Norepinephrine depletion facilitates recovery of function after focal ischemia in the rat
Abstract
Previous studies have suggested that increased norepinephrine plays an important role in recovery of function after brain injury; however, the majority of these studies used drugs that are known to also affect other monoamines to increase or decrease norepinephrine. The purpose of the present study was to determine if norepinephrine is required to promote recovery after ischemia. A form of enriched rehabilitation was used to rehabilitate animals after ischemia and the neurotoxin N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine was used to selectively destroy norepinephrine projections from the locus coeruleus. Three sensorimotor tests were used to evaluate the recovery of the animals. Depletion of norepinephrine improved sensorimotor recovery in standard-housed animals and did not impede recovery in the rehabilitation groups. Dopamine beta hydroxylase staining was used to confirm N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine-depleted terminal norepinephrine levels. The amount of norepinephrine terminal staining negatively correlated with recovery of function in the staircase test after ischemia. In addition, enriched rehabilitation increased, but depletion of norepinephrine had no effect on, brain-derived neurotrophic factor protein levels, which have also been linked to improved recovery of function. Together the above findings question the previously postulated role of norepinephrine in recovery of function after stroke.
Introduction
Physiotherapy is the best treatment option for patients disabled as a result of stroke. Recent evidence suggests that following brain injury pharmacological as well as environmental factors can markedly alter neuronal plasticity and augment rehabilitation (Gladstone & Black, 2000; Biernaskie & Corbett, 2001; Johansson & Belichenko, 2002; Biernaskie et al., 2004).
Amphetamine paired with motor training promotes recovery of function on a beam-walking task after brain injury (Feeney et al., 1982; Hovda & Fenney, 1984; Goldstein & Davis, 1990). Although amphetamine increases other monoamines, studies suggest that increased norepinephrine (NE) is probably responsible for the positive actions of amphetamine on recovery. For example, intraventricular infusion of dopamine or NE improves beam walking after cortical injury but the effect is lost if the conversion of dopamine to NE is blocked (Boyeson & Feeney, 1990). Other drugs that increase NE (i.e. atipamezole) also facilitate sensorimotor recovery after middle cerebral artery occlusion (MCAo) in the rat (Jolkkonen et al., 2000; Butovas et al., 2001; Puurunen et al., 2001).
We have previously found that environmental enrichment improves forelimb motor function after MCAo (Biernaskie & Corbett, 2001) and others have shown that mice reared in an enriched environment for 40 days had significantly higher brain levels of NE than control animals (Naka et al., 2002). Environmental enrichment facilitates neuroplasticity, neurogenesis and increases several neurotrophins, such as brain-derived neurotrophic factor (BDNF), which may explain why it facilitates recovery of motor function after brain injury (Pham et al., 1999; Ickes et al., 2000; Biernaskie & Corbett, 2001; Johansson & Belichenko, 2002; Mohammed et al., 2002; Gobbo & O'Mara, 2004). Similarly, amphetamine and other drugs that increase NE have also been shown to facilitate plasticity, neurogenesis and neurotrophin increases (Stroemer et al., 1998; Malberg et al., 2000; Russo-Neustadt et al., 2001; Butefisch et al., 2002), whereas decreasing NE pharmacologically can decrease BNDF and plasticity associated with exercise (Garcia et al., 2003) and training (Sawaki et al., 2003).
Lesioning of the locus coeruleus (LC), the largest nucleus of noradrenergic neurons in the central nervous system (Nakamura & Sakaguchi, 1990), prior to motor cortex injury impairs motor recovery (Goldstein & Bullman, 1997) as does selective degeneration of the NE projections from the LC using N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) (Goldstein et al., 1991; Boyeson et al., 1992).
It is important to note that most of the studies demonstrating the importance of NE have been performed using drugs (i.e. amphetamine, antidepressants and prazosin) that also have effects on other neurotransmitters. In addition, it has yet to be determined whether NE is actually required to promote or increase recovery after ischemia. In order to more directly assess the involvement of NE in functional recovery, the present study used DSP-4 to selectively deplete NE projections from the LC at 1 week after MCAo in rats. Environmental enrichment combined with a rehabilitation task was used to promote recovery and three different sensorimotor tests were used to assess recovery. In addition, the effect of depleting NE on BDNF levels was measured.
Materials and methods
Subjects
A total of 139 male Sprague Dawley rats (Charles River, Montreal, QC, Canada), weighing approximately 300 g at the time of surgery, were used in this study. Initially, all animals were socially housed in pairs in standard Plexiglas cages on a reverse day/night cycle (see housing and treatment groups below). Behavioral testing was performed during the dark phase. Food and water were provided ad libitum except during behavioral testing periods when food was restricted to 12–15 g/day. All procedures were in accordance with guidelines set by the Canadian Council on Animal Care and approved by the Memorial University Animal Care Committee. Every effort was made to reduce animal numbers.
Surgical procedures
Animals were anesthetized with 3% isoflurane in 30% oxygen and 70% nitrous oxide and maintained with 1.5% isoflurane. Their temperature was maintained at between 36.5 and 37.5 °C throughout the surgery by using a self-regulating heating blanket (Harvard Apparatus, Holliston, MA, USA). Animals underwent endothelin-1 (ET-1) MCAo as outlined in previous studies (Biernaskie & Corbett, 2001), except that an increased concentration of ET-1 (human and porcine, Calbiochem, Cedarlane, Hornby, ON, Canada) was employed. Briefly, a single injection of ET-1 (600 pmol in 3 µL sterile H2O) was placed adjacent to the middle cerebral artery (anterioposterior, +0.9 mm; mediolateral, −5.2 mm; dorsoventral, −8.7 mm). All stereotaxic measurements are relative to bregma (Paxinos & Watson, 1986). The control group included sham animals that underwent the same surgery up to and including the drilling of the burr hole but did not receive ET-1. Based on combined behavioral and histological criteria a success rate of ∼ 60% is achieved with this ischemic model (Biernaskie et al., 2004; Windle et al., 2006). Animals that showed less than a 20% reduction in reaching ability on staircase testing at 5 days post-stroke were excluded from treatment groups. Of these animals, those with reaching performance between 80 and 95% of pre-stroke levels were excluded from the study, as such animals typically show spontaneous recovery. Finally, those rats with minimal reaching impairments (i.e. ≥ 95% of pre-stroke levels) and an absence of infarcts upon histological examination were assigned to the control group in order to reduce animal numbers.
Norepinephrine depletion
In order to deplete the noradrenergic projections from the LC, rats were given a single i.p. injection of 50 mg/kg of DSP-4 (Sigma-Aldrich, St Louis, MO, USA) dissolved in sterile saline (50 mg/mL) at 6 days after ischemia. The dose of DSP-4 was based on previous studies that have shown long-term depletion of NE (Ross, 1976; Fritschy & Grzanna, 1991). Animals were closely monitored and given mash and lactated ringers (5 mL) if needed. Most animals appeared healthy and were able to commence their housing treatment (see below) the next day; however, a small number of animals were kept separate until they began to gain weight (usually 2–3 days). Weight loss occurred equally in all of the treatment groups and the slight delay in starting housing treatment did not affect outcome (data not shown). Animals that did not receive DSP-4 received an equivalent volume (1 mL/kg) of sterile saline.
Housing and treatment groups
A timeline of the experimental protocol is given in Fig. 1. At 5 days after ischemia, rats were tested on three behavioral tests of motor coordination and forelimb function (see below), and grouped according to the severity of impairment on the staircase test. We have previously shown that performance in the staircase test at 5 days after surgery is a reliable predictor of infarct volume (Windle & Corbett, 2005). Once grouped for severity, animals were randomly assigned to one of eight treatment groups: ischemic + enriched rehabilitation, ischemic + standard housing (I + St), control + enriched rehabilitation, control + standard housing, ischemic + enriched rehabilitation + DSP-4, ischemic + standard housing + DSP-4 (I + St + DSP-4), control + enriched rehabilitation + DSP-4 and control + standard housing + DSP-4. Post-treatment testing was carried out at 2, 6 and 9 weeks after the injection of DSP-4 and the start of housing treatment. In addition to the animals listed in the above groups, an additional 30 animals in the four ischemic groups (n = 7–8/group) were used without behavioral testing for the BDNF portion of the study and seven animals in the ischemic + enriched rehabilitation + DSP-4 and six animals in the I + St + DSP-4 group were tested behaviorally but killed at 1 week post-DSP-4. Animals were killed wih an overdose of Somnotol®.
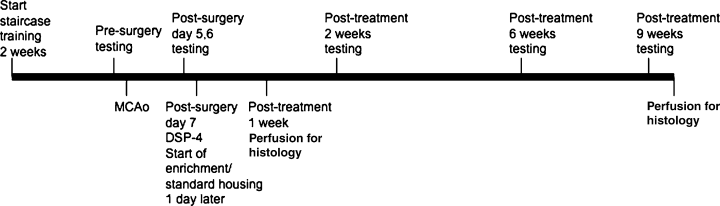
Timeline of behavioral testing, drug administration and housing treatment. MCAo, middle cerebral artery occlusion; DSP-4, N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine.
Enriched rehabilitation
A similar protocol was followed as used previously (Biernaskie & Corbett, 2001). Animals were housed in groups of six to eight rats in large metal cages equipped with ropes, beams, platforms and various toys. Twice per week the cages were cleaned and the types of toys and the orientation of objects were changed. In addition, animals were given a 6-h rehabilitation session 5 days per week (with the exception of testing days) for a total of 9 weeks. The animals were placed individually in a standard cage containing a Plexiglas reaching apparatus containing Noyes precision pellets (45 mg, Research Diets Inc., New Brunswick, NJ, USA). The apparatus was such that the animal must use its impaired limb to retrieve the pellets.
Standard housing
Animals were housed in pairs in standard Plexiglas cages. In addition to their regular chow they were fed the average amount of Noyes pellets eaten by the enriched rehabilitation (ER) rats each day (approximately 12–14 g).
Behavioral assessment
Staircase reaching test
The staircase reaching test (Montoya et al., 1991) consisted of a chamber with a central platform for the rat to climb onto and a set of seven steps on each side. Each step held three 45 mg Noyes precision pellets. The rats remained in the staircase for 15 min and the total number of pellets eaten on each side was recorded. This test provides a sensitive measure of skilled reaching ability of the forepaw and also of sensory neglect. The animals were pre-trained twice per day over a 14-day period ending 2 days prior to the ischemic injury. Animals that failed to consistently retrieve over 55% of the total available pellets were excluded from the study. Animals were retested for two trials per day on days 5 and 6 after ischemia (pre-treatment) as well as over 2 days at 2, 6 and 9 weeks after DSP-4 (post-treatment). Reaching ability was expressed as a percentage of the last four pre-ischemia reaching trials for each of the test points.
Forelimb asymmetry test
Animals were tested for limb preference and their ability to support weight on either forelimb by placing the animals in a 20 × 35-cm (diameter × height) clear Plexiglas cylinder for 5 min (Schallert et al., 1997). As the animal reared to explore the environment, the number of bilateral paw placements, placements of the paw ipsilateral to the lesion and placements of the paw contralateral to lesion were counted. Animals were required to have a minimum of 20 contacts. If 20 contacts were not achieved in the 5-min timeframe the animal was observed until 20 contacts had been made. Normal animals tended to use each limb more or less equally, whereas ischemic animals favored their ipsilateral forelimb after injury (Schallert et al., 1997). The percent of ipsilateral limb use was calculated by using the equation: (ipsilateral contacts + 0.5 bilateral contacts/total contacts) × 100%. Paw contacts were videotaped from below using an angled mirror and later analysed in a blinded fashion. Animals were tested once prior to ischemia and once at each subsequent timepoint.
Ladder-rung walking test
Animals were tested for forelimb and hindlimb function as well as motor coordination by crossing a ladder with an irregular rung pattern (Metz & Whishaw, 2002). Animals were trained to cross the ladder over four trials in 1 day. At each test point, including prior to ischemia, the animals were filmed while crossing the ladder on four trials. Slips and misplacements of paws were scored for a 1-m segment of the ladder. To give an indication of impairment the number of slips was added to the placement errors and divided by the number of steps taken for a total number of errors/step. The rung pattern was changed for each test point.
All of these tests have been shown to be sensitive indicators of forelimb deficits following focal ischemic injury (Montoya et al., 1991; Biernaskie & Corbett, 2001; Metz & Whishaw, 2002).
Histological procedures and assays
Histology
Animals were killed at either 1 or 9 weeks post-DSP-4 treatment. At the conclusion of each study, animals were injected with an overdose of Somnotol® and perfused transcardially with heparinized saline followed by 4% paraformaldehyde for 5 min. Brains were removed and placed in 4% paraformaldehyde for 90 min then transferred to a 20% sucrose solution in phosphate-buffered saline (PBS) and allowed to sink (approximately 3 days). Brains were frozen on dry ice and 40-µm sections were cut using a cryostat (CM 3050 S, Leica, Germany). Every eighth section was mounted and stained with cresyl violet for infarct volume assessment. All other sections were stored in cryoprotectant at −20 °C until processed for immunohistology.
Infarct measurement
Slides were scanned and every third slice was analysed microscopically; the healthy tissue in the cortex, striatum and total hemisphere for each side of the brain was traced using image j software (NIH). The volume of injury was calculated by subtracting the area measured in the ischemic hemisphere from the contralateral hemisphere and multiplying that value by the distance between the measured slices. In order for animals to be considered as controls no visible damage was seen apart from a needle tract.
Immunohistochemistry
Every eighth section was stained for dopamine beta hydroxylase (DβH). Sections were washed in PBS followed by 3 min in 3% H2O2. Slices were then washed in PBS (3 × 10 min), incubated in 5% normal goat serum in PBS with 0.25% Triton-X for 1 h and then incubated overnight at 4 °C in a 1 : 1000 dilution of mouse anti-DβH (Chemicon, Temecula, CA, USA) in PBS with 0.25% Triton-X. The next day slices were washed in PBS, incubated in a 1 : 500 dilution of biotinylated goat anti-mouse (Jackson Immuno Research Laboratories Inc., West Grove, PA, USA) in PBS with 0.25% Triton-X for 1 h, washed in PBS, incubated in 10 µg/mL extravadin (Sigma-Aldrich, St Louis, MO, USA) in PBS with 0.25% Triton-X for 1 h, washed in PBS and reacted for 5 min in a 3,3′-diaminobenzidine tablet set (Sigma-Aldrich). Negative controls were run with each batch of staining.
Measurement of dopamine beta hydroxylase staining
Loss of DβH staining has been shown to represent degeneration of LC axons and has been confirmed by morphological techniques, staining for NE antibodies and measuring NE using high-performance liquid chromatography (Fritschy & Grzanna, 1991). Neuronal projections stained with DβH were traced and analysed with neurolucida software (MicroBrightfield Inc., Williston, VT, USA) at 40× magnification (DMRXE light microscope, Leica Microsystems Canada, Richmond Hill, ON, Canada). The total length of axonal projections in a given area was calculated using NeuroExplorer (MicroBrightfield Inc.). Projections were analysed in four different regions of interest (ROIs) in the hemisphere contralateral to the MCAo (in order to avoid infarcted tissue): the frontal cortex (ROI 1), forelimb region of the motor cortex (ROI 2), parietal cortex (ROI 3) and CA1 region of the hippocampus (ROI 4). Three counts were taken in each ROI (150 × 150 µm2) and averaged, as shown in Fig. 6.
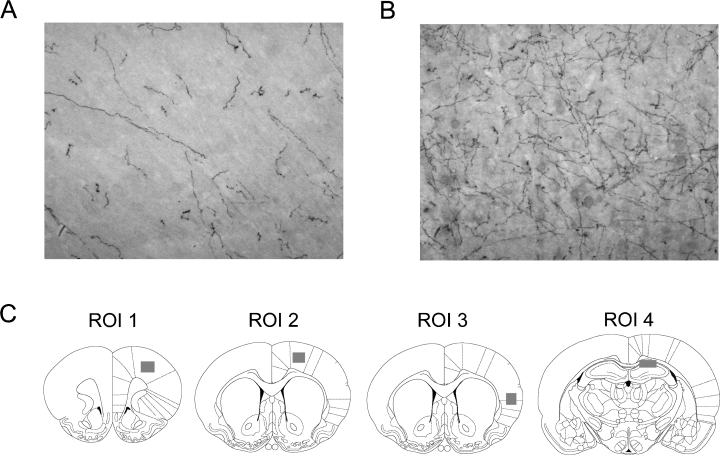
Dopamine beta hydroxylase (DβH) staining of motor cortex contralateral to the infarct in (A) N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine- and (B) saline-treated animals (20× magnification). (C) Regions of interest (ROIs) in which DβH staining was measured. Note that the shaded boxes represent the approximate area in which three measurements were taken.
Brain-derived neurotrophic factor immunoassay
Animals in this portion of the study were killed by decapitation at 9 weeks under light isoflurane anesthesia. Both hippocampi and a portion of motor cortex from the intact, contralateral hemisphere (to avoid infarcted tissue) were quickly removed, weighed and flash frozen in liquid nitrogen. Samples were stored at −80 °C until further processing. Tissue was homogenized in a 7× volume of ice-cold homogenization buffer (100 mm Tris/HCl, pH 7, containing 2% bovine serum albumin, 1 m NaCl, 4 mm EDTA, 2% Triton X-100, 0.1% sodium azide and protease inhibitor cocktail, Sigma-Aldrich) and centrifuged at 15 000 g for 30 min at 4 °C. Supernatants were collected and further diluted with buffer for a final dilution of 70×. Samples were frozen and stored at −20 °C until further processing. BDNF levels were measured using ELISA (Chemicon) according to the manufacturer's protocol. Using the optical densities of known BDNF concentrations to create a standard curve, the mean optical densities of samples (in duplicate) were used to calculate BDNF concentrations. Each plate contained samples from all four groups tested in this portion of the study.
Statistics
Behavioral and histological data were analysed using repeated measures anova or two-way anova where appropriate. A Fisher's PLSD test was used to determine differences between groups and Student's t-test was used to determine differences within groups over time, where P < 0.05 was considered significant. Regression analysis was used to determine the relationship between the amount of DβH staining and staircase performance as well as staircase performance and infarct volume. All values are given as mean ± SEM.
Results
Infarct volume
Values for infarct volume are provided in Table 1. There was no effect of DSP-4 or housing on infarct volume although there was a trend for animals in the I + St group to have larger cortical, and thus total, infarcts. All three measures of infarct volume correlated with the pre-treatment staircase score (R = 0.516, 0.615 and 0.654 for striatal, cortical and hemisphere, respectively, P < 0.0001 for all three). The range of injury is shown in Fig. 2 with most animals sustaining damage of the lateral striatum, parietal and insular cortex with sparing of the forelimb and hindlimb regions of cortex. This injury pattern is typical for the MCAo ET-1 and suture models of focal ischemia (Windle et al., 2006).
| Group | Infarct volume (mm3) | ||
|---|---|---|---|
| Striatum | Cortex | Hemisphere | |
| I + St | 26.53 ± 2.63 | 73.04 ± 9.76 | 106.44 ± 16.03 |
| I + St + DSP-4 | 18.78 ± 3.30 | 59.47 ± 7.44 | 83.70 ± 11.61 |
| I + ER | 27.13 ± 1.99 | 50.87 ± 7.61 | 78.44 ± 10.05 |
| I + ER + DSP-4 | 25.99 ± 1.99 | 55.22 ± 6.52 | 91.60 ± 10.61 |
- Values are given as mean ± SEM. DSP-4, N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine; I + ER, ischemic + enriched rehabilitation; I + ER + DSP-4, ischemic + enriched rehabilitation + DSP-4; I + St, ischemic + standard housing; I + St + DSP-4, ischemic + standard housing + DSP-4.
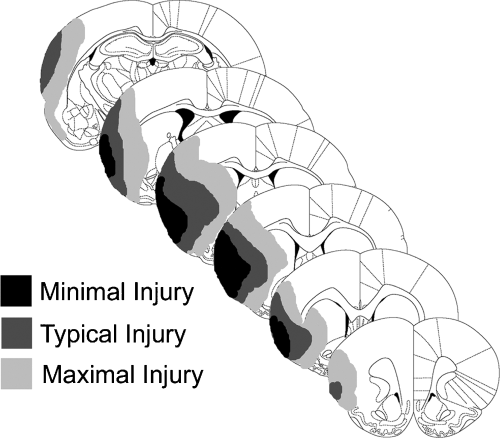
Schematic illustration of the minimal, average and maximal amount of ischemic damage after endothelin-1-induced middle cerebral artery occlusion.
Behavioral results
No differences were found between control animals with regard to housing condition or drug treatment and therefore they were pooled into a single control group. In order to be considered a control, animals must have scored ≥ 95% on the first staircase test after stroke. Eight animals that had reached this criterion were later eliminated from the control group because of the presence of ischemic injury during histological analysis. Animals with successful ischemia had at least a 20% reduction in performance in the first staircase test after surgery. Three animals were excluded at the end of study despite meeting the behavioral criterion due to lack of histological evidence of ischemia. Seven animals were excluded because deficits were either too mild (i.e. < 20%) or too pronounced (> 5%) to be included in ischemic or control groups, respectively. Finally, two animals were excluded because they inexplicably lost weight late in the study. The final behavioral group numbers were: ischemic + enriched rehabilitation, n = 14; I + St, n = 14; ischemic + enriched rehabilitation + DSP-4, n = 18; I + St + DSP-4, n = 14 and control, n = 16. In summary, 20 of the initial 139 animals were excluded from the study.
Staircase reaching test
There was a significant effect of group (F4,71 = 24.445, P < 0.0001) and time (F3,64 = 7.15, P = 0.0001) as well as a significant time × group interaction (F12,71 = 2.209, P = 0.0124). All ischemic groups were severely impaired in the staircase test compared with control animals at all timepoints (P = 0.0001). Although the I + St group did not improve over the 9 weeks, the ischemic + enriched rehabilitation group improved slightly and the two DSP-4-treated groups improved significantly (P < 0.05) from pre-treatment (Fig. 3). At 9 weeks post-treatment both ER groups as well as the I + St + DSP-4 group exhibited significantly better recovery than the I + St group (P = 0.035).
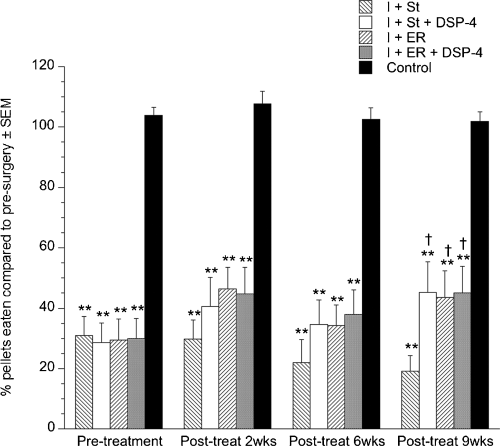
Performance in the staircase reaching task. All ischemic groups were impaired at pellet reaching with the contralateral limb compared with control animals. Enriched rehabilitation (ER) as well as depletion of norepinephrine using N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) resulted in enhanced recovery compared with animals housed in standard cages. Values are given as mean ± SEM [**P < 0.0001 compared with control group; †P < 0.05 compared with the ischemic + standard housing (I + St) group]. I + ER, ischemic + ER; I + ER + DSP-4, ischemic + ER + DSP-4; I + St + DSP-4, ischemic + standard housing + DSP-4.
Forelimb asymmetry test
There was no overall effect of group for this test although all ischemic groups were significantly impaired compared with control animals at 6 days after ischemia (pre-treatment) (P < 0.001). All of the ischemic groups improved so that by 6 weeks no groups were different from the control group. Although all groups showed recovery on this task the I + St group was the slowest to recover, returning to control levels at 6 weeks rather than at 2 weeks like the other ischemic groups (Fig. 4).
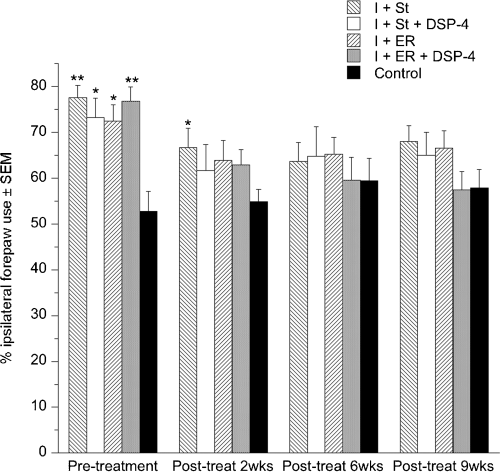
Ipsilateral forepaw use in forelimb asymmetry test. All ischemic groups displayed increased reliance on the ipsilateral forepaw after ischemia but returned to normal levels within the first 2 weeks of post-ischemic testing with the exception of the ischemic + standard housing (I + St) group. Values are given as mean ± SEM (*P < 0.05, **P < 0.0001 compared with control group). DSP-4, N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine; I + ER, ischemic + enriched rehabilitation; I + ER + DSP-4, ischemic + enriched rehabilitation + DSP-4; I + St + DSP-4, ischemic + standard housing + DSP-4.
Ladder-rung walking test
There was no effect of group for this task with all groups recovering over the course of the study (Fig. 5).
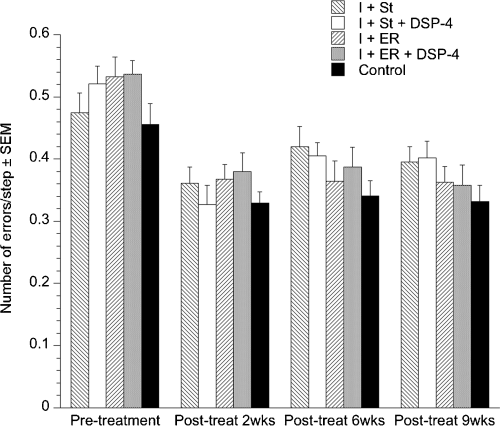
Mean number of errors/step in the ladder task. All groups improved post-ischemia (P < 0.01). Values are given as mean ± SEM. DSP-4, N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine; I + ER, ischemic + enriched rehabilitation; I + ER + DSP-4, ischemic + enriched rehabilitation + DSP-4; I + St, ischemic + standard housing; I + St + DSP-4, ischemic + standard housing + DSP-4.
Dopamine beta hydroxylase staining
Figure 6 illustrates DβH terminal staining in a DSP-4- and a saline-treated animal. There was a significant effect of group (F5,70 = 13.736, P < 0.0001) and the ROI measured (F3,70 = 107.361, P < 0.0001) as well as a group × region interaction (F15,70 = 4.968, P < 0.0001) for DβH staining. All groups that received DSP-4 showed significantly decreased staining for DβH compared with the groups that received saline (P < 0.05) (Fig. 7A). Table 2 includes the mean length of DβH-stained fibres for each group and the percent decrease in staining that resulted from DSP-4 treatment. There was a trend for the ischemic + enriched rehabilitation + DSP-4 group to have more staining than the I + St + DSP-4 group, which reached significance in ROI 1 (frontal cortex) (P < 0.05). In addition, it was found that DβH staining in DSP-4- and saline-treated groups was significantly decreased in ROI 4 (hippocampus) compared with the other regions (P < 0.05).
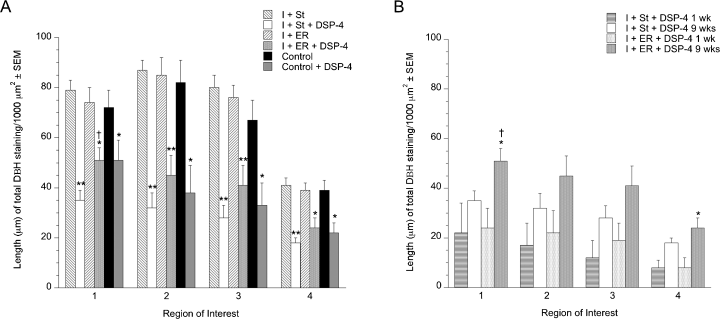
Mean length (µm) of dopamine beta hydroxylase (DβH) staining/1000 µm2 of noradrenergic fibres. At 9 weeks after N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) treatment there was a significant decrease in staining in all regions measured. (A) In addition, it was found that, among saline-treated animals, there was significantly less staining in region of interest 4 compared with the other regions examined [*P < 0.05, **P < 0.0001 compared with the respective saline-treated group, †P < 0.05 compared with ischemic + standard housing + DSP-4 (I + St + DSP-4)]. (B) A comparison of staining at 1 and 9 weeks after DSP-4 treatment in ischemic animals revealed that staining increased over time (*P < 0.05 compared with the same group at 1 week, †P < 0.05 compared with I + St + DSP-4 at the same timepoint). Values are given as mean ± SEM. I + ER, ischemic + enriched rehabilitation; I + ER + DSP-4, ischemic + enriched rehabilitation + DSP-4; I + St, ischemic + standard housing.
| Group | Length of dopamine beta hydroxylase staining (µm/1000 mm2) | |||
|---|---|---|---|---|
| ROI 1 | ROI 2 | ROI 3 | ROI 4 | |
| I + St | 79 ± 4 | 87 ± 4 | 80 ± 5 | 41 ± 3 |
| I + St + DSP-4 | 35 ± 4 | 32 ± 6 | 28 ± 5 | 18 ± 2 |
| Decrease (%) | 56 | 63 | 65 | 56 |
| I + ER | 74 ± 6 | 85 ± 7 | 76 ± 5 | 39 ± 3 |
| I + ER + DSP-4 | 51 ± 5 | 45 ± 8 | 41 ± 8 | 24 ± 4 |
| Decrease (%) | 31 | 47 | 46 | 38 |
| Control | 72 ± 7 | 82 ± 9 | 67 ± 8 | 39 ± 4 |
| Control + DSP-4 | 51 ± 8 | 38 ± 11 | 33 ± 9 | 22 ± 4 |
| Decrease (%) | 29 | 54 | 51 | 44 |
- Values are given as mean ± SEM. DSP-4, N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine; I + ER, ischemic + enriched rehabilitation; I + ER + DSP-4, ischemic + enriched rehabilitation + DSP-4; I + St, ischemic + standard housing; I + St + DSP-4, ischemic + standard housing + DSP-4; ROI, region of interest.
To investigate the possibility that NE projections were regenerated during the course of the study and whether housing had an effect on this, a subgroup of animals was killed at 1 week after DSP-4 injection. Comparison of DβH staining at 1 and 9 weeks post-treatment revealed that there was a trend towards increased staining at 9 weeks in the standard-housed animals and a near-significant or significant increase in staining in the ER animals depending on the ROI (P = 0.006, 0.0586, 0.0534 and 0.0072 for ROI 1, 2, 3 and 4, respectively) (Fig. 7B).
Regression analysis revealed that, among all animals, decreased DβH staining correlated with a greater improvement in the staircase test (pre-treatment score subtracted from the score at 9 weeks) for all regions except ROI 3 (R = 0.315, 0.245, 0.185 and 0.249 and P = 0.0057, 0.0327, 0.1097 and 0.0302 for ROI 1, 2, 3 and 4, respectively). When the animals were separated into their respective housing conditions, the correlation only remained for the standard-housed ischemic animals (R = 0.597, 0.507, 0.51 and 0.528 for ROI 1, 2, 3 and 4, respectively, P < 0.005 for all).
Brain-derived neurotrophic factor
No significant differences in the amount of BDNF protein were found between the four ischemic groups in either hippocampi or the cortex contralateral to the lesion. There was, however, an effect of region (F4,26 = 384.786, P < 0.0001) due to the cortex containing significantly less BNDF than both hippocampi (P < 0.0001). As there was a trend for the animals exposed to environmental enrichment to have higher levels of BDNF, DSP-4- and saline-treated animals were pooled for housing conditions showing a significant effect of group (F1,28 = 4.952, P = 0.0343). ER resulted in significantly higher levels of BDNF in the contralateral hippocampus (Fig. 8). Pooling animals for drug treatment showed that DSP-4 had no effect on BDNF levels in the regions measured (data not shown).
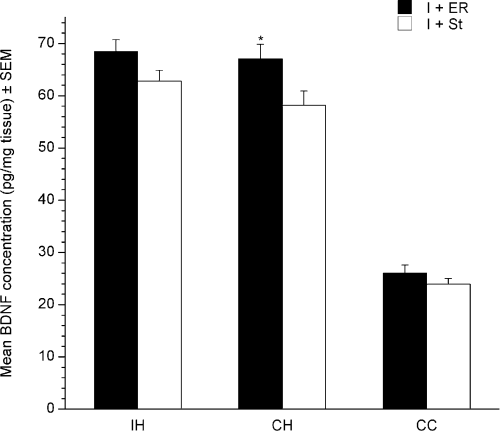
Effects of housing on brain-derived neurotrophic factor (BDNF) protein levels in the ipsilateral hippocampus (IH), contralateral hippocampus (CH) and contralateral cortex (CC). Animals housed in enriched rehabilitation (ER) showed increased BDNF levels in the CH. Values are given as mean ± SEM (*P < 0.05). I + ER, ischemic + ER; I + St, ischemic + standard housing.
Discussion
The goal of the present study was to determine if NE is required for recovery of function after focal ischemia in rats. Depleting NE by injecting DSP-4 at 1 week after MCAo resulted in no difference in infarct volumes between the groups that received DSP-4 and those that received saline. DSP-4 was given after MCAo as DSP-4 given prior to ischemia can affect injury outcome (Blomqvist et al., 1985; Nishino et al., 1991; Nellgard et al., 1999a). There was a trend for the animals exposed to ER or DSP-4 to have slightly smaller infarcts than the I + St-housed animals, suggesting that lesion size might account for the observed differences in behavioral recovery. This is unlikely because all groups were carefully balanced for severity of reaching deficits and found not to differ prior to being assigned to respective treatment groups. However, ER (and possibly DSP-4) treatments are known to increase cortical thickness due to increased dendritic branching and synaptogenesis (Biernaskie & Corbett, 2001; Jones et al., 1999; Kleim et al., 2002). This could have increased the area of intact cortical tissue relative to the area of infarction in these groups resulting in an apparent reduction in infarct volume.
We have shown here that ER facilitates recovery of function as previously found (Biernaskie & Corbett, 2001; Biernaskie et al., 2004) and that depleting NE did not reduce the benefits of ER. Similarly, it has been shown that NE depletion does not prevent the beneficial effects of enriched environment on cortical thickening and cognitive performance of animals without ischemic injury (Brenner et al., 1985; Murtha et al., 1990). In addition, we have shown that depleting NE facilitates recovery of function at 9 weeks post-treatment regardless of housing. These results are at odds with those previously reported in short-term (12 and 19 days, respectively) survival studies (Goldstein et al., 1991; Boyeson et al., 1992). In those studies the DSP-4 animals were more impaired than the respective controls at the start of behavioral testing, perhaps as a consequence of giving DSP-4 prior to the cortical injury (Goldstein et al., 1991; Boyeson et al., 1992). Further, infarct volumes were not measured in the study of Boyeson et al. (1992) and no volumetric measures were provided in the study of Goldstein et al. (1991). In the present study, all groups were balanced for equal behavioral impairments prior to the initiation of treatments. Despite claims that depleting NE impeded recovery, the DSP-4 animals in the study of Boyeson et al. (1992) did fully recover if given an extra 5 days of training. Given that these DSP-4 animals began the study with a more severe impairment, the rate of recovery appears to be approximately the same as their control animals. In the study of Goldstein et al. (1991), the DSP-4 animals did not recover as much as the saline controls but, by the 12th day, appeared similar to sham animals given DSP-4. This suggests that recovery was not impeded by DSP-4 as previously suggested but instead DSP-4 affected the baseline performance of the animals. In addition, these studies used only a beam-walking test to evaluate functional outcome (Goldstein et al., 1991; Boyeson et al., 1992) and spontaneous recovery was seen after the somatosensory cortex lesion.
Clinical stroke can result in a large variety of motor deficits but commonly involves persistent upper extremity deficits (Parker et al., 1986; Nakayama et al., 1994). The present study used several tasks that have previously been shown to reveal different impairments in animal models of stroke, including forelimb reaching deficits, and show little spontaneous recovery (Montoya et al., 1991; Schallert et al., 1997; Biernaskie & Corbett, 2001; Metz & Whishaw, 2002). The most pronounced effects of NE depletion were seen in the staircase reaching task. Animals housed in standard cages did not show recovery on the staircase test and it was only through intervention, such as ER and/or NE depletion, that some recovery occurred.
The use of DSP-4 has been reported to be a reliable tool for depleting LC-NE terminals for as long as 8 months without permanent effects on dopamine, serotonin or peripheral NE systems (Ross, 1976; Jonsson et al., 1981; Fritschy & Grzanna, 1991; Harro et al., 2003). In the present study, staining for DβH increased in both ischemic groups given DSP-4 over the course of the study but NE remained significantly depleted at 9 weeks after DSP-4 administration. The present data show that LC-NE depletion facilitates forelimb function after ischemia as standard-housed ischemic animals that received DSP-4 performed better in the staircase test than their saline-treated counterparts. There was a negative correlation between DβH terminal staining and improvement in staircase performance at the end of the study but this reached significance only in ischemic animals housed in standard conditions. This may be because animals housed in ER showed a greater increase in DβH staining over time or because the benefit of ER and DSP-4 treatment is not additive. The negative correlation supports the hypothesis that DβH fiber loss is the significant variable in improved recovery for standard-housed animals.
The mechanisms by which DSP-4 treatment facilitates recovery are unknown. Effects on other systems, such as enhanced D2 receptor density and sensitivity, that might facilitate motor function (Harro et al., 2000, 2003) is one possibility. Alternatively, DSP-4 may enhance NE function in a way that would be beneficial to motor recovery. NE infusion into the cerebellum of animals with a unilateral lesion of the LC resulted in a heightened behavioral response (Boyeson et al., 1993). This could have occurred because of selective increases in NE receptors, supersensitization of remaining receptors, sprouting of remaining NE terminals or a combination of all three. Several studies have shown that DSP-4 treatment can up-regulate and increase sensitivity of α1- and β-adrenergic receptors in the cortex and hippocampus, which could facilitate the action of NE released from the remaining terminals (Dooley et al., 1983; Dunwiddie et al., 1983; Mogilnicka, 1986; Zahniser et al., 1986; Theron et al., 1993; Wolfman et al., 1994). It is unknown how long the receptor changes persist but even transient changes may play a key role in shaping recovery.
Important to the hypothesis that DSP-4 facilitates NE function is that the drug treatment did not totally eliminate DβH fiber staining. Regenerative sprouting of residual LC axons has been noted previously and even hyperinnervation of the frontal cortex has been observed at 6 months after DSP-4 treatment (Fritschy & Grzanna, 1992). NE terminals were not completely removed in the present study and indeed there was a slight increase in DβH staining from 1 to 9 weeks post-DSP-4 (see Fig. 7B), which might be important in the functional benefits that were seen. As there are fibers remaining, an increase in NE turnover could compensate for decreased innervation. Microdialysis studies have shown that extracellular NE levels are unchanged (Kask et al., 1997; Nellgard et al., 1999b) or even increased in areas of decreased tissue NE after DSP-4 treatment (Logue et al., 1985; Hughes & Stanford, 1998). Thus, physiologically effective NE release acting on supersensitive β-adrenergic receptors might provide an enhancement of NE function that would facilitate motor recovery as seen in the present study. Enhanced NE release is also consistent with the reported down-regulation or decreased sensitivity of α2-adrenoreceptors following DSP-4 (Heal et al., 1993; Kask et al., 1997; Prieto & Giralt, 2001). This hypothesis is speculative as the microdialysis studies were conducted within 1 week of DSP-4 treatment and it is unknown how long such levels are sustained. However, as mentioned earlier, even early events may shape later recovery.
Environmental enrichment has previously been shown to increase NE in mice (Naka et al., 2002). In addition, repeated mild stress has also been shown to increase terminal sprouting in NE axons from the LC (Nakamura et al., 1989). ER could be viewed as a mild stress and in the present study ER animals treated with DSP-4 did tend to have higher levels of DβH staining than standard-housed animals treated with DSP-4. In addition, there was a larger increase in DβH staining from 1 to 9 weeks in the ER animals. The significance of this increased staining in ER animals is unclear as DSP-4 treatment neither improved nor impeded the recovery seen.
Brain-derived neurotrophic factor has been suggested to be crucial for experience-dependent plasticity as it can increase other mediators of plasticity such as synapsin I and growth-associated protein 43 (Gomez-Pinilla et al., 2002). Rehabilitative therapies, such as environmental enrichment (Falkenberg et al., 1992; Gobbo & O'Mara, 2004) and exercise (Russo-Neustadt et al., 2000; Gomez-Pinilla et al., 2002; Vaynman et al., 2003; Ploughman et al., 2005), increase BDNF levels. Similarly, NE has been suggested to be a modulator of BDNF, given that several noradrenergic antidepressants increase BDNF in the brain (Nibuya et al., 1995; Russo-Neustadt et al., 2000) and that NE depletion attenuates exercise-induced increases in BDNF (Garcia et al., 2003). In the present study we found that ER increased BDNF levels in the contralateral hippocampus compared with standard housing. It is unclear if this contributes to the improved functional recovery seen with ER in this study as depleting NE with DSP-4 did not alter BDNF levels. It is possible that NE function was maintained and therefore no change in BDNF would be expected but it is also possible that changes in BDNF took place much earlier than when samples were taken (9 weeks post-DSP-4). Thus, it may be that BDNF levels were transiently altered and then returned to normal; however, this cannot be determined without further investigation.
Contrary to previous studies, NE depletion did not impede the recovery associated with ER and furthermore facilitated recovery in standard-housed animals. It remains possible that these effects are due to an NE-induced receptor supersensitivity but this interpretation awaits further analysis.
Acknowledgements
The authors would like to thank Sue Evans for her assistance with behavioral testing and Garry Chernenko for his assistance with the BDNF assay. Research funding was provided by grants from the Canadian Institute for Health Research, Canadian Stroke Network (CSN) and Canada Research Chairs Program to D.C. and from the CSN Focus on Stroke Program to V.W.
Abbreviations
-
- BDNF
-
- brain-derived neurotrophic factor
-
- DβH
-
- dopamine beta hydroxylase
-
- DSP-4
-
- N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine
-
- ER
-
- enriched rehabilitation
-
- ET-1
-
- endothelin-1
-
- I + St
-
- ischemic + standard housing
-
- I + St + DSP-4
-
- ischemic + standard housing + N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine
-
- LC
-
- locus coeruleus
-
- MCAo
-
- middle cerebral artery occlusion
-
- NE
-
- norepinephrine
-
- PBS
-
- phosphate-buffered saline
-
- ROI
-
- region of interest.