Cocaine- and amphetamine-regulated transcript peptide (CART) is present in peptidergic C primary afferents and axons of excitatory interneurons with a possible role in nociception in the superficial laminae of the rat spinal cord
Abstract
Cocaine- and amphetamine-regulated transcript peptides (CART) have been implicated in the regulation of several physiological functions, including pain transmission. A dense plexus of CART-immunoreactive fibres has been described in the superficial laminae of the spinal cord, which are key areas in sensory information and pain processing. In this study, we used antibody against CART peptide, together with markers for various types of primary afferents, interneurons and descending systems to determine the origin of the CART-immunoreactive axons in the superficial laminae of the rat spinal cord. Calcitonin gene-related peptide (CGRP), a marker for peptidergic primary afferents in the dorsal horn, was present in 72.6% and 34.8% of CART-immunoreactive axons in lamina I and II, respectively. The majority of these fibres also contained substance P (SP), while a few were somatostatin (SOM)-positive. The other subpopulation of CART-immunoreactive boutons in lamina I and II also expressed SP and/or SOM without CGRP, but contained vesicular glutamate transporter 2, which is present mainly in excitatory interneuronal terminals. Our data demonstrate that the majority of CART-immunoreactive axons in the spinal dorsal horn originate from peptidergic nociceptive primary afferents, while the rest arise from excitatory interneurons that contain SP or SOM. This strongly suggests that CART peptide can affect glutamatergic neurotransmission as well as the release and effects of SP and SOM in nociception and other sensory processes.
Introduction
Cocaine- and amphetamine-regulated transcript (CART) was discovered as a novel mRNA in the rat striatum, which is upregulated following acute administration of psychomotor stimulants (Douglass et al., 1995; Douglass & Daoud, 1996). CART peptides of different sizes derived from the transcript have been identified (Adams et al., 1999; Kuhar & Yoho, 1999), and the biologically active fragments have been implicated in the regulation of several physiological functions, including reward, food intake, neuroendocrine functions and pain transmission (Fekete & Lechan, 2006).
CART mRNA and peptides are expressed throughout the brain and the spinal cord (Couceyro et al., 1997). CART-immunoreactive cell bodies and dense plexus of fibres have been described in the superficial laminae of the spinal cord in rats and mice (Koylu et al., 1998; Dun et al., 2000; Ohsawa et al., 2000); however, the characteristics of these neurons and the origin of these fibres remained unknown.
The spinal dorsal horn is the key area of sensory information processing and pain transmission. Aδ and C primary afferents, most of which are nociceptors, terminate in lamina I and II, and a few of them reach deeper laminae (Réthelyi, 1977; Light & Perl, 1979; Sugiura et al., 1986). C fibres can be subdivided into two overlapping populations: peptidergic afferents, containing calcitonin gene-related peptide (CGRP) with substance P (SP) or somatostatin (SOM); and non-peptidergic C fibres that bind isolectin B4 from Bandeiraea simplicifolia (IB4: Ju et al., 1987; Hunt et al., 1992; Lawson, 1992; Kitchener et al., 1993; Wang et al., 1994). Although glutamate is the principal neurotransmitter in all primary afferents (De Biasi & Rustioni, 1988; Broman et al., 1993), different vesicular glutamate transporter (VGLUT) isoform combinations are expressed in different types of them (Oliveira et al., 2003; Todd et al., 2003; Alvarez et al., 2004). The majority of neurons in lamina I–III are interneurons (Todd & Spike, 1993; Todd & Koerber, 2005), and only a few cells in lamina I and III project to supraspinal areas (Lima & Coimbra, 1988; Spike et al., 2003). One-third of interneurons in lamina I–III are inhibitory and immunoreactive for γ-aminobutyric acid (GABA) and/or glycine (Todd & Sullivan, 1990; Polgár et al., 2003), while the rest are thought to be glutamatergic (Todd & Spike, 1993) and express VGLUT2 in their axon terminals (Todd et al., 2003).
Serotoninergic and noradrenergic pathways descending from the brainstem also terminate in the dorsal horn, and can modulate sensory (including nociceptive) transmission (Ruda et al., 1981; Millan, 2002; Suzuki et al., 2004; Yoshimura & Furue, 2006).
The functional importance of CART peptides in the spinal cord has been revealed in behavioural studies. Intrathecal application of CART(55–102) causes hyperalgesia in acute pain conditions (Ohsawa et al., 2000), attenuates hyperalgesia and allodynia in neuropathic (but not inflammatory) pain (Damaj et al., 2006), and enhances the spinal analgesic actions of morphine (Damaj et al., 2004).
We used antibodies against the biologically active CART peptides with markers for primary afferents, excitatory and inhibitory interneurons, and descending systems to determine the origin of the CART-immunoreactive axons in the superficial laminae of the rat spinal cord.
Materials and methods
All animal experiments were performed in accordance with European Communities Council Directive of 24 November 1986 (86/609/ECC) and were approved by the Committee on Animal Experiments, Semmelweis University, Budapest. All efforts were made to minimize the number of animals used.
CART antisera
Monoclonal CART antibody was raised in mouse against purified recombinant CART(54–102) peptide (Thim et al., 1998), as described by Vrang et al. (1999). The antibody reacted equally well with CART(54–102), CART(61–102) and CART(62–102) peptides (Singru et al., 2007). Polyclonal CART antibody was raised in rabbit against the CART(79–102) fragment, as reported by Koylu et al. (1997). This antibody reacted with the immunizing peptide but not by other CART peptide fragments, such as the CART(2–27), CART (31–52) or CART(55–76). No immunostaining was detected when primary antibodies were preabsorbed with their immunizing peptides (Koylu et al., 1997; Vrang et al., 1999).
In our study, further controls were carried out by omitting either the primary or secondary antibodies: the immunostaining did not differ from the background. Immunoperoxidase staining was also abolished by preabsorption of the monoclonal CART antibody with the naturally occurring, biologically active CART(55–102) fragment (American Peptide Company; 0.01 mm). Perfect matching of the staining patterns of the mouse and rabbit CART antibodies was also demonstrated by dual immunofluorescent labelling (see Supplementary material, Fig. S1).
Immunofluorescence staining for CART: distribution in the spinal cord
Three adult male Wistar rats were transcardially perfused under deep ketamine/xylazine (75 mg/7.5 mg/kg, i.m.) anaesthesia with a fixative consisting of 4% (para)formaldehyde and 4% acrolein.
Transverse 50-µm sections from the cervical, thoracic, lumbar and sacral spinal segments were cut with a Vibratome. Sections were first treated in 50% ethanol to enhance antibody penetration, and then transferred to 0.1 m phosphate buffer with 1% sodium borohydride to eliminate free aldehyde groups. Sections were incubated for 72 h in the monoclonal CART antibody raised in mouse (1 : 1500), and for 24 h in species-specific donkey anti-mouse IgG conjugated to Alexa-488 (Invitrogen-Molecular Probes, Budapest, Hungary; 1 : 500). Finally, sections were mounted in Vectashield (Vector Laboratories) and scanned through a 4 × lens on a Nicon Eclipse E800 microscope attached to a Bio-Rad Radiance 2100 Rainbow confocal laser scanning system. Confocal images were converted into grey-scale and then inverted to give a black-on-white image.
Labelling of Aδ myelinated afferents
Cholera toxin β-subunit (CTb) was used as a trans-ganglionic tracer in a further two adult male Wistar rats for selective labelling of myelinated primary afferents (Aδ fibres in the superficial laminae). Animals were deeply anaesthetized as described above and 5 µL of the tracer (final concentration: 1%) was injected into the left sciatic nerve. After 3 days survival, rats were perfused with a fixative containing 4% (para)formaldehyde and 1% acrolein. Sections from the L3–L5 segments, which are the main termination zone of the sciatic nerve, were processed for immunofluorescent labelling.
Multiple immunofluorescent labelling for CART peptide with markers for primary afferents, local interneurons and descending fibres
Sections of lumbar segments from the three rats used for studying the CART peptide distribution and from the two that had CTb injections were processed for multiple fluorescent immunolabelling. Sections were treated as described above, and were incubated for 72 h in primary antibody cocktails, which contained:
(I) mouse antibody against CART peptide together with: (i) goat-anti-CGRP, rat-anti-SP and guinea pig-anti-VGLUT2; (ii) goat-anti-CGRP, rabbit-anti-SOM and guinea pig-anti-VGLUT2; (iii) goat-anti-CGRP and biotinylated IB4; (iv) rabbit-anti-CGRP and goat-anti-CTb; (v) guinea pig-anti-VGLUT2 and rabbit-anti-neurotensin (NT); (vi) rabbit-antibody against vesicular GABA transporter (VGAT) and guinea pig-anti-glycine transporter 2 (GLYT2); (vii) rabbit-anti-serotonin transporter (5-HTT); and
(II) rabbit antibody against CART peptide together with mouse-anti-dopamine β hydroxylase (DβH).
Then sections were incubated for 24 h in a mixture of the appropriate species-specific secondary antibodies conjugated to fluorescent dyes (raised in donkey and conjugated to Alexa-488 or Alexa-555, Invitrogen-Molecular Probes; and Cy5 or FITC, Jackson ImmunoResearch). In the case of (iii), IB4 was revealed with Pacific Blue-conjugated Streptavidin (Invitrogen-Molecular Probes, Budapest, Hungary; 1 : 500). All the primary and secondary antibodies were diluted in phosphate-buffered saline containing 0.3% Triton-X, and finally sections were mounted with Vectashield (Vector Laboratories).
For the details of primary antibodies used for confocal microscopy, see Table 1.
| Antibody | Species | Dilution | Source |
|---|---|---|---|
| Cocaine- and amphetamine-regulated transcript (CART) | Mouse | 1 : 1500 | gift from Jes Thorn Clausen, Novo Nordisk, Bagsvaerd, Denmark |
| Rabbit | 1 : 1500 | gift from Michal J. Kuhar, Emory University, Atlanta, GA, USA | |
| Calcitonin gene-related peptide (CGRP) | Goat | 1 : 300 | Santa Cruz Biotechnology, Heidelberg, Germany |
| Rabbit | 1 : 300 | Merck (Calbiochem), Budapest, Hungary | |
| Cholera toxin β-subunit (CTb) | Goat | 1 : 5000 | List Biological, Campbell, CA, USA |
| Dopamine β hydroxylase (DβH) | Mouse | 1 : 150 | Millipore (Chemicon), Budapest, Hungary |
| Glycine transporter 2 (GLYT2) | Guinea pig | 1 : 2000 | Millipore (Chemicon), Budapest, Hungary |
| Neurotensin (NT) | Rabbit | 1 : 1000 | Bachem AG (Peninsula Laboratories), Bubendorf, Switzerland |
| Serotonin transporter (5-HTT) | Rabbit | 1 : 250 | Merck (Oncogene Research Products), Budapest, Hungary |
| Somatostatin (SOM) | Rabbit | 1 : 1000 | Bachem AG (Peninsula Laboratories) |
| Substance P (SP) | Rat | 1 : 200 | AbD Serotec (Oxford Biotechnology), Budapest, Hungary |
| Rabbit | 1 : 300 | Merck (Oncogene Research Products) | |
| Vesicular GABA transporter (VGAT) | Rabbit | 1 : 1000 | Synaptic Systems, Goettingen, Germany |
| Vesicular glutamate transporter 2 (VGLUT2) | Guinea pig | 1 : 5000 | Millipore (Chemicon) |
- GABA, γ-aminobutyric acid.
Immunofluorescent staining of dorsal root ganglia (DRG)
L2–L4 DRGs were used from the three rats that were perfused with 4% paraformaldehyde and 4% acrolein. Longitudinal 50-µm-thick sections were cut on a Vibratome, and multiple fluorescent immunostaining was carried out with the primary antibody cocktail consisting of mouse-anti-CART peptide, goat-anti-CGRP and rabbit-anti-SP, while the corresponding secondary antibodies were conjugated to Alexa-488, Alexa-555 and Cy5.
Analysis of immunofluorescence
For each antibody combination, four sections from L2–L4 were selected randomly. In each of these sections, two overlapping fields of approximately 600 × 600 µm covering the whole dorsal horn were scanned with a 20 × lens using light transmitted through a darkfield objective in order to allow accurate identification of the borders of laminae I and II. Then in each section, three fields of 133 × 133 µm representing the medial, intermediate and lateral part of the superficial dorsal horn containing lamina I and II were scanned with a 60 × oil-immersion lens. For each field, 24 optical sections were acquired at a z-separation of 0.5 µm.
The Neurolucida for Confocal 4.34 software (MicroBrightField, Williston, VT, USA) was used to analyse the colocalization of CART immunoreactivity with markers for primary afferents, interneuronal axons and terminals of descending pathways. In the series of images acquired with the laser line corresponding to the CART peptide labelling, 25–50 (average 30) immunoreactive boutons were selected randomly in both lamina I and lamina II. Efforts were made not to select boutons that obviously belonged to the same axon. Then the selected boutons were checked in the corresponding optical sections of the image stacks, and the presence or absence of colocalization with CART was determined manually bouton by bouton.
The lack of colocalization of CART peptide with IB4, GLYT2, CTb, 5-HTT and DβH became clear after finishing the analysis in a few sections from each rat. So in these obvious cases the analysis was not continued.
Altogether for each antibody combination (excluding those containing IB4, GLYT2, CTb, 5-HTT or DβH), 900–1050 CART-immunoreactive boutons were investigated both in lamina I and lamina II.
Results
Distribution of CART peptide in the rat spinal cord
The distribution of CART-immunoreactivity was examined along the length of the rat spinal cord. As reported earlier (Koylu et al., 1998; Dun et al., 2000; Ohsawa et al., 2000), CART staining was present from cervical to sacral segments, and CART-stained fibres and terminals were found in each lamina and in the dorsolateral fasciculus including the lateral spinal nucleus (Fig. 1). The densest network of CART-positive fibres was observed in the superficial laminae, especially in lamina I, and the CART-positive axon density dramatically decreased away from the superficial laminae. CART-immunoreactive cell bodies were found around the central canal, and a few pale neurons appeared in the dorsal horn. Strongly stained neurons and fibres were also located in the intermediolateral cell column of the thoracic cord, as reported previously (Koylu et al., 1998; Ohsawa et al., 2000).
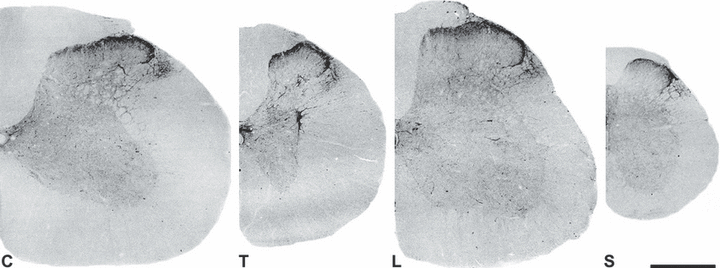
Distribution of CART immunoreactivity in sections from cervical (C), thoracic (T), lumbar (L) and sacral (S) segments of the rat spinal cord. Low-power confocal images showing fluorescent CART immunostaining. (Confocal images are converted into grey-scale and then inverted to give a black-on-white image.) CART immunoreactivity is concentrated in the superficial laminae, but strong staining is also found around the central canal, in the dorsolateral funiculus and in the intermediolateral cell column in the thoracic cord. Some scattered fibres and terminals can be seen throughout the grey matter. Scale bar: 0.5 mm.
Colocalization of CART with various immunocytochemical markers in the superficial laminae
The majority (72.6%) of CART-positive axons in lamina I and a smaller population (34.8%) of those in lamina II were found to be immunoreactive for CGRP (Fig. 2A–P). The proportion of CART terminals in laminae I and II that contained both CGRP and SP was 62.70 ± 0.90% and 33.90 ± 1.30%, respectively (Fig. 2E–H). A few CART terminals were immunoreactive for both CGRP and SOM (9.89 ± 1.17% in lamina I and 0.94 ± 0.34% in lamina II; Fig. 2I–L). CART immunoreactivity was not found in non-peptidergic primary afferent terminals, which were detected by IB4 and occupied mainly the inner part of lamina II (Fig. 2A–D). CTb injected into the sciatic nerve labelled the myelinated afferents and gave a similar staining pattern to that reported earlier (LaMotte et al., 1991; Rivero-Melian & Grant, 1991; Woolf et al., 1995). CTb-labelled Aδ fibres and terminals were found in lamina I, and in a band extending ventrally from the ventral half of lamina II to lamina V, but hardly any were present in the dorsal half of lamina II. There was no colocalization between CTb-stained axons and the CART peptide (Fig. 2M–P).
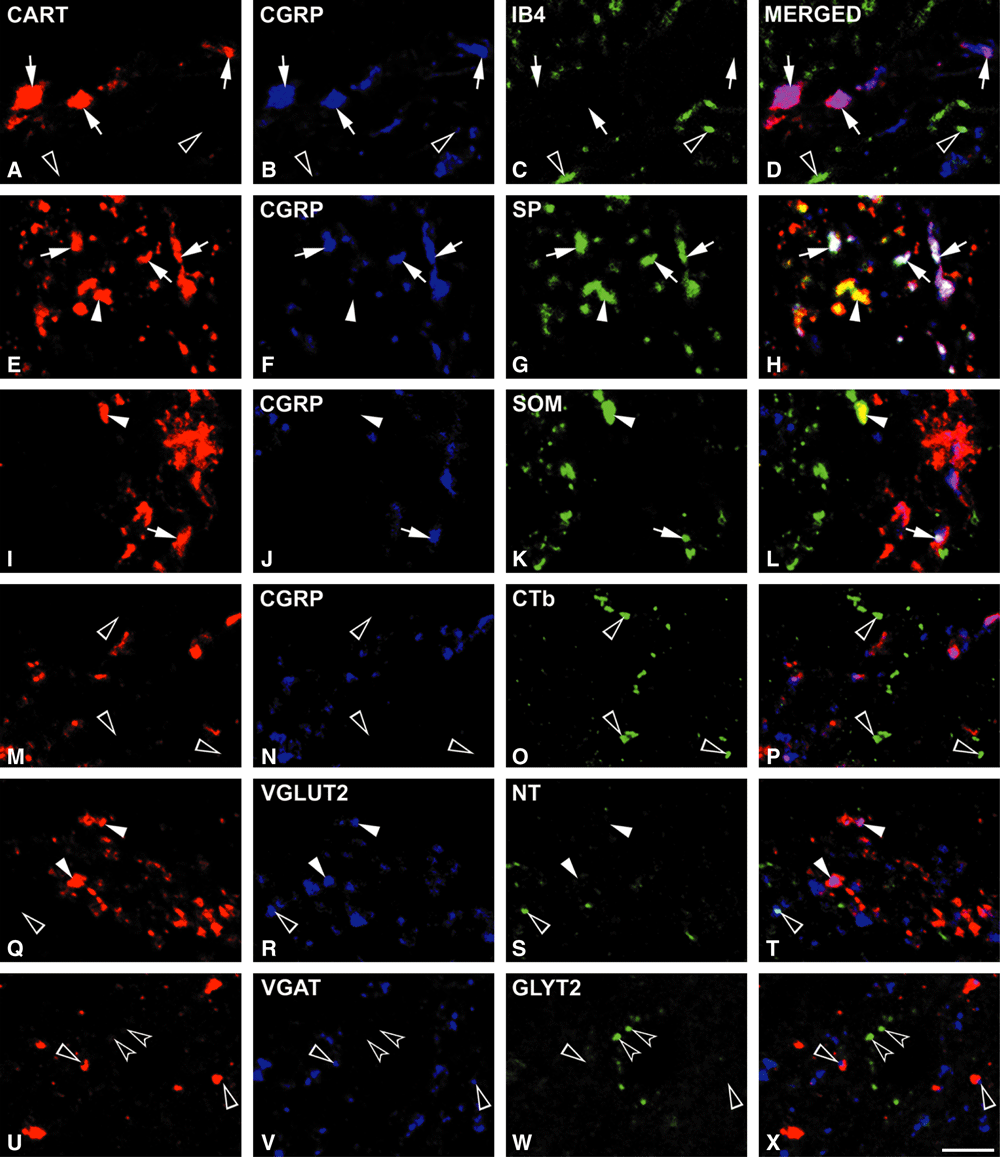
Cocaine- and amphetamine-regulated transcript (CART) immunoreactivity in axons belonging to various types of primary afferents and local interneurons in the superficial laminae. Each row contains single optical sections from the same field. Many CART-immunoreactive terminals are labelled with calcitonin gene-related peptide (CGRP; A–D; arrows), a marker of peptidergic primary afferents. No colocalization is found with isolectin B4 (IB4; A–D; empty arrowheads), a marker for non-peptidergic C fibres. The majority of peptidergic C fibres also contain substance P (SP; E–H; arrows) and a smaller population is somatostatin (SOM)-positive (arrows, I–L). These rows also show the CART-positive interneuronal axons containing SP or SOM (E–L; arrowheads). None of the CART terminals is filled with cholera toxin β-subunit (CTb), which selectively labels myelinated fibres (M–P: empty arrowheads). A population of CART-immunoreactive axons express vesicular glutamate transporter 2 (VGLUT2; Q–T: arrowheads). There is little or no colocalization with neurotensin (NT; Q–T; empty arrowhead), which is present in an excitatory interneuronal population, and with vesicular GABA transporter (VGAT) or glycine transporter 2 (GLYT2; U–X; empty and concave arrowheads, respectively) that are characteristic for inhibitory interneurons. Scale bar: 5 µm.
Many of the CART-immunoreactive axons showed VGLUT2 immunoreactivity (57.22 ± 4.29% in lamina I and 48.77 ± 8.36% in lamina II; Fig. 2Q–T).
Examination of the extent of colocalization of CART peptide-containing axons with interneuronal markers revealed that only a few of the former were immunoreactive for VGAT (2.42 ± 0.42% and 6.57 ± 1.71% in lamina I and II, respectively), and no colocalization was found with GLYT2 (Fig. 2U–X). In order to identify the subpopulations of excitatory interneurons that coexpress CART and VGLUT2, we used SP, NT and SOM antibodies in triple- and quadruple-labelling studies. These experiments showed that many of the CART-immunopositive boutons lacking CGRP were immunoreactive for SP (17.10 ± 0.10% and 33.9 ± 2.70% in lamina I and II, respectively; Fig. 2E–H) and/or for SOM (15.33 ± 1.83% and 25.5 ± 1.39% in lamina I and II, respectively; Fig. 2I–L), while the number of NT-labelled CART-ergic terminals was negligible (3.23 ± 0.55% and 3.38 ± 0.15% in lamina I and II, respectively; Fig. 2Q–T).
CART immunoreactivity was never colocalized with 5-HTT or DβH (see Supplementary material Fig. S2), which are markers of descending serotoninergic and noradrenergic pathways, respectively.
For the details of the quantitative analysis, see Table 2.
| Markers | Lamina I | Lamina II | |||
|---|---|---|---|---|---|
| Relative extent | Colocalizationof CART (%) | Relative extent | Colocalization of CART (%) | ||
| Primary afferents | |||||
| Peptidergic afferents | |||||
| Substance P-containing | CGRP+, SP+ | +++ | 62.70 ± 0.90 | ++ | 33.90 ± 1.30 |
| Somatostatin-containing | CGRP+, SOM+ | + | 9.89 ± 1.17 | – | 0.94 ± 0.34 |
| Non-peptidergic C afferents | CGRP–, IB4+ | – | 0.0 | – | 0.0 |
| Aδ nociceptive afferents | CTb+ | – | 0.0 | – | 0.0 |
| Primary afferent and interneuronal terminals with glutamate | VGLUT2+ | +++ | 57.22 ± 4.29 | ++ | 48.77 ± 8.36 |
| Non-primary afferents containing | |||||
| Substance P | CGRP–, SP+ | + | 17.10 ± 0.10 | ++ | 33.90 ± 2.70 |
| Somatostatin | CGRP–, SOM+ | + | 15.33 ± 1.83 | ++ | 25.55 ± 1.39 |
| Neurotensin | NT+ | – | 3.23 ± 0.55 | – | 3.38 ± 0.15 |
| GABA | VGAT+ | – | 2.42 ± 0.42 | + | 6.57 ± 1.71 |
| Glycine | GLYT2+ | – | 0.0 | – | 0.0 |
| Descending axons | |||||
| Serotonin-containing | 5-HTT+ | – | 0.0 | – | 0.0 |
| Noradrenalin-containing | DβH+ | – | 0.0 | – | 0.0 |
- All values are given as percentage, mean ± SD. The relative extent of colocalization is defined as: –, < 5%; +, 5–20%; ++, 21–50%; +++, > 50%. 5-HTT, serotonin transporter; CGRP, calcitonin gene-related peptide; CTb, cholera toxin β-subunit; DβH, dopamine β hydroxylase; GABA, γ-aminobutyric acid; GLYT2, glycine transporter 2; IB4, isolectin B4; NT, neurotensin; SOM, somatostatin; SP, substance P; VGAT, vesicular GABA transporter; VGLUT2, vesicular glutamate transporter 2.
CART immunoreactivity in the DRG
A relatively small number (less then 10%) of CART-immunoreactive neuronal perikarya and axons were detected in sections from DRG. The stained cells belonged to the ‘small’ cell group. Most of them were also CGRP- and SP-positive, but there were a few cells that contained neither of these two peptides (Fig. 3).
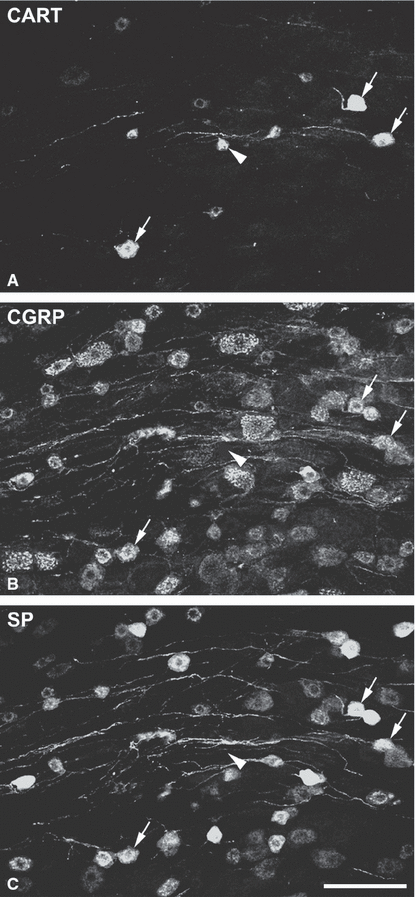
Cocaine- and amphetamine-regulated transcript (CART)-containing neurons in the L4 DRG. Confocal images, built from five optical sections 1 µm apart, showing (A) CART, (B) calcitonin gene-related peptide (CGRP) and (C) substance P (SP) immunoreactivity. CART-positive cells that contain CGRP and SP are indicated with arrows. Note that few cells that are only CART-immunoreactive (arrowhead) are also found in the ganglion. Scale bar: 100 µm.
Discussion
The main findings of this study are that the majority of CART-immunoreactive fibres in the superficial laminae of the dorsal horn originate from peptidergic C primary afferents, containing CGRP with SP or SOM. Most of these axons are thought to be nociceptors. The remaining CART-positive axons belong to local excitatory interneurons, which also express SP and/or SOM.
Fixation and antibody specificity
The CART antibodies used in this study have been used frequently and are well characterized (Vrang et al., 1999; Koylu et al., 1997, 1998; Smith et al., 1999; Singru et al., 2007). Because the best CART staining appeared when the fixative contained 4% paraformaldehyde and 4% acrolein, we had to test the other markers with this fixative. All antibodies used showed a staining pattern in the spinal cord similar to that reported previously using paraformaldehyde-based fixatives without acrolein (Todd & Spike, 1993; Koylu et al., 1998; Ohsawa et al., 2000).
In our studies, we did not characterize the CART-positive cell bodies as these were rare and very weakly immunoreactive, which would have made neurochemical characterization unreliable.
Subpopulations of CART-immunoreactive terminals in lamina I and II
The neurochemical diversity of CART-containing fibres and cell bodies has been shown in various areas of the central and peripheral nervous systems. Coexistence of CART peptide with nitric oxide synthase or calbindin in intracardiac neurons (Richardson et al., 2006), with SP in neurons of the nucleus accumbens (Hubert & Kuhar, 2005), and with choline acetyltransferase in sympathetic preganglionic neurons of the thoracolumbar spinal cord (Dun et al., 2000) have all been demonstrated.
We have shown that the majority of CART-containing fibres in lamina I, together with a smaller population in lamina II, express CGRP. CGRP is a marker for peptidergic primary afferents, most of which are unmyelinated, and is never found in spinal interneurons (Carlton et al., 1987; Ju et al., 1987). In agreement with these findings, CART-positive small neurons expressing CGRP (and SP) were also found in DRG. However, the small number of CART-immunoreactive cells in the DRG seems to contradict the large number of CART/CGRP/SP axon terminals in the superficial laminae. As in the case of galanin (Zhang et al., 1993), a possible explanation could be that many DRG neurons synthesize CART peptide, but because axonal transport of the peptide to the terminals is very fast the peptide is undetectable in the cell bodies of most of them. A similar situation in the spinal cord probably explains the low number of CART-immunoreactive cell bodies in the dorsal horn.
Previous studies have reported that although most of the peptidergic afferents do not express VGLUT2 at detectable levels, some of the peptidergic fibres show a low level of immunoreactivity (Li et al., 2003; Oliveira et al., 2003; Todd et al., 2003; Alvarez et al., 2004; Landry et al., 2004). Because in our experiments we did not distinguish between the weak and strong VGLUT2 immunoreactivity, this could result in the relatively high proportion of VGLUT2-positive CART terminals in lamina I.
While the majority of CART axons in lamina I originate from primary afferents, colocalization of CART peptide with interneuronal markers becomes dominant in lamina II. Approximately half of the CART-immunoreactive terminals in lamina II contained VGLUT2, and more than 60% of the CART axons were immunoreactive for SP (34%), SOM (25%) or NT (3%) without CGRP (Table 2). It has been reported that the SOM- and NT-immunoreactive cells form two different sets of excitatory interneuronal subpopulations in the dorsal horn (Proudlock et al., 1993; Todd & Koerber, 2005), and almost all of the SP-, SOM- and NT-positive interneuronal terminals express VGLUT2 (Todd et al., 2003). In contrast to Tuchscherer & Seybold (1989), who reported no colocalization of SP and SOM in varicosities of the spinal dorsal horn, we found previously that a small proportion of CART terminals coexpress SP and SOM without CGRP (Kozsurek & Puskár, unpublished observation). The cross-reactivity between SP and SOM antibodies was excluded with radioimmunoassay applications performed by the suppliers. Differences in the fixatives and the resolution of the microscopes in the z-axis may yield these divergent results. This suggests, however, that while SP and SOM are present in two different populations of primary afferents (Hökfelt et al., 1976), they may be colocalized in a small population of interneurons, and this can explain the discrepancy in our quantitative data.
Functional implications
Physiological studies suggest that all SP-containing primary afferents function as nociceptors (Hylden & Wilcox, 1983; Kellstein et al., 1990; Lawson et al., 1997). Our results demonstrate that most of the CART axons in the superficial laminae belong to this type of nociceptor. It has also been demonstrated that one population of SP-containing primary afferents express mu opioid receptor on which morphine acts (Li et al., 1998). A possible mechanism of the potentiation of morphine action by CART peptide, reported by Damaj et al. (2004), could be the facilitation of the presynaptic morphine effect on CART/SP-containing primary afferents. To test this hypothesis, it will be necessary to test for the existence of mu-receptors on CART-positive afferents.
As the receptor for the CART peptide has not yet been identified, and because no appropriate antagonists are available, it is difficult, at present, to determine the exact role of the peptide in the spinal cord. However, it has been reported in behavioural studies that intrathecal application of CART(55–102) causes hyperalgesia in acute pain states (Ohsawa et al., 2000), and attenuates hyperalgesia and allodynia in neuropathic but not in inflammatory pain (Ohsawa et al., 2000; Damaj et al., 2004, 2006). In in vivo experiments, when the effect of CART peptide on thermal hyperalgesia induced by N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) was studied in rats, the peptide alone had no effect on the tail flick latency. However, CART significantly enhanced NMDA- but not AMPA-induced nociceptive effects (Hsun Lin et al., 2005). In in vitro studies, using whole-cell patch-clamp measurements on substantia gelatinosa neurons in spinal cord slices, CART peptide alone caused no significant change of resting membrane potential, but increased the amplitude of NMDA-induced depolarization in substantia gelatinosa neurons and had little effect on AMPA-induced responses (Hsun Lin et al., 2005). These studies demonstrate that exogenously applied CART peptide selectively facilitates NMDA receptor-mediated glutamatergic sensory and nociceptive transmission. Although the exact roles of the excitatory interneuron populations containing SP, SOM or NT have not been determined, the subpopulations that express CART peptide, together with the CART-containing primary afferents, could be involved in the NMDA-mediated physiological effects including nociception.
The fact that both the C fibres and the interneurons that are CART-immunoreactive express SP and/or SOM raises the possibility that CART can affect not only glutamatergic neurotransmission but also the release and effects of SP or SOM.
Acknowledgements
We thank Professor A. J. Todd for helpful discussion and advice, and Drs Jes Thorn Clausen and Michael J. Kuhar for the gift of CART antibodies.
This work was supported by the Scientific Council of Health, Ministry of Health, Government of the Republic of Hungary (ETT 463/2006).
Abbreviations
-
- 5-HTT
-
- serotonin transporter
-
- AMPA
-
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
-
- CART
-
- cocaine- and amphetamine-regulated transcript peptides
-
- CGRP
-
- calcitonin gene-related peptide
-
- CTb
-
- cholera toxin β-subunit
-
- DβH
-
- dopamine β hydroxylase
-
- DRG
-
- dorsal root ganglia
-
- GABA
-
- γ-aminobutyric acid
-
- GLYT2
-
- glycine transporter 2
-
- IB4
-
- isolectin B4 from Bandeiraea simplicifolia
-
- NMDA
-
- N-methyl-d-aspartate
-
- NT
-
- neurotensin
-
- SOM
-
- somatostatin
-
- SP
-
- substance P
-
- VGAT
-
- vesicular GABA transporter
-
- VGLUT2
-
- vesicular glutamate transporter 2.