major parameters primarily affected in the course of ageing, it appeared necessary to investigate the profile of CalB secretion in the SCN in aged mammals.
The grey mouse lemur (Microcebus murinus), a nonhuman primate, exhibits alterations of the rest–activity rhythm during ageing which are similar to those documented in humans (Huang et al., 2002; Cayetanot et al., 2005b). In addition to the age-related effects on the locomotor activity rhythm, the daily variation in the expression of AVP and VIP in the SCN are shifted in aged mouse lemurs (Cayetanot et al., 2005a). These results suggest that neurochemical pathways within the SCN may underlie some of the age-related changes in output rhythms of the circadian clock.
As CalB is a good potential candidate for neurochemical regulation of the locomotor activity rhythm, the present study examined the expression of CalB in SCN neurons and the daily pattern of such expression in adult and aged mouse lemurs.
Materials and methods
Subjects
Gray mouse lemurs (Microcebus murinus) used in this study were born and kept in captivity in the laboratory breeding colony of the CNRS at Brunoy (MNHN, France; license approval N° A91.114.1). General conditions of captivity were maintained constant with respect to ambient temperature (24–26 °C) and relative humidity (55%). Food (fresh fruits, milk, porridge and insects) and water were provided ad libitum. Mouse lemurs exhibit seasonal and circadian rhythmicity dependent on photoperiod in most of their biological functions. The mean life span of the mouse lemur is 8–10 years in captivity (Perret, 1997). The animals reach sexual maturity at the age of 6 months, before entering the period of long days (breeding season). Both physiological and behavioural activity parameters show a decrease after 5 years (Aujard & Perret, 1998; Némoz-Bertholet & Aujard, 2003). The mouse lemur has been found to be one of the most powerful nonhuman primate models for the study of ageing (for review see Bons et al., 2006). As is usual in the breeding colony, the animals used in our study had been exposed since birth to a two-season regimen consisting of alternating periods of 6 months of short days (10 : 14 h LD) and 6 months of long days (14 : 10 h LD) every year. Animals were all studied under long photoperiod exposure. Two groups of males were used, 15 adult mouse lemurs (2.2 ± 0.2 years) and 15 aged ones (6.2 ± 0.2 years). They were housed on long days with artificial illumination (cool fluorescent lamps, 250–380 lux, and dim red light, 0.002 lux, were provided during daytime and nighttime, respectively).
All experiments were carried out in accordance with the European Community Council Directive of November 24, 1986 (86/EEC) and were approved by the CNRS. Care was taken to minimize the number of animals used in this study.
Telemetry recording
Locomotor activity rhythm was recorded by telemetry (Dataquest LabPro System; Data Sciences International, Transoma Medical, St Paul, MN, USA) using radio sensor–transmitters (TA10TA-F20; Data Sciences International) implanted in the visceral cavity under anaesthesia (ValiumR, 2 mg/100 g, i.m.; Imalgen 500R, 10 mg/100 g, i.p.). Fifteen adult (2–4.5 years) and 15 aged mouse lemurs (5–9 years) were used. Animals were isolated during the entire experiment. Locomotor activity was recorded over 15 days with a long photoperiod (14 h of light; LD 14 : 10). Data were collected using a computerized data acquisition system (LabPro v.3.11; Data Sciences International). Locomotor activity was quantified in arbitrary units (counts). The daily repartition of the locomotor activity was determined, by distinguishing mean nocturnal and diurnal amounts of locomotor activity. The daily activity onset was determined as the first six successive bins where activity was greater than the mean diurnal locomotor activity level.
Perfusion and histology
The animals were killed at five different times of the day. Zeitgeber time (ZT) 0 is defined as the time of lights on. Three animals per age group were killed at each of the following time points: ZT2, 8, 11, 15 and 20 h. Three time points during the daytime and two time points during the nighttime were studied, according to the duration of each phase (14 h of light, 10 h of dark). Mouse lemurs were deeply anaesthetized with sodium pentobarbital (100 mg/kg; i.p.) and then transcardially perfused with saline followed by a fixative solution containing 4% paraformaldehyde in phosphate-buffered saline (PBS; 0.1 m, pH 7.4). A block containing the hypothalamus was dissected out from the brain, postfixed overnight in the same fixative solution and then stored for 24 h in a cryoprotectant solution containing 30% sucrose in phosphate buffer, pH 7.4.
Serial coronal sections through the hypothalamus of all animals were cut on a freezing microtome at a thickness of 30 µm and collected in two series. One series (corresponding to one every second section) was processed for CalB staining.
Immunohistochemistry
Series of sections from adult and aged animals were reacted in parallel. Labelling was performed exploiting the 3-3′diaminobenzidine tetrahydrochloride (DAB) chromogens in the last step of the reaction intensified with nickel (resulting in a black reaction product).
Briefly, free-floating sections through the hypothalamus were immersed in one bath of peroxidase water (1.5%), followed by a 1-h blocking step in PBS containing 0.3% Triton X-100 (PBST) and 0.5% bovine serum albumin; the sections were incubated for 2 days at 4 °C with mouse monoclonal antibodies against the CalB protein (Sigma), diluted 1 : 20 000 in PBST containing 0.25% bovine serum albumin. The sections were then incubated at room temperature for 2 h in biotinylated horse antimouse immunoglobulins and for 1 h in the avidin–biotin–peroxidase complex solution (Vectastain Elite; Vector Laboratories). Peroxidase was detected with 0.02% DAB, 0.1% nickel ammonium sulphate and 0.01% hydrogen peroxide in 0.05 m Tris buffer, pH 7.6.
Standard control experiments were performed by omission of primary antibody. No staining due to the omitted antibody was seen in these conditions.
At the end of the procedures, the sections were mounted on gelatin-coated slides, air-dried, dehydrated, cleared with xylene and coverslipped with Depex. The material was examined under the light microscope.
Data analysis
Quantitative analysis was performed evaluating different parameters in the SCN for the purpose of a comparison between adult and aged animals at each time point. The number of CalB-positive cells and the percentage of CalB-positive cells with a CalB-immunoreactive (ir) nucleus was identified, and the level of peptide expression in the cytoplasm and nucleus of CalB cells as reflected by the immunostaining intensity was quantified. The analysis was conducted blindly as to the animal's age group and time of killing. In addition, cell size was measured by outlining the contour of cell body profiles with an image analysis digital system (analySIS® vs4; analySIS Soft imaging System GmbH, Münster, Germany), which calculated cell areas and mean diameters.
CalB cells and CalB cells with ir nuclei were visually counted in each section collected through the SCN, and therefore throughout the rostrocaudal extent of the nucleus, using an Olympus microscope BX51 (Olympus France SAS, Rungis, France). The boundaries of the SCN were clearly delineated by the immunoreactivity so that the counting strategy was consistent across sections. Counting in alternate sections and the small size of immunostained SCN neurons (see further) ensured that the same cells were not counted twice in different sections. Each field was screened with a 20× objective, and entire cell profiles in the focal plane (using as reference lightly stained or unstained cell nucleus) were then counted using a 40× objective, in two focal planes, following the optical dissector principle (Coggeshall & Lekan, 1996). The number of analysed sections (n = 6 or 7) was similar in adult and aged animals.
To evaluate the intensity of immunoreactivity, the relative optical density (ROD) of the nucleus and cytoplasm was measured in 16–30 CalB-positive cells for each animal. Densitometric analysis was performed using a digital camera (Olympus DP50-CU; Olympus Optical Co.) and the image analysis system mentioned above, using a 100× oil-immersion objective. The zero value of ROD was assigned in each section to the background defined as the intercellular spaces within the SCN outside the CalB cell group. All measurements were done keeping constant light intensity to standardize ROD measurements across the analysis. The contour of cell body profiles was outlined for cytoplasmic ROD measurements, using consistent random sampling within the SCN. Inside the cytoplasmic contour, the nuclear contours were outlined for nuclear ROD measurement and, finally, cytoplasmic ROD was substracted from nuclear ROD. The mean RODs for the nucleus and the cytoplasm were calculated for each animal.
Results are given as mean (number of positive cells and percentage of cells with ir nucleus, or ROD units per section) ± SEM. The influence of ageing and daily time was evaluated by two-way anova . This analysis was aimed at determining whether the parameters (cell number or percentage of cells with ir nucleus, and ROD values) underwent rhythmic changes during 24 h. The effect of time on the number and immunostaining intensity of CalB-positive cells or percentage of cells with CalB-positive nucleus in either age group was assessed with the post hoc Tukey HSD test for paired comparison between different time points in each group. Significance was set at P < 0.05.
Results
Representative average profiles of the daily locomotor activity rhythm of one aged and one adult mouse lemur showed age-related changes (Fig. 1). Compared to adult animals, aged mouse lemurs exhibited an advance of the activity phase onset (t28 = 2.283, P = 0.03). Moreover, the percentage of diurnal activity increased in aged compared to younger mouse lemurs (t28 = −3.353, P = 0.002).
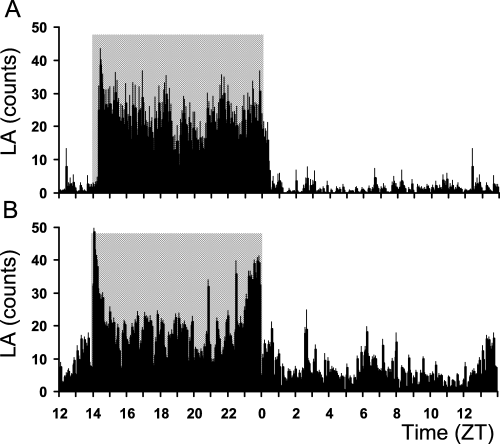
Representative average daily profile (average ± SEM) of locomotor activity (LA) obtained from 10 days of telemetric recording in (A) an adult and (B) an aged mouse lemur held under a 14 : 10 h light–dark cycle. The dark phase is indicated by the grey shaded area.
The immunocytochemical staining revealed a distribution of CalB-positive cells in the ventral part of the SCN. The surface area of CalB cells was 157 ± 16 µm2 whatever the age of the animals.
There was a distinct pattern of CalB immunoreactivity within the SCN (Fig. 2). In the rostral part of the SCN, CalB-ir cells were relatively sparse. In the middle of the SCN, CalB-ir neurons increased in number and were mainly localized within the central region of the nucleus. In the caudal SCN, CalB-ir cells became less clustered. The most centrally located CalB-ir cells were more densely concentrated and more darkly labelled than the CalB-ir neurons in the more peripheral regions of the SCN.
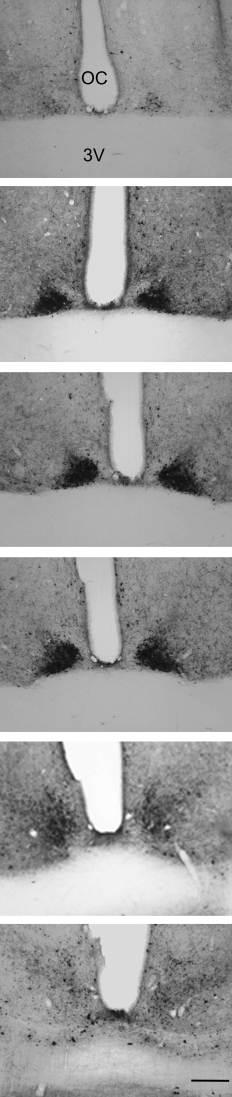
Distribution of CalB immunoreactivity through the SCN of one mouse lemur exposed to a 14 : 10 h LD cycle and killed at ZT2. Serial 30-µm sections are arranged from the rostral (top) to the caudal (bottom) extent of the SCN. Most CalB cells are in the mid-SCN region. CalB neurons are also located in the rostral and caudal part of the SCN but cells are sparse. OC, optic chiasm; 3V, third ventricle. Scale bar, 200 µm.
The two-way anova did not reveal any influence of time on the number of cells that expressed CalB (F4,20 = 0.34, P = 0.84; Fig. 3A). Ageing did not affect the number of CalB-ir neurons (two-way anova, F1,20 = 2.76, P = 0.11) and no significant time × age interaction was notified (two-way anova, F4,20 = 0.08, P = 0.98). However, time-dependent variation in the cytoplasmic ROD was close to significance (F4,20 = 2.78, P = 0.055), with no significant effect of age (two-way anova, F1,20 = 0.59, P = 0.45) or time × age on this parameter (two-way anova, F4,20 = 0.98, P = 0.43; Fig. 3B). Post hoc tests did not reveal any point-by-point difference in the cytoplasmic ROD of adult mouse lemurs, while significant differences could be found in aged mouse lemurs. Indeed, a peak in cytoplasmic ROD at ZT2 was observed in the aged group, characterized by a significant increase in cytoplasmic ROD from ZT20 to ZT2 (P < 0.05) and a significant decrease from ZT2 to ZT8 (P < 0.05).
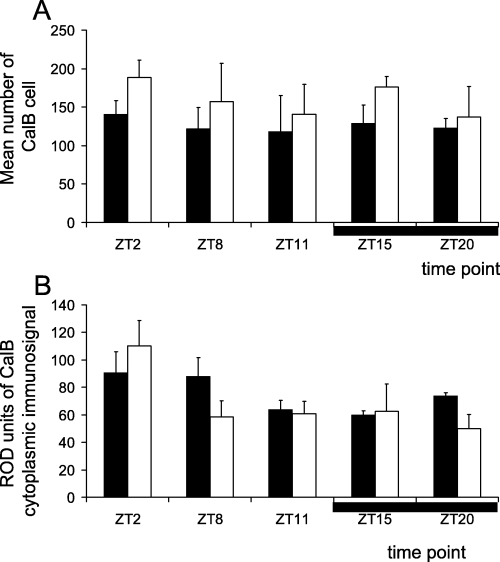
Oscillation of CalB immunoreactivity in the suprachiasmatic nucleus of adult (filled bars) and aged (empty bars) mouse lemurs at the sampled time points (the horizontal black bar indicates the nighttime). Data obtained (A) with cell counts and (B) with densitometric analysis on cytoplasm (ROD measured in arbitrary units). Note that the number of CalB-positive cells and ROD do not exhibit a daily rhythm whatever the age in mouse lemurs. Statistical values are given in the text.
The percentage of cells with a CalB-ir nucleus was significantly modified by ageing (two-way anova, F1,20 = 4.50, P < 0.05) and by time of day (F4,20 = 7.00, P < 0.005; Fig. 4A). In adult mouse lemurs, the percentage of cells with a CalB-ir nucleus underwent changes over 24 h. The percentage of cells with a CalB-ir nucleus was high during the daytime (ZT11, 31.7 ± 4.0%) and decreased to significantly lower values during the nightime (ZT15, 9.3 ± 2.8; and ZT20, 15.8 ± 4.8% of cells with immunopositive nucleus; post hoc tests, P < 0.005 and P < 0.01, respectively, compared to ZT11; Fig. 5). No significant time-of-day variation could be observed in the aged group.
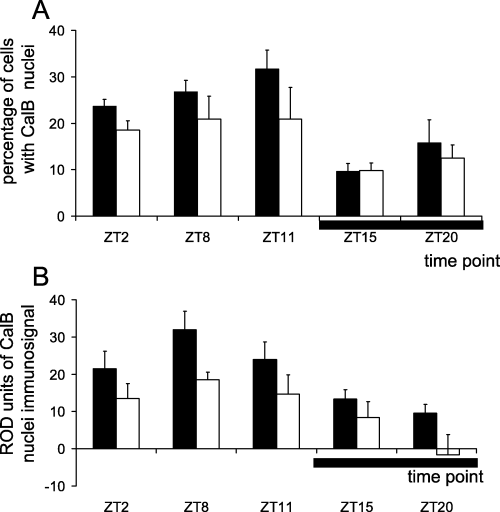
Oscillation of the percentage of CalB cells with CalB-positive nucleus in the suprachiasmatic nucleus of adult (filled bars) and aged (empty bars) mouse lemurs at the sampled time points (the horizontal black bar indicates the nighttime). Data obtained (A) with cell counts and (B) with densitometric analysis on the nuclear compartment of the cells (ROD measured in arbitrary units). Note that the percentage of cells with CalB-ir nuclei and ROD in the nucleus exhibit a daily rhythm in adult mouse lemurs, with higher values during the daytime and lower values during the night (ZT15 for percentage of cells with CalB-positive nuclei, and ZT20 for ROD in CalB-positive nuclei). In aged mouse lemurs, the immunosignal intensity was decrease at each time point compared with adults. Statistical values are given in the text.
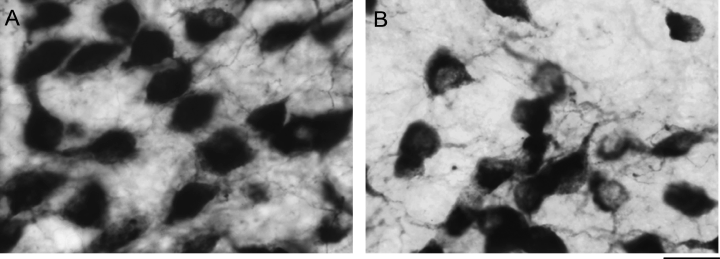
Photomicrograph of CalB immunoreactivity in the SCN of an adult mouse lemur (A) during the daytime (ZT11) and (B) during the night (ZT15). Note the decrease in the number of CalB cells with CalB-ir nuclei during the night. Scale bar, 20 µm.
Two-way anova indicated that time of day had a significant effect on the nuclear CalB ROD (F4,20 = 7.89, P < 0.005; Fig. 4B). In adult animals, nuclear ROD peaked at ZT8, in the middle of the day, and decreased to significantly lower values at the beginning of the night (ZT15: P < 0.01). The nuclear ROD was significantly modified by ageing, with a general decrease in CalB intensity in aged animals compared to adults (two-way anova, F1,20 = 12.04, P < 0.005). Therefore, the daily variation in nuclear ROD was damped in aged animals. The peak in intensity during the daytime was absent in aged animals, and a significant decrease in intensity did not occur until the end of the nighttime (ZT20: P < 0.05). To illustrate the age-related damping in CalB intensity, representative CalB immunostainings in the cytoplasm and nucleus of SCN cells from one adult and one aged animal during the middle of the daytime (ZT8) are presented in Fig. 6.
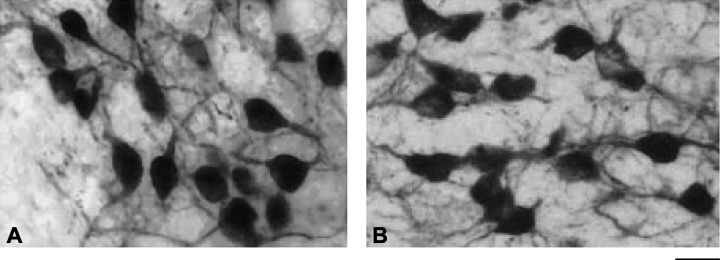
Photomicrograph of CalB immunoreactivity in the SCN of (A) an adult and (B) an aged mouse lemur during the daytime (ZT8). Note a decrease in nuclear immunosignal intensity in the aged animal compared to the adult. Scale bar, 20 µm.
Discussion
We examined in a nonhuman primate, under the LD cycle, the daily fluctuation of CalB in SCN neurons, and the effect of ageing on such oscillations within the circadian pacemaker. The main findings of our investigations are: (i) the presence of a CalB cell group in the SCN of a prosimian primate; (ii) evidence for a daily rhythm of CalB immunoreactivity in the cell nuclei and in the percentage of CalB cells with ir nuclei in the SCN of adult mouse lemurs; (iii) reduced daily variation in nuclear CalB immunoreactivity with ageing in mouse lemurs.
The SCN of the mouse lemur, a nocturnal primate, contains a CalB cell group. CalB-ir cells are distributed rostrocaudally, with sparse CalB-ir neurons in the rostral part, a dense cluster of CalB-ir cells in the middle of the nucleus and a less clustered distribution of CalB-ir neurons in the caudal section of the SCN. CalB protein is found in the SCN of humans and in the two nonhuman primates studied so far: the marmoset and the squirrel monkey. The distributions of the CalB-positive cells in the SCN of these two nonhuman primate species differ from the one observed in the mouse lemur. In marmoset, CalB is expressed in all part of the SCN (Costa & Britto, 1997) whereas CalB-positive cells are scattered in the SCN of squirrel monkey (Fortin & Parent, 1997). In humans, a cluster of CalB-cells is present in the central and the anterior area of the SCN (Mai et al., 1991). The distribution of CalB-ir neurons in the SCN in mouse lemurs is similar to the distribution described in hamsters (Silver et al., 1996) and Arvicanthis but differs from that of Rattus norvegicus in which the repartition of CalB cells was sparse throughout the SCN (Mahoney et al., 2000).
Daily rhythm of calbindin in SCN neurons of adult mouse lemurs
In adult animals, time of day had no effect on the number of CalB-containing cells or on cytoplasmic CalB immunoreactivity. This finding is in accordance with previous studies in rats (Rattus norvegicus) and Arvicanthis (Mahoney et al., 2000) and in hamsters (Hamada et al., 2003), which reported that the number of CalB cells or the CalB-cytoplasmic immunoreactivity remained constant during the LD cycle. In contrast, the number of CalB-positive cells was lower during the daytime compared to nighttime in another study in Wistar rats (Arvanitogiannis et al., 2000). Hamada et al. (2003) showed that a circadian rhythm of CalB was expressed in the nuclei of CalB cells in the hamster SCN. In this species, the protein expression was detected at a constant level at all times in the cytoplasm and was low in the nucleus during the nightime and elevated during the daytime in light–dark condition or in constant darkness. Our results show that mouse lemurs exhibited a daily rhythm in CalB nucleus content, with a higher percentage of cells with CalB-positive nucleus during the daytime and a low percentage of cells with CalB-nucleus immunoreactivity during the nocturnal phase in LD conditions. The presence of CalB in cell nuclei has been reported previously in rodents (German et al., 1997), but the function of the nuclear CalB is unknown. CalB proteins are involved in the regulation of intracellular calcium concentrations and calcium transport (German et al., 1997). CalB has a high affinity for calcium and may act as an intracellular calcium buffer in the cell nucleus. Consequently, nuclear CalB could be involved in the regulation of processes mediated by calcium during the daytime in the SCN, when the level of positive cells with nuclear CalB immunoreactivity is high.
Several lines of evidence suggest that CalB-immunoreactive neurons within the SCN are critical for the maintenance of circadian rhythms in hamster (LeSauter & Silver, 1999; Kriegsfeld et al., 2004). In hamsters, SCN CalB-ir neurons receive retinal innervations (Bryant et al., 2000) and are responsive to light (Silver et al., 1996). CalB cells may have a pacemaker function in the SCN and may act in photic entrainment. Indeed, the number of CalB-positive cells changed according to the light conditions, increasing in a manner dependant on the time of exposure to constant darkness in hamsters (LeSauter et al., 1999). CalB-ir cells contain GABA (Jobst et al., 2004) and are interconnected with other populations of neurons within the SCN itself (LeSauter et al., 2002; Jobst et al., 2004). The colocalization of GABA with CalB may suggest that this calcium-binding protein is implicated with GABA in the synchronization of the outputs from different populations of SCN neurons (Jobst et al., 2004). Nevertheless, the CalB-ir cells themselves do not exhibit a spontaneous daily rhythm in c-fos expression (Silver et al., 1996) or a circadian rhythm in firing rate (Jobst & Allen, 2002). Moreover, lesion of CalB cells in the SCN leads to a disruption of the circadian rhythm in body temperature, heart rate, cortisol and melatonin, suggesting that CalB is necessary for sustaining circadian rhythms (Kriegsfeld et al., 2004).
Daily rhythm of calbindin in SCN neurons of aged mouse lemurs
It is the first time that the influence of ageing on CalB expression in SCN cells has been studied in a primate. In aged mouse lemurs, as in adults, the number of CalB-positive cells in the SCN did not exhibit a significant daily rhythm. Our results on CalB protein expression are similar to a recent study on CalB mRNA expression in hamsters, which showed no difference in mRNA levels in SCN between aged and adult animals (Duncan & Franklin, 2007). CalB expression in the nuclei of CalB-ir cells in the SCN tended to be modified by ageing. Although the sample size per time point was limited by the use of a primate species, a general decrease in CalB intensity in aged animals compared to adults was observed. The amplitude of daily variation in CalB expression was damped. The peak in intensity during the daytime observed in adults was not observed in aged animals, and the significant decrease in intensity was delayed, compared to adults, to the end of the nighttime. The dynamic of transfer through the nucleus membrane could be impaired with ageing, leading to the accumulation of CalB in the cytoplasm and a reduced mobilization within the nucleus.
Modifications of the daily rhythm in locomotor activity have been observed in aged mouse lemurs, with a decrease in amplitude, an increase in fragmentation and a phase advance of the onset of nocturnal activity (Cayetanot et al., 2005b). Moreover, previous findings in aged mouse lemurs corroborate the hypothesis that the SCN function is altered with ageing. First, a reduced stimulation of the SCN in response to light has been demonstrated with ageing in mouse lemurs (Aujard et al., 2001a). Second, the free-running period decreases with ageing in mouse lemurs, demonstrating that the mechanism within the master circadian clock changes with ageing (Cayetanot et al., 2005b). The attenuation of daily rhythm in nuclear CalB immunoreactivity may be in part responsible for the modification of the daily rhythm in locomotor activity observed in aged mouse lemurs. In hamsters, the destruction of the CalB cell group in the SCN in adults leads to a loss of rhythmicity in locomotor activity; this could be restored by an SCN graft from pups that contains CalB cells (LeSauter & Silver, 1999). This suggests a relation between CalB function in the SCN and the expression of a locomotor activity rhythm. A recent model suggests that the calbindin cell region in the SCN provides a daily signal that maintains synchronization among the clock cells in the SCN shell (Antle & Silver, 2005). CalB-positive neurons do not exhibit a circadian rhythm in their spontaneous firing rate, but these cells receive a dense retinal innervation, suggesting that they may play a critical role on the circadian system by gating photic input to pacemaker cells of the SCN core. Disruption of the CalB rhythm in the SCN could affect the synchronization between cells within the SCN in aged individuals.
A study using tracer coupling has identified gap junctions in neurons within the core region that contains CalB-positive cells (Jobst et al., 2004). This gap junction coupling between rhythmic cells and CalB neurons provides a pathway by which the latter can interact with pacemaker oscillators and a direct mechanism by which light information could be rapidly transmitted to rhythmic cells in the SCN. Gap junction coupling may be involved in the mechanism of synchronization between the cells that receive retinal input and the other populations of cells in the SCN. It has been demonstrated that individual SCN neurons from aged mice lose their spontaneous circadian electrical activity rhythm in vitro and that this alteration is associated with an altered circadian pattern of locomotor activity in vivo (Aujard et al., 2001b). In humans, the daily rhythm of rest–activity and core body temperature or the relationship between cognitive performance and core body temperature deteriorate with ageing (Monk & Kupfer, 2000; Van Someren et al., 2002). Age-related changes in the circadian timing system in humans, as in other species of mammals, may be linked to the alteration of the message received by the SCN and/or to a dysfunctioning of the SCN cells themselves (van Someren et al., 2002; Hofman & Swaab, 2006). Thus, with ageing, the alteration of locomotor activity rhythm, like some other daily or circadian rhythms, may depend on the synchronization of SCN cells. Without this synchronization, the output message of the SCN is modified and this would lead to a disturbed rhythm in behaviour.
There are several arguments to say that both CalB and VIP cell groups are implicated in the synchronization of SCN cells on photic information (Aton et al., 2005): first, CalB and VIP neurons receive retinal input; second, these cell groups are located in the ventrolateral part of the SCN; third, they both send efferent projections to the entire SCN. In the mouse lemur, VIP daily rhythm in the cells of the core SCN is modified with ageing and exhibits a delay of the peak value at the beginning of the daytime in comparison with adults, which had their peak value during the nighttime (Cayetanot et al., 2005a). VIP injections into the SCN produce a phase shift of the locomotor activity rhythm in Syrian hamsters in constant lighting conditions (Piggins et al., 1995) and a phase shift in SCN electrical activity (Reed et al., 2001). In mouse lemurs, it has been suggested that the delay of the VIP peak observed in aged mouse lemurs would be implicated in the advance of the locomotor activity phase observed in the long-days photoperiod in aged animals (Aujard et al., 2006). In addition, the present results suggest that the attenuation of the daily rhythm in nuclear CalB expression may participate to the modification of the locomotor activity rhythm in aged animals. Indeed, the expression of a clear locomotor activity rhythm or of other output rhythms may depend on interactions between neurotransmitters or neuromodulators present in the SCN, such as VIP or CalB protein. As reciprocal connections between CalB cells and VIP cells exist in the SCN (LeSauter et al., 2002), we may hypothesize that the absence of a nuclear peak in CalB immunointensity in aged animals would participate in the delayed peak in VIP expression in the SCN.
To conclude, our results showed that CalB-positive cells in the SCN exhibited a daily rhythm in nuclear content and not in cytoplasmic content in adult mouse lemurs, and that CalB nuclear rhythmicity in the SCN seems to deteriorate with ageing in the mouse lemur. The attenuation of the nuclear CalB signal in the SCN cells in aged mouse lemurs associated with the shift of the VIP peak previously observed in this species may be implicated in a loss of synchronization between the SCN cells, leading to less daily behavioural organization.
Acknowledgements
This work was supported by grants from the European Commission QLK6-CT-2002-02258, ATC Ageing and ATC Time and Brain of the French Ministry of Research, Institute for Longevity and Foundation for Medical Research.
Abbreviations
-
- AVP
-
- arginine vasopressin
-
- CalB
-
- calbindin-D28K
-
- ir
-
- immunoreactive
-
- LD
-
- light–dark
-
- ROD
-
- relative optical density
-
- SCN
-
- suprachiasmatic nucleus
-
- VIP
-
- vasoactive intestinal polypeptide
-
- ZT
-
- zeitgeber time