Expression patterns of promoters for NPY Y1 and Y5 receptors in Y5RitTA and Y1RVenus BAC-transgenic mice
Abstract
In the rat brain, neuropeptide Y (NPY) Y1 and Y5 receptors are coexpressed in various forebrain regions where they mediate several NPY-activated functions, including feeding behaviour, anxiety, neuronal excitability and hormone secretion. We studied the distribution pattern and cellular colocalization of the Y1 and the Y5 receptor gene expression in the mouse brain by using transgenic mice with genomically integrated BAC clones, where the coding regions of the Y1 and Y5 receptor genes were replaced by Venus and the synthetic transcription factor itTA reporter genes, respectively (TgY5RitTA/Y1RVenus mice). Analysis of Venus fluorescence and itTA-mediated activation of Cre recombinase revealed copy number-dependent expression levels, between the lines, but similar expression patterns. In three transgenic lines the BAC encoded Y5 receptor promoter induced strong Cre expression in the olfactory system, cerebral cortex, hippocampus and basal ganglia. Weaker expression was found in most of the hypothalamic nuclei of line 25, the highest-expressing transgenic line. Activation of Cre was itTA-dependent and could be regulated by doxycycline. The Y1 receptor promoter-induced Venus fluorescence was intense, widely present through the brain and colocalized with Cre immunostaining in neurons of distinct brain regions, including the cerebral cortex, basolateral amygdala, dentate gyrus and paraventricular nucleus. These data provide a detailed and comparative mapping of Y1 and Y5 receptor promoter activity within cells of the mouse brain. The TgY5RitTA/Y1RVenus-transgenic mice generated here also represent a genetic tool for conditional mutagenesis via the Cre lox system, particularly of genes involved in feeding behaviour, neuronal excitability and hormone secretion.
Introduction
Neuropeptide Y (NPY) is a 36 amino acid peptide widely distributed in the nervous system. In the brain, the Y1 and Y5 receptor (Y1R, Y5R) subtypes for NPY have received significant attention because of their ability to reduce anxiety, modulate neuronal excitability, increase food intake and regulate hormone secretion (Wieland et al., 2000; Elmquist, 2001; Chamorro et al., 2002; Baraban, 2004; Heilig, 2004; Kalra & Kalra, 2004; Woldbye & Kokaia, 2004; Acosta-Martinez et al., 2007; Carvajal et al., 2006; Eva et al., 2006a, 2006b).
The Y1R is highly expressed in several forebrain regions (Naveilhan et al., 1998; Kopp et al., 2002; Kishi et al., 2005), including the cerebral cortex and the hippocampus, and several amygdaloid, thalamic and hypothalamic nuclei. In addition, high levels of Y1R mRNA have been detected in numerous brainstem nuclei (Kopp et al., 2002; Kishi et al., 2005).
In the rat brain, Y5R mRNA and immunoreactivity are fairly widely distributed although to a lower extent than those of Y1R (Gerald et al., 1996; Parker & Herzog, 1998; Nichol et al., 1999; Durkin et al., 2000; Campbell et al., 2001). Additionally, Y1R and Y5R are colocalized in several forebrain regions (Wolak et al., 2003) and their coexpression may possibly reflect a common cis-acting transcriptional control as the two genes map to the same locus on human chromosome 4q31–q32 (Herzog et al., 1997; Nakamura et al., 1997).
Conversely, in the mouse brain, low levels of Y5R mRNA were found (Naveilhan et al., 1998; Xin & Huang, 1998). However, in absence of immunohistochemical studies, it remains to be determined whether the restricted Y5R distribution in mice is due to species differences or to dissimilar sensitivity of detection systems. Furthermore, it needs to be resolved whether Y1R and Y5R are coexpressed in the same neurons in the mouse brain. Given that the activations of Y1R and Y5R contribute to similar functions, a detailed analysis of their expression patterns is essential for understanding the contribution of each receptor subtype in physiological processes.
To map Y1R and Y5R promoter activity at the cellular level we generated transgenic mice which express Y1R and Y5R promoter-controlled reporter genes. In a single bacterial artificial chromosome (BAC) we could replace the Y1R and the Y5R coding exons with coding regions for the ‘improved’ tTA (itTA; Krestel et al., 2004) and the Venus-fluorescent protein (Nagai et al., 2002), respectively (Fig. 1). We generated BAC-transgenic mouse lines and analysed the Y1R promoter activity using Venus fluorescence and the Y5R promoter activity using immunoreactive staining of ‘improved’ (i-) Tetracycline (Tet)-dependent transcription factor (tTA; itTA)-induced Cre in the tTA reporter line TgLC1 (Schonig et al., 2002). Cre recombinase labelled the nuclei of Y5R receptor neurons in several telencephalic and diencephalic regions and Cre expression could be regulated by doxycycline (Dox). Venus fluorescence distribution was widespread and recapitulated Y1R expression in the mouse brain. By comparing expression patterns and cellular colocalizations of Cre and Venus we provided further insight into the cellular distribution and overlapping activity of Y1R and Y5R in the mouse brain.
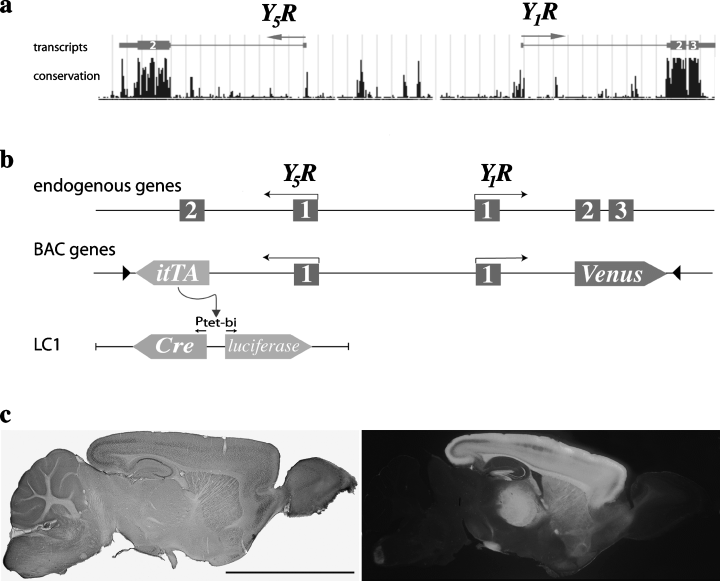
Genomic BAC construct used for specific coexpression of itTA and Venus by the Y1R and Y5R promoters, respectively. (a) Schematic drawing of the genomic fragment encoding theY1R and the Y5R genes in opposite orientation (arrows) as encoded by mouse chromosome 8; pos. 69 608 028–69 634 897 (modified blast search output from UCSC at http://www.genome.ucsc.edu). The premRNA sequences are given with introns (thin lines), noncoding exons (boxes) and coding exons (thick boxes). Conserved sequences between different species are indicated in the lower line as degree of conservation. (b) Schematic comparison between the endogenous Y1R/Y5R gene locus (upper panel) and the BAC (middle panel) where Y5R exon 2 and Y1R exons 2/3 were replaced by coding exons for itTA and Venus, respectively. The Ptet-bi-controlled tTA reporter genes of transgene LC1 are depicted in the lower panel. Arrows, transcriptional starts; black triangles, frt sites. (c) Stereomicroscopic visualization of Cre (left) and Venus-fluorescent protein (right) expression in brain sagittal sections from line 25. Sections were taken from double-transgenic mice TgY5RitTA/Y1RVenus/LC1 that carried the Cre recombinase controlled by the itTA-responsive promoter Ptet-bi to permit the visualization of Cre activity by immunostaining. Overview shows dominant expression pattern of both transgenes in the forebrain. Scale bar, 500 µm.
The TgY5RitTA/Y1RVenus-transgenic mice are useful for physiological studies, including those directed at temporally and regionally controlled manipulation of genomes in vivo.
Materials and methods
Animal experiments
Animal care was in compliance with the European Community Council Directive of November 24, 1986 (86/609/EEC) and in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication no. 85–23, revised 1996) and the European Community guidelines for the use of experimental animals. The pronucleus injections were performed by Frank Zimmermann at the IBF, INF 347, D-69120 Heidelberg, Germany, according to a license (35–9185.81/G-4/02) of the Regierungspräsidium Karlsruhe, Germany. The experimental protocol was approved by the animal investigation committee of the Ministero dell'Istruzione dell'Università e della Ricerca, Italy.
Y1R gene BAC screening
A mouse 129 BAC library (BAC Mouse ES release I, BAC4921, Genome Systems, St Louis, MO, USA) was screened at Genome Systems with a 2-kb DNA probe obtained from XbaI digestion of a pBSY6-7 plasmid containing the Y1R gene (Eva et al., 1992). Two clones were obtained: 22673 and 22674, both of which were fingerprinted with several restriction enzymes and hybridised with the probe mentioned above. All the work was carried out on clone number 22674.
Plasmid construction of pBS.htTApA.2frtneo
The neomycin resistance of the pCR4-TOPO (Invitrogen, Karlsruhe, Germany) was amplified with overhang primer which flanked the resulting PCR product with the 34-bp minimal flippase recombinase (Flp) target (Frt) sequences in the same orientation and direction. This fragment was digested with BglII and EcoRI and inserted downstream of the human growth hormone polyadenylation (pA) signal of the plasmid pBS.itTApA (Krestel et al., 2004) to create the plasmid pBS.itTApA.2frtneo.
Plasmid construction of pBS-Venus 2frtneo
The Venus gene was excised with HindIII-EcoRV digestion from plasmid pCS2-Venus (Nagai et al., 2002) and subcloned in HindIII-EcoRV, in pBS.iCrepA.2frtneo plasmid in place of the iCre gene. Correct insertion of the Venus gene was confirmed by sequence analysis using oligonucleotides sitting outside of the insertion site.
Recombinogenic targeting of BAC
The Y1R BAC clone containing the Y1R and the Y5R genes in opposite orientation and, in between, their potential 25-kb regulatory region, was modified using the E.coli-based chromosome system adapted for recombinogenic targeting and subcloning of BAC DNA (Lee et al., 2001). The EL250 strain (kindly provided by Dr Neal Copeland) used in this study, originating from DH10B E.coli cells, has homologous recombination (λ red genes) and site-specific recombination (Flp) functions with the former controlled by temperature and the latter by arabinose.
First recombinogenic step
The BAC DNA clone 22674, containing Y1R and Y5R, was electroporated into EL250 bacteria (see below); 10 chloramphenicol-resistant colonies were selected. NotI and EcoRI digestion of their BAC DNA was analysed with Pulse Field Gel Electrophoresis on 1% agarose gel in 0.5× TBE (45 mm Tris-borate and 1 mm EDTA, pH 8.0).
The Venus-FRT-neo-FRT targeting cassette was PCR-amplified with Platinum Pfx (Invitrogen, San Giuliano Milanese, Milano, Italy) from pBS-Venus 2frtneo plasmid using a pair of 68-bp chimeric oligonucleotides: VenusY1L (5′-AGAACTATAACTGTCCATTTATCTAATCGGTAACAACAAAACATAAAAAAATGGTGAGCAAGGGCGAG-3′) and NeofrtY1 (5′-TCAGACTTTTTCATTGTCATTCATACTGATTTTTTTAAATGCGACTGGGCGTGGGCAGATCTATGCAT-3′), which are homologous to the amplification cassette (Venus gene of pBS-Venus 2frtneo; 50 bp, in roman type above) and to the targeted sequence (Y1R coding sequences on the BAC; 18 bp, in italics above). PCR products were purified using a Qiaex II gel extraction kit (Qiagen, Milano, Italy) and digested with DpnI to remove contaminating template. Three hundred nanograms of the Venus-FRT-neo-FRT cassette were electroporated in EL 250 bearing the BAC Y5R/Y1R DNA and spread out on kanamycin–chloramphenicol plate. About 25 resistant colonies were analysed for recombination by whole-cell PCR using primer external to the recombination region: Y1int1S3 (5′-GTATGCTGAAGATTTGATCCG-3′ and KM2RT (5′-CAAGGTGAGATGACAGGAGATC-3′). Platinum Pfx (Invitrogen, San Giuliano Milanese, Milano, Italy) PCR-amplified products were fully sequenced to detect point mutation or recombination errors.
To remove the neo marker, one EL 250 clone carrying the modified BAC-Y5R/Y1R Venus was incubated for 1 h at 32 °C in LB broth recipe (Luria-Bertoni broth) containing 0.1%l-arabinose in order to induce flp recombinase expression. Neo removal was assessed both by whole-cell PCR and by negative control, checking cell growth on kanamycin-containing plates.
Second recombinanat step
Further on, a second round of recombination followed on BAC-Y5R/Y1RVenus in order to replace the Y5R gene with the human itTA gene. The itTA-FRT-neo-FRT targeting cassette was PCR-amplified from pBS-itTA2frtneo plasmid. Amplification primers for targeting were: Y5itTAET2 (5′-GTTCTTCAAGCTGCTAATGGACACTGTCTTCTTCCAAGCAGGACTCTAGTATGTCTAGACTGGACAAG-3′) and Y5itTAneoKm2 (5′-TCATGACATGTGTAGGCAGTGGATAAGGGCTCTCAAGTCTGCTTTGATAGTGGGCAGATCTATGCAT-3′), which are homologous to the amplification cassette (itTA-FRT-neo-FRT; 50 bp, in roman type above) and to the targeted sequence (Y5R coding sequences on the BAC; 18 bp, in italics above). Again, positive clones were analysed by whole-cell PCR using external primers Y5E (5′-TCTAGATGTTAGT-TGTGTTCTGAG-3′) and itTA2RT (5′-CAACTCAATAGCTTGCCGCAG-3′) and induced with arabinose for neo removal.
After sequence analysis, 30% glycerol stocks were made from the positive EL 250 clones carrying the mutated BAC Y5RitTA/Y1RVenus and stored at −80 °C.
Preparation of electrocompetent cells and generation of recombinants
For BAC modification, 100 mL LB broth recipe medium was inoculated with 2 mL of overnight culture from single colonies of bacteria containing the BAC and grown to 0.5–0.7 optical density 600. Cultures were then induced for Beta, Exo and Gam expression by shifting the cells to 42 °C for 15 min and immediately chilling for 20 min on ice. Cells were then centrifuged for 5 min at 5500 g at 4 °C and washed three times with 100 mL of ice-cold sterile 10% glycerol. Electrocompetent cells were then resuspended in 1 mL of ice-cold sterile water and electroporated.
Cell transformation was performed by electroporation of 100–300 ng linear DNA into 50 µL of ice-cold competent cells in cuvettes (0.1 cm) using a Bio-Rad gene pulser set at 1.75 kV, 25 mF, with a pulse controller set at 200 Ω. One millilitre of LB broth recipe medium was added after electroporation. Cells were incubated at 32 °C for 1.5 h with shaking, and then spread on appropriate selective agar media.
Production of BAC-transgenic mice
Modified BAC was digested by NotI to free the 150-kb insert, containing the Y5RitTA/Y1RVenus sequence, from the BAC backbone. The insert was purified on a Sepharose CL-4B gradient (Amersham, Milano, Italy), using a microinjection buffer containing (in mm) Tris-HCl, pH 7.6, 10; EDTA, 0.1; and NaCl, 100. BAC insert (∼ 0.5–1 ng) was microinjected into the pronucleus of (C57BL/6 × DBA2)F1 zygotes. Founder mice were analysed by PCR using primers Y151 (5′-CTGCTGTTGACACCATTTGTCTAACACTTGG-3′) and Venus2 (5′-CTTCAGCTCGATGCGGTTCAC-3′), and by Southern blot analysis of phenol–chloroform-extracted tail or liver DNA, digested with MscI or with BglII and hybridised with a DNA probe obtained by PCR on BAC Y5RitTA/Y1RVenus using Y151 and Venus2 primers.
Generation of double-transgenic mice and genotyping
Transgenic mice TgY5RitTA/Y1RVenus were crossed with TgLC1 (Schonig et al., 2002). Pups were genotyped by PCR of tail DNA with specific primers. TgLC1 rspCre1 (5′-ACCAGG TTC GTT CAC TCA TGG-3′) and rspCre2 (5′-AGG CTA AGT GCC TTC TCT ACAC-3′), 200 bp; TgY5RitTA/Y1RVenus, itTA2RT (5′-CAACTCAATAGCTTGCCGCAG-3′) and itTA (5′-TGCCTTGGAGCTCCTGAATGAAGTTG-3′), 500 bp.
Regulation with Dox treatment
In double-transgenic mice, TgY5RitTA/Y1Rvenus- and TgLC1-positive Cre recombinase expression was suppressed from conception until postnatal day (P)0 with Dox (Sigma-Aldrich, Milano, Italy) in the drinking water (which contained 1% sucrose and 20 (line 4 and 36) and 50 mg (line 25) Dox/L) of pregnant females; it was subsequently induced by transferring pups to Dox-naïve foster mothers (Krestel et al., 2001).
After PCR tail genotyping, positive pups were analysed for Cre immunoreactivity at P20, P30, P40 and P52.
Immunohistochemistry
Mice at 4–5 weeks of age were given an anaesthetic overdose (Avertin; 0.5 mg per gram of body weight, i.p.) and transcardially perfused with 4% paraformaldehyde in PBS. Brains were removed, postfixed for 2 h and placed in 2% agarose in 1 × PBS. Cre immunostaining was carried out using the ABC Vectastain kit (Vector Laboratories, Burlingame, CA, USA) on 50-µm vibratome coronal sections. After endogenous peroxidase blocking in 0.5% H2O2 in 0.1 m PBS for 15 min, sections were blocked with 10% normal goat serum in DayI buffer (PBS containing 0.3% Triton X-100 and 1% bovine serum albumin) for 1 h and incubated overnight at room temperature with primary Cre antibody, a polyclonal rabbit anti-Cre recombinase antiserum (Novagene, Madison, WI, USA) at 1 : 3000 dilution in DayI solution. After washing in DayII buffer (1 : 3 dilution of DayI in 0.1 m PBS), sections were incubated for 1 h with a secondary biotinylated goat antirabbit antibody (1 : 500; Vector) followed by the ABC reagent (Vector). Peroxidase was reacted with 0.05% diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich, Milano, Italy), and 0.003% hydrogen peroxide, mounted on slides and air-dried overnight. DAB-developed slices were coverslipped with DPX mounting medium and analysed with a Leica microscope. For colocalization studies, Cre immunofluorescence detection was performed using the above protocol with some modifications: the primary Cre antibody dilution was lowered to 1 : 1500, the secondary antibody incubation was performed in a modified DayI solution containing 0.15% Triton X-100 in PBS, and was followed by 1 h incubation with Avidin Texas Red (1 : 600; Vector). The extension of the immunostaining was independently evaluated by two observers on an ordinal scale, using four different levels: high (+ + +) when > 50% of cellular elements of a brain region were positive to the histochemical reaction; intermediate (+ +) when between 20 and 50% of the elements were stained; low (+) when < 20% of the elements were stained; and very low (+/–) when only sparse positive elements were detected (Table 1).
| CNS region | Transgenic lines | Dox until P0 | References | ||||
|---|---|---|---|---|---|---|---|
| 25 | 4 | 36 | P5225 | P404 | P4036 | ||
| Telencephalon | |||||||
| Anterior olfactory n. | + + + | + + | + + | + + + | + + | + + | b |
| Olfactory tubercle | + + + | + + + | + + | + + + | + + + | + + | a,e |
| Cingulate cortex | + + + | + + + | + + + | + + + | + + + | + + + | b,e |
| Cortex | |||||||
| Piriform cortex | + + + | + + + | + + + | + + + | + + + | + + + | a,c,d,e |
| Layers 2 and 3 | + + | + + | + + | + + | + + | + + | c,d |
| Layer 4 | + + + | + + + | + + + | + + + | + + + | + + + | c,d |
| Layer 5 | + | + | +/– | + | nd | nd | c |
| Layer 6 | + + + | + + + | + + + | + + + | + + + | + + + | c |
| Hippocampus | |||||||
| CA1 | + + + | + + + | + + + | + + + | + + + | + + + | a,b,c,e,g |
| CA2 | + + + | + | + | + + | + | + | c,d,e,g |
| CA3 | + + + | + + | + | + + + | + + | + | b,c,d,e,g |
| GrDG | + + + | + + + | + + + | + + + | + + + | + + + | a,b,c,d,e,g |
| Amygdaloid area | |||||||
| Basolateral amygdala | + + + | + + | + + | + + | + | + | c,e |
| Medial amygdala | + + | + | + | + | + | + | e |
| Central amygdala | + | + | + | + | + | + | c,e |
| Anterior cortical | |||||||
| amygdaloid n. | + + | + + | + + | + + | + + | + + | e |
| Claustrum | + + | + + | + + | + | + | + + | c |
| Caudate–putamen | + + + | + + | + + | + + + | + + | + + + | b,c |
| Nucleus accumbens | + + + | + + | + + | + + + | + + | + | c |
| Ventral pallidum | + + + | + | + | + + | + | + | |
| Bed nucleus stria terminalis | + | + | + | + | nd | + | e |
| Septum | |||||||
| Lateral septum n. | + + | + | + | + | + | + | b,c |
| Septofimbrial n. | + + | + + | + + | + | + | + | c |
| Islands of Calleja | + | + | nd | + | + | nd | a,c |
| Tenia tecta | + + + | + + | + + | + + | + + | + + | e |
| Medial preoptic area | + + | nd | + | + | nd | + | c |
| Diencephalon | |||||||
| Hypothalamus | |||||||
| Suprachiasmatic n. | + + + | + + + | + + | + + + | + + | + + | c,e,f |
| Anterior hypothalamus | + | nd | +/– | + | nd | +/– | e |
| Lateral hypothalamic | |||||||
| area | + | nd | +/– | + | nd | +/– | c,d,e,f |
| Periventricular n. | + | nd | +/– | +/– | nd | +/– | |
| Paraventricular n. | + + | nd | nd | + | nd | nd | c,e,f |
| Ventromedial n. | + | nd | nd | nd | nd | nd | b,d,e,f |
| Dorsomedial n. | + + | + | + | + + | + | + | b,d,e,f |
| Arcuate n. | + | nd | nd | + | nd | nd | a,b,c,d,e |
| Mammillary n. | + + | + | + + | + | nd | +/– | c,d,e |
| Subependymal cells | |||||||
| of the 3rd ventricle | + + + | + + + | + + + | + + + | + + + | + + + | |
| Thalamus | |||||||
| Anterodorsal n. | +/– | nd | nd | nd | nd | nd | c,e |
| Paraventricular n. | + | nd | +/– | +/– | nd | nd | c,d,e |
| Reticular n. | nd | nd | nd | nd | nd | nd | c,e |
| Reuniens n. | + + | nd | +/– | + | nd | nd | b,d |
| Lateral geniculate n. | + + | nd | +/– | nd | nd | nd | c,d,e |
| Lateral habenula | + + + | nd | +/– | nd | nd | nd | c,d,e |
| Mesencephalon | |||||||
| Substantia nigra | + + | nd | +/– | nd | nd | nd | c,d,e |
| Superior colliculus | + | ° | ° | ° | ° | ° | |
| Inferior colliculus | + + | ° | ° | ° | ° | ° | |
| Metencephalon | |||||||
| Spinal vestibular n. | + | ° | ° | ° | ° | ° | |
| Vestibulocerebellar n. | + | ° | ° | ° | ° | ° | |
| Solitary tract n. | + | ° | ° | ° | ° | ° | b,c |
| Parabrachial n. | + | ° | ° | ° | ° | ° | |
| Spinal trigeminal tract | + + | ° | ° | ° | ° | ° | c,e |
- Cre expression was detected by immunohistochemical staining and graded as very low (+/–), low (+), intermediate (+ +) or high (+ + +); nd, not detected; °, not determined. References for the endogenous expression of the rat and mouse Y5R mRNA or immunoreactivity are: a, Naveilhan et al. (1998); b, Xin & Huang (1998); c, Wolak et al. (2003); e, Parker & Herzog (1999); f, Larsen & Kristensen (1998); g, Parker & Herzog (1998).
Analysis of Venus expression
Vibratome coronal sections (50 µm) of brains from perfused mice were mounted on slides and used directly for fluorescence distribution analysis using a Leica DM LB2 fluorescence microscope equipped with a DFC 320 Leica camera and an Olympus Bx51WI or a Zeiss LSM5 Pascal confocal laser-scanning microscope. The intensity of fluorescence was independently evaluated by two observers on an ordinal scale as described for immunostaining of Cre.
Results
Y1R and Y5R promoter-controlled reporter gene expression in four transgenic lines
The Y5RitTA/Y1RVenus BAC, containing transcription units for Venus and itTA driven by the Y1R and the Y5R regulatory regions, respectively, was used to generate TgY5RitTA/Y1RVenus-transgenic mice (Fig. 1B). Six founder lines were identified by Southern blot analysis of genomic DNA, and five founders showed the expected hybridization pattern (data not shown). To monitor Y5R promoter activity using itTA, the founder mice were crossed with TgLC1-transgenic mice that carry Ptet-bi controlled Cre recombinase and Luciferase as tTA reporter genes (Schonig et al., 2002). Ptet-bi is a bidirectional tTA-responsive promoter composed of an array of seven Tet operator sequences flanked by two human cytomegalovirus promoter IE minimal promoters. Ptet-bi is activated by tTA and initiates transcription of the Cre recombinase and the Luciferase transgenes in opposite directions (Fig. 1B). Analysis of Cre expression was performed by immunohistochemical staining of coronal brain sections from 4-week- to 2-month-old transgenic mice. Specific Cre expression was detected in offspring from founders 4, 25, 36 and 39 but with different reporter levels. Cre and Venus expression levels were correlated with the relative copy number of the transgene, which was found in Southern blots to be 1 : 2 : 2 : 2 : 6 between the lines 27, 39, 4, 36 and 25. In low-expressing mice of founder 39, Cre immunoreactivity was observed in some dispersed cells of the cerebral cortex and hippocampus (data not shown). Lines 4 and 36 showed similar Cre-expression patterns. In both lines, moderate to high Cre expression was present in the olfactory system, cerebral cortex, hippocampus, basal ganglia and suprachiasmatic nuclei (SCh; the data are summarised in Table 1 and in Fig. 2). Lower Cre expression was found in the medial amygdala (Me) and central amygdala, and weak Cre immunostaining was observed in a few thalamic and hypothalamic nuclei.
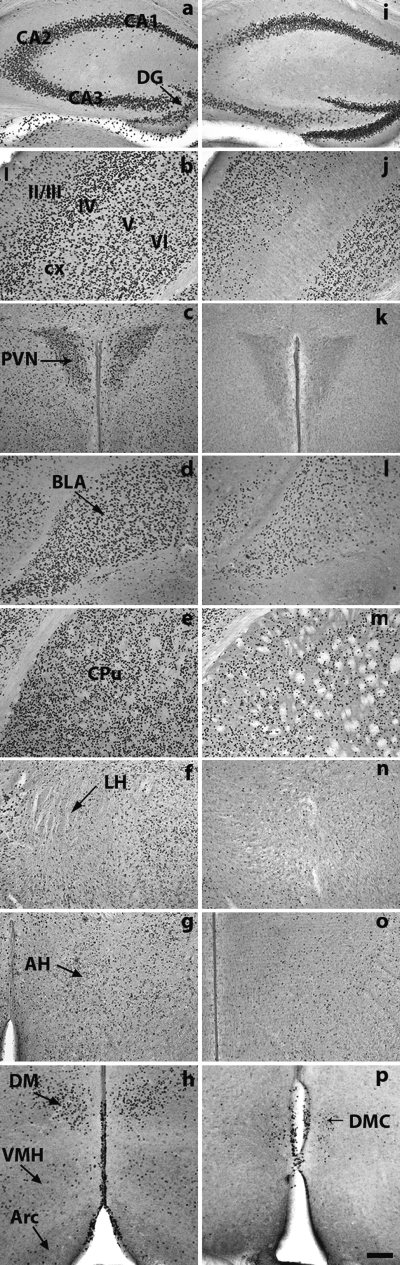
Y5R promoter-controlled itTA activity, visualized by Cre immunostaining of brain coronal sections from (a–h) line 25 and (i–p) line 36. Sections from double-transgenic mice TgY5RitTA/Y1RVenus/LC1 were taken between P40 and P60. (a and i) Pyramidal cell layer of the CA1-CA3 regions (CA1-CA3) and dentate gyrus (DG), hippocampus; (b and j) layers I, II/III, IV, V and VI of the cerebral cortex (cx); (c and k) paraventricular hypothalamic nucleus (PVN); (d and l) basolateral amygdala (BLA); (e and m) CPu; (f and n) lateral hypothalamic nucleus (LH); (g and o) anterior hypothalamus (AH); (h) dorsomedial nucleus (DM), ventromedial nucleus (VMH) and arcuate nucleus (Arc); (p) dorsomedial nucleus, compact (DMC). All pictures are at the same enlargement. Scale bar, 100 µm.
In mice of the highest-expressing line (line 25), the Cre expression pattern overlapped the one reported in the literature for the rat and mouse Y5R in most of the brain regions (Larsen & Kristensen, 1998; Naveilhan et al., 1998; Parker & Herzog, 1998, 1999; Xin & Huang, 1998; Broberger et al., 1999; Wolak et al., 2003). The highest Cre expression was found in the olfactory system, layers II/III, IV and VI of the cerebral cortex, stratum pyramidale of the CA1-CA3 fields, granule cell layer of the dentate gyrus (GrDG), basolateral amygdala (BLA), caudate–putamen (CPu), nucleus accumbens, ventral pallidum, tenia tecta, lateral habenula and the ependymal–subependymal cell layer of the third ventricle (3V; Table 1, 1, 2). Moderate to low Cre expression was also found in the layer V of the cerebral cortex, Me, central amygdala and anterior cortical amygdaloid nucleus, claustrum, bed nucleus of stria terminalis, septum, islands of Calleja, medial preoptic area and lateral habenula. In the hypothalamus, Cre-immunoreactive staining is also similar to endogenous rat Y5R gene expression. High levels of Cre expression were found in SCh, paraventricular (PVN) and dorsomedial (DM) hypothalamic nuclei and mammillary nuclei (MM), whereas low levels of Cre immunostaining were observed in the lateral hypothalamic area (LH), anterior hypothalamus (AH), ventromedial (VMH), arcuate (Arc) and periventricular (Pe) hypothalamic nuclei (Fig. 2 and Table 1). Conversely, in the thalamus, moderate to low Cre expression was detected in the anterodorsal, paraventricular, reuniens and lateral geniculate nuclei, while there was no Cre expression in the central, anteromedial, reticular or mediodorsal thalamic nuclei, in the ventral group or in the zona incerta, all thalamic regions in which the Y5R is expressed in the rat (Wolak et al., 2003).
Although we did not map Cre expression in the brainstem as rigorously as we did in the higher structures, we noted a low to moderate expression of Cre immunoreactivity confined to the substantia nigra, colliculi and a few metencephalic nuclei.
Conditional Cre expression in forebrain
Ptet-bi controlled Cre and Luciferase expression can be prevented by Dox treatment which inhibits the binding of itTA to Ptet-bi, permitting a temporal control of Cre-mediated recombination (for review see Sprengel & Hasan, 2007). Therefore, we investigated the Tet sensitivity of Y5R-itTA-activated TgLC1 mice by Dox treatment of lines 25, 4 and 36. Our results demonstrate that the extent of Cre expression could be restricted by Dox. When double-transgenic mice were raised with Dox from conception, itTA induction was prevented, keeping Cre at undetectable levels (Fig. 3). If Dox was applied from conception until birth (P0), the Cre-specific immunosignal slowly increased over time, reflecting a slow unsilencing of the Ptet-bi of the TgLC1 transgene (Mack et al., 2001; Krestel et al., 2004; Zhu et al., 2007). In mice with Dox suppression during intrauterine development, a weak expression of Cre was observed at P20 when compared with the untreated lines 25 and 36 at P40–60 in histochemical stains (Fig. 3) indicating that at P20 the TgLC1 responder genes were not yet fully activated. In all lines, Cre expression levels similar to those observed in untreated mice were reached by P40 in the olfactory system, cerebral cortex, GrDG and CA1-CA3, basal ganglia, septal area, medial preoptic area, SCh, DM and the ependymal–subependymal cells of the 3V (Table 1 and Fig. 3). Further analysis of mice from line 25 at longer time windows for Cre reactivation showed that, in the BLA, in the reuniens and paraventricular thalamic nuclei, and in most of the hypothalamic nuclei (AH, LH, Pe, PVN, Arc and MM), the reactivation of Cre was reached by P52 but Cre expression was still limited to a minor subset of neurons as compared to Dox-naïve mice (Table 1 and Fig. 3). Furthermore, no Cre induction was evident in the VMH, lateral habenula and geniculate thalamic nucleus at this age of mice.

Dox-regulated itTA activity, monitored in responder lines 25 and 36. Coronal brain sections of TgY5RitTA/Y1RVenus/LC1 double-transgenic mice derived from lines 25 and 36 were immunostained for Cre. Activation by itTA in animals prenatally and postnatally treated with Dox is compared with that in littermates for which Dox was removed at P0 and killed at P20 and at P40. In the hypothalamus of line 25, Dox treatment and removal leads to a slower Cre induction that was evident only by P52. CA1, pyramidal cell layer of the CA1 region; cx, cerebral cortex; CPu, caudate putamen; SCh, suprachiasmatic nuclei; BLA, basolateral amygdala; PVN, paraventricular hypothalamic nucleus; LH, lateral hypothalamus; AH, anterior hypothalamus; DM, dorsomedial nucleus; Arc, arcuate nucleus. All pictures are at the same enlargement, scale bar:100 µm.
Expression of the Y1R promoter as monitored by Venus in five transgenic lines
As mentioned above, BAC copy number-dependent Venus expression could be detected in all five transgenic lines. According to their location and aspect and to the lack of glial fibrillary acidic protein immunoreactivity (data not shown), the majority of the examined cells were neurons and Venus fluorescence was present in both cell bodies and cell processes. In offspring from founders 27 and 39, Venus-fluorescent cell bodies and fibres were observed in the cerebral cortex, central amygdala and hypothalamus (data not shown). In mice of lines 4, 25 and 36, Venus fluorescence was intense and widely expressed through the brain, with an overlapping pattern of distribution among the three transgenic lines (Table 2, 1, 4). We found that, in general, the Venus fluorescence recapitulated the endogenous Y1R expression in mouse, although some discrepancies were observed in the cerebral cortex and in the hippocampal formation (Naveilhan et al., 1998; Kopp et al., 2002; Fetissov et al., 2004; Kishi et al., 2005; Fig. 4 and Table 2).
| CNS regions | Transgenic lines | References Y1RI and mRNA | |||||
|---|---|---|---|---|---|---|---|
| 25 | 4 | 36 | |||||
| Cellbodies | Fibres | Cellbodies | Fibres | Cellbodies | Fibres | ||
| Telencephalon | |||||||
| Anterior olfactory n. | + + | + + + | + + | + + | + + | + + | b,c,a |
| Olfactory tubercle | nd | + + + | nd | + | nd | + + | b,c,a |
| Cingulate cortex | + + + | + | + + + | + + | + + + | + | b,a |
| Cortex | |||||||
| Piriform Cortex | + + | + + + | + | + | + | + | b,c,a |
| Layers 2 and 3 | + + + | + + + | + + + | + + + | + + + | + + + | b,c,a |
| Layer 4 | nd | + | nd | + + + | nd | + | b,c,a |
| Layer 5 | nd | + + + | nd | + + | nd | + + + | b,c |
| Layer 6 | + + | + + + | + | + + + | + | + + + | b,c,a |
| Hippocampus | |||||||
| CA1 | + | nd | + + | nd | + | nd | b,c,a |
| CA2 | nd | + + + | nd | + + + | nd | + + | b,c,a |
| CA3 | nd | + + + | nd | + + + | nd | + + + | b,c |
| GrDG | + + + | nd | + + + | nd | + + + | nd | b,c |
| MolDG | nd | + + + | nd | + + + | nd | + + + | b,c |
| PoDG | nd | + + + | nd | + + + | nd | + + + | b,c |
| Amygdaloid area | |||||||
| Basolateral amygdala | + | + + + | + | + + + | + | + + + | b,c |
| Medial amygdala | + + | + + | + | + + | + + | + | b,c |
| Central amygdala | + | + + | + | + + | + | + + | b,c,a |
| Anterior cortical amygdaloid n. | + + | + + + | + | + + | + | + | b,c,a |
| Amygdalohippocampal area | + + + | + + | + + + | + + | + + + | + + | c |
| Claustrum | + | + + + | + | + + | + | + + | b,c,a |
| Endopyriform n. | + | + | nd | + | nd | + | b,c, |
| Globus pallidus | nd | + + | nd | + | nd | + | b,c, |
| Caudate putamen | nd | + + + | nd | + + + | nd | + + + | b,c |
| Nucleus accumbens | nd | + + | nd | + | nd | + + | b, |
| Diagonal band of Broa | + | nd | ° | ° | + | nd | c |
| Bed nucleus stria terminalis | nd | + + + | nd | + + | nd | + + + | b,c |
| Septum | |||||||
| Lateral septal n. | nd | + + + | nd | + + | nd | + + | b,c |
| Septofimbrial n. | nd | + + + | nd | + + | nd | + + + | b,c |
| Medial septal n. | nd | + | nd | + | nd | + | b,c |
| Subfornical organ | nd | + + + | nd | + + + | nd | + + + | b,c |
| Tenia tecta | + | + | nd | + | + | + | b,c |
| Medial preoptic area | + + + | + + | + + | + + | + + + | + + | b,c,a |
| Vascular organ, lamina terminalis | + + + | nd | nd | + + | ° | ° | |
| Diencephalon | |||||||
| Hypothalamus | |||||||
| Suprachiasmatic n. | + + + | + | + + + | + | + + + | + | b,c,a,d |
| Anterior hypothalamus | + + | + + | + | + + | + + | + + | b,c |
| Lateral hypothalamic area | + + | + + | + + | + + | + + | + + | b,c,d |
| Periventricular n. | + + | nd | + | nd | nd | + | b,c,a,d |
| Paraventricular n. | + + + | + + | + + | + | nd | + | b,c,d |
| Ventromedial n. | nd | + + | nd | + + | nd | + | b,c,d |
| Dorsomedial n. | + + + | + | + + | + | + + | + | b,c,d |
| Arcuate n. | + + | + | + + | + | + + | + | b,c,a,d |
| Mammillary n. | + + | + | + + + | + | + + | + | b,c,a,d |
| Subependymal cells | |||||||
| of the 3rd ventricle | + + + | nd | + + + | nd | + + + | nd | d |
| Thalamus | |||||||
| Anterodorsal n. | nd | + + | nd | + | nd | + | b, |
| Paraventricular n. | nd | + | nd | nd | nd | + | b |
| Reticular n. | nd | + + | nd | + | nd | + | b |
| Reuniens n. | + | + + | + | + | + + | + | b,c |
| Lateral geniculate n. | nd | + + + | nd | + + | nd | + + | b,c |
| Laterodorsal n. | nd | + + | nd | + | nd | + | b,c |
| Ventrolateral n. | nd | + + + | nd | + + | nd | + + | b,c |
| Lateral habenula | + + | + | + + | + | + | + + | b,c, |
| Mesencephalon | |||||||
| Substantia nigra pars reticulata | nd | + + | nd | + + | ° | ° | b,c |
| Olivary pretectal n. | + + | + | ° | ° | + + | + | b |
| Ventral tegmental area | + | + | + | + | ° | ° | b,c |
| Periaqueductal grey | + | + + | nd | + | ° | ° | b,c |
| Superior colliculus | + | + | nd | + | ° | ° | b,c |
| Inferior colliculus | + | + | ° | ° | ° | ° | b,c |
| Metencephalon | |||||||
| Vestibular n. | + | + + | ° | ° | ° | ° | b,c |
| Solitary tract n. | + | + + | nd | + | ° | ° | b,c |
| Parabrachial n. | nd | + + | ° | ° | ° | ° | b,c |
| Spinal trigeminal n. Principal sensory | + + + | + + | nd | + + | ° | ° | b,c, |
| trigeminal n. | + | + + | ° | ° | ° | ° | b,c,a |
| Raphe magnus n. | nd | + + | ° | ° | ° | ° | b,c |
- Venus fluorescence in cell bodies and fibres was graded as low (+), intermediate (+ +) or high (+ + +); nd, not detected; °, not determined; MolDG, molecular cell layer of the dentate gyrus; PoDG, polymorphic cell layer of the dentate gyrus. References for the endogenous expression of the mouse Y1R mRNA or immunoreactivity are: a, Naveilhan et al. (1998); b, Kopp et al. (2002); c, Kishi et al. (2005); d, Fetissov et al. (2004).

Photomicrographs of the Venus-fluorescent protein distribution in the telencephalon and the hypothalamus of TgY5RitTA/Y1RVenus mice from (a–i) line 25 and (j–r) line 36. (a and j) In the hippocampus, cells in the granule layer of the dentate gyrus (GrDG) are densely labelled. Venus-fluorescent processes are evident in the polymorphic layer (PoDG) and in the molecular layer (MolDG) of the dentate gyrus and in the CA3 region.Venus-fluorescent perikarya are evident in (b and k) the cingulate cortex (Cg) and in (c and l) layers II/III and VI of the cerebral cortex (cx). Venus-fluorescent processes can be seen within (c and l) the other cortical layers (lamina IV) and in (d and m) the CPu. In the amygdaloid complex, Venus-fluorescent perikarya and fibres are present in (e and n) the medial (Me) and (f and o) the basolateral (BLA) divisions. In the hypothalamus, Venus fluorescence is present in cells and fibres in (g and p) the medial preoptic area (MPA), (h and q) the dorsomedial (DM) and (i and r) the arcuate (Arc) nuclei. Venus-positive cells are observed in (g and p) the periventricular nucleus (Pe). Scale bars, 100 µm.
In the olfactory region, strong Venus fluorescence was monitored in neurons of the anterior olfactory nucleus, medial part (AOM) and in fibres of the AOM and olfactory tubercle. Prominent fluorescence was present in neocortical areas, exhibiting a clear laminated distribution of individually labelled cells. High levels of Venus expression were seen in neurons and fibres of cingulate cortex, piriform cortex and layers II/III and VI of the cerebral cortex. However, in layer IV of the cerebral cortex, a cortical region which contains high levels of Y1R immunoreactivity and mRNA, Venus expression was limited to a small number of fibres.
In the hippocampus, the highest number of Venus-fluorescent cell bodies was found in the GrDG with densely stained axon fibres projecting to CA3. Conversely, we did not note the presence of Venus-fluorescent cell bodies in the CA2 and CA3 fields, although these areas contain a low to moderate number of Y1R-immunoreactive neurons. In the amygdaloid area, moderate to high expression of Venus was seen in cell bodies and fibres of BLA, central amygdala, Me and anterior cortical amygdaloid nucleus. Densely stained fibres were seen in the basal ganglia and fluorescent cell bodies were seen in claustrum, endopyriform nucleus and the diagonal band of Broca, but not in the bed nucleus of stria terminalis, a region where Y1R-immunoreactive neurons have been identified (Kopp et al., 2002; Kishi et al., 2005). In the septal area and in the thalamus we found the presence of fluorescent fibres, whereas cell bodies were devoid of or exhibited only weak fluorescent signal. In the hypothalamus, moderate to high Venus fluorescence was observed in cell bodies and fibres of several hypothalamic nuclei (SCh, AH, LH, Pe, PVN, DM, Arc and MM), with the exclusion of VMH, where fibres but not cell bodies were observed (Fig. 4 and Table 2).
Coexpression of Cre and Venus in the mouse brain
As described above, most brain regions positive for Venus also expressed Cre (Table 3). To take the colocalization analysis of Venus and Cre to a cellular level, we analysed brains derived from line 25 using confocal microscopy. The highest degree of colocalization was found in the GrDG, PVN (both in the parvocellular and magnocellular division) and subependymal layer of the 3V, where ∼ 80–90% of cells were positive for Venus and Cre (5, 6) whereas in the AOM, BLA and the reuniens thalamic nucleus a smaller cell population (∼ 60%) displayed colocalization of Venus and Cre (Fig. 7). In other brain regions there were regional differences in the relative number of cells that coexpressed Cre and Venus. For instance, analysis of sections from different animals suggests that, in layers II/III and VI of the cerebral cortex and in the CA1 region of the hippocampus, most of the Venus-fluorescent cells coexpressed Cre whereas only 40–50% of Cre-immunofluorescent cells in these locations were Venus-positive (Fig. 5). Conversely, in the AH, DM and ventrolateral part of SCh, 50–70% of Cre-immunoreactive cells were also positive for Venus while only a subpopulation of Cre-immunoreactive cells coexpressed Venus (6, 7).
| Forebrain regions | Venusfluorescence | itTA-directedCre activity | Venus–Cre coexpression |
|---|---|---|---|
| Telencephalon | |||
| Anterior olfactory n. | + + | + + + | * |
| Olfactory tubercle | nd | + + + | |
| Cingulate cortex | + + + | + + + | |
| Cortex | |||
| Piriform cortex | + + | + + + | |
| Layers 2 and 3 | + + + | + + | * |
| Layer 4 | nd | + + + | |
| Layer 5 | nd | + | |
| Layer 6 | + + | + + + | * |
| Hippocampus | |||
| CA1 | + | + + + | * |
| CA2 | nd | + + + | |
| CA3 | nd | + + + | |
| GrDG | + + + | + + + | * |
| Amygdaloid area | |||
| Basolateral amygdala | + | + + + | * |
| Medial amygdala | + + | + + | |
| Central amygdala | + | + | |
| Anterior cortical | |||
| amygdaloid n. | + + | + + | * |
| Amygdaloid hippocampal area | + + + | nd | |
| Claustrum | + | + + | |
| Endopyriform n. | + | nd | |
| Caudate–putamen | nd | + + + | |
| Nucleus accumbens | nd | + + + | |
| Ventral pallidum | nd | + + + | |
| Diagonal band of Broca | + | nd | |
| Bed nucleus of | |||
| stria terminalis | nd | + | |
| Septum | |||
| Lateral septal n. | nd | + + | |
| Septofimbrial n. | nd | + + | |
| Islands of Calleja | nd | + | |
| Tenia tecta | + | + + + | |
| Medial preoptic area | + + + | + + | |
| Vasc. organ, lamina terminalis | + + + | nd | |
| Diencephalon | |||
| Hypothalamus | |||
| Suprachiasmatic n. | + + + | + + + | * |
| Anterior hypothalamus | + + | + | * |
| Lateral hypothalamic area | + + | + | |
| Periventricular n. | + + | + | |
| Paraventricular n. | + + + | + + | * |
| Ventromedial n. | nd | + | |
| Dorsomedial n. | + + + | + + | * |
| Arcuate n. | + + | + | |
| Mammillary n. | + + | + + | |
| Subependymal cells | |||
| of the 3rd ventricle | + + + | + + + | * |
| Thalamus | |||
| Anterodorsal n. | nd | + /– | |
| Paraventricular n. | nd | + | |
| Reuniens n. | + | + + | * |
| Lateral geniculate n. | nd | + + | |
| Lateral habenula | + + | + + + | |
- Comparison of expression pattern of Venus fluorescence and itTA-directed Cre in cell bodies of the forebrain from line 25. Intensity of staining was graded as very low (+/–), low (+), intermediate (+ +) or high (+ + +); nd, not detected; *, nuclei where coexpression of Venus and Cre in the same cells was observed.
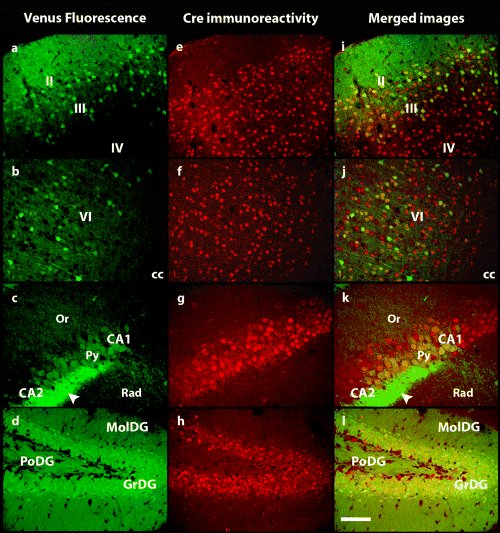
Confocal images showing the distribution of (a–d) Venus fluorescence (green) and (e–h) Cre immunostaining (red) in cells of (a and e) layers II/III and IV and (b and f) layer VI of the cerebral cortex, (c and g) the CA1 and CA2 regions of the hippocampus and (d and h) the polymorphic (PoDG), molecular (MolDG) and granule cell (GrDG) layers of the dentate gyrus. (i–l) Merged images showing the degree of colocalization (yellow) of Venus and Cre. Arrowhead indicates mossy fibre terminals. cc, Corpus callosum; Or, oriens layer, hippocampus; Py, pyramidal cell layer, hippocampus; Rad, stratum radiatum, hippocampus. All pictures are at the same enlargement. Scale bar, 100 µm.
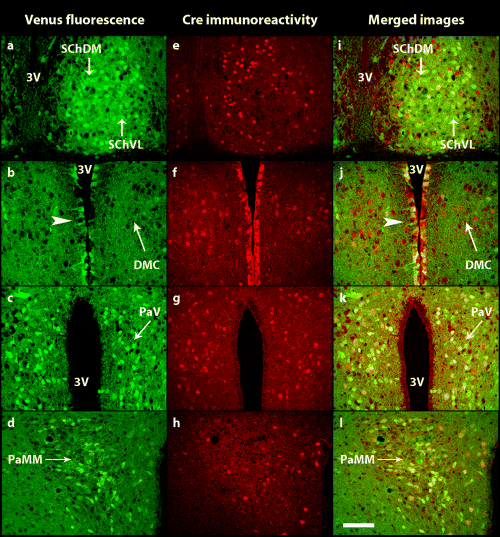
Confocal images showing the distribution of (a–d) Venus fluorescence (green) and (e–h) Cre immunostaining (red) in cells of (a and e)the dorsomedial (SChDM) and ventrolateral (SChVL) parts of the suprachiasmatic nuclei, of (b and f) the dorsomedial nucleus, compact (DMC) and of (c and g) the parvicellular (PaV) and (d and h) magnocellular (PaMM) divisions of the paraventricular nucleus. (i–l) Merged images showing the degree of colocalization (yellow) of Venus and Cre. Arrowhead indicates the ependymal–subependymal cell layer of the third ventricle (3V). All pictures are at the same enlargement. Scale bar, 100 µm.

Optical sections of multiple confocal scans, showing the distribution of (a–d) Venus fluorescence (green) and (e–h) Cre immunostaining (red) in cells of (a and e) the anterior hypothalamus (AH), (b and f) anterior olfactory nucleus (AOM), (c and g) anterior cortical amygdaloid nucleus (ACo) and (d and h) dorsomedial nucleus (DM). (i–l) Merged images showing the degree of colocalization (yellow) of Venus and Cre. 3V, third ventricle. All pictures are at the same enlargement. Scale bar, 100 µm.
No colocalization of Venus and Cre was found in the olfactory tubercle, layers IV and V of the cerebral cortex, CA2 and CA3 regions, CPu, nucleus accumbens, ventral pallidum, bed nucleus of stria terminalis, septum, islands of Calleja, VMH or anterodorsal, paraventricular or lateral geniculate thalamic nuclei, which displayed immunoreactive staining for Cre but were devoid of Venus-fluorescent cell bodies. In contrast, in the amygdaloid hippocampal area, the diagonal band of Broca and the vascular organ of the lamina terminalis only single-labelled Venus-fluorescent cells were observed.
Discussion
In the present study we generated and describe transgenic mice with genomically integrated BAC clone which (i) confines Tet regulation via itTA to the forebrain regions under the control of the Y5R promoter and (ii) allows permanent marking of Y1R-positive neurons via the Venus-fluorescent protein. We have shown that the use of a long genomic segment of one BAC results in faithful expression of both Y1R and Y5R promoter-controlled reporter genes, indicating that all the regulatory elements of the Y1R and Y5R genes were maintained in their native configuration on our BAC clone. As previously described for BAC or YAC transgenes (Schedl et al., 1993; Yang et al., 1999), the BAC TgY5RitTA/Y1RVenus-transgenic lines described here gave copy number-dependent expression, implying that lines with fewer BAC copies give low levels of itTA and Venus expression. In low copy number lines itTA-induced Cre signals were not widespread whereas in the same animals the Venus fluorescence reached the brightness and expression pattern of mice with higher BAC copy number. This might reflect a difference in Y1R and Y5R promoter strength but is also caused by the different reporter genes. Venus expression is directly controlled by the Y1R promoter whereas Cre expression is induced by Y5R promoter-controlled itTA. As itTA and the itTA-activated reporter levels have no linear correlation (Bond et al., 2000), higher copy numbers of BAC itTA leads to a much stronger Cre expression than the same BAC itTA with low copy number.
In three out of five BAC-transgenic lines both Cre and Venus expression were strong enough to demonstrate that the Y1R promoter-controlled Venus and the Y5R promoter-controlled itTA were expressed in patterns similar to those of the endogenous Y5R and Y1R genes, respectively. Only minor discrepancies could be observed. First, for the Y1R there was no expression of Venus in cell bodies of layer IV of the cerebral cortex or in the pyramidal cell layer of the CA1 and CA3 regions, areas where the mouse Y1R is expressed (Naveilhan et al., 1998; Kopp et al., 2002; Kishi et al., 2005). As all three lines showed these differences a BAC integration effect can be excluded. It might be due to low levels or post-transcriptional regulation of Venus in these areas. An antibody stain against Venus should distinguish between these options.
For the BAC-encoded Y5R promoter activity we found in mice of line 25 an itTA-directed Cre expression which had an almost complete overlap with the rat Y5R in the telencephalic, diencephalic and mesencephalic structures (Gerald et al., 1996; Larsen & Kristensen, 1998; Nichol et al., 1999; Parker & Herzog, 1999; Durkin et al., 2000; Campbell et al., 2001; Wolak et al., 2003; Fetissov et al., 2004). Therefore, the previously described rather restricted distribution of Y5R mRNA in the mouse brain when compared to rats was due to low sensitivity of the employed techniques rather than to species differences. Similarly, the moderate expression of the Y5R receptor in some thalamic neurons and in the medial habenula of the rat brain was not picked up by our BAC-transgenic mice, as low itTA levels were insufficient for detectable CRE expression (see Discussion above).
Using the high-expressing TgY5RitTA/Y1RVenus mice we found a similar distribution of Y1R and Y5R promoter activity in several telencephalic and diencephalic structures, these being the AOM, layers II/III and VI of the cerebral cortex, CA1 and GrDG of the hippocampus, amygdaloid area, medial preoptic area, hypothalamus and lateral habenula (Table 3; Wolak et al., 2003). Co-localization of itTA and Venus was also identified in several brain regions, including cerebral cortex, AOM, BLA, GrDG, reuniens thalamic nucleus and hypothalamus. The coexpression of Y1R and Y5R in neurons may reflect a common cis-acting transcriptional control, as the Y5R and the Y1R gene share a common bidirectional promoter region on mouse chromosome 8B3-C2 (Fig. 1). The functional implications of coordinated Y1R and Y5R expression are currently not resolved and changes in NPY-induced cellular responses of Y1R, Y5R or Y1R–Y5R mixed receptors remains to be investigated. The regional differences in the relative number of itTA- and Venus-expressing cells might reflect differences in Y1R and Y5R responses in a given region.
It should also be emphasised that the TgY5RitTA/Y1RVenus-transgenic mice represent a powerful tool for Cre/lox-mediated gene regulation in Y5R neurons to address various issues regarding gene function in the forebrain. Pharmacological and genetic evidence suggests that Y5R is involved in several NPY-activated functions. The Y5R stimulates food intake (Elmquist, 2001; Chamorro et al., 2002; Kalra & Kalra, 2004) and it contributes to the inhibitory actions of NPY on reproductive hormone secretion (Raposinho et al., 1999; Elmquist, 2001). Y5R is also involved in the anticonvulsant effect of NPY and plays a prominent role in inhibiting excitatory neurotransmission in mice, at least in the CA3 region (Baraban, 2004; Woldbye & Kokaia, 2004; Woldbye et al., 2005). Consistently with the physiology of Y5R in the hypothalamus, the itTA-directed Cre expression was observed within AH, LH, PVN, DM, VMH and Arc, providing an optimal tool for genetic strategies aimed toward understanding the regulation of hormone secretion as well as the role of anabolic and catabolic circuits in the hypothalamus. Furthermore, because itTA-directed recombinase activity is present in most of the GrDG and in the CA1-C3 regions, the TgY5RitTA/Y1RVenus-transgenic mice represent a suitable model for the study of hippocampal function in neuronal excitability, synaptic plasticity and learning in adult mice. Additionally, given that the Y2 receptor subtype is considered to function as a presynaptic autoreceptor on NPYergic terminals and it is involved in feeding, anxiety and neuronal excitability (Eva et al., 2006b), the TgY5RitTA/Y1RVenus-transgenic mice could provide an interesting new model for assessing the relationship between the Y2 receptor and the Y1R and Y5R. Lastly, the Venus fluorescence of Y1R-expressing cells permits detailed electrophysiological analysis of Y1 receptor-positive neurons in acute brain slice preparation and in vivo.
In summary, we have generated transgenic mouse lines expressing itTA and Venus under the control of the Y5R and of the Y1R promoters, respectively. By comparing the Venus- and itTA-controlled Cre distribution we provided further insight into the cellular localization of Y1R and Y5R in the mouse brain, giving new information about which receptor subtype could signal the different effects of NPY in the nervous system. Considering the rapidly increasing number of mouse lines with loxP-flanked genes, the TgY5RitTA/Y1RVenus-transgenic mice generated here also represent a genetic tool for conditional knockout approaches to study the contribution of a specific gene to physiological functions, including emotional and feeding behaviour, hormone secretion and neuronal excitability.
Acknowledgements
We thank Dr Neal G. Copeland for generously providing EL250 bacterial strains and protocols for BAC targeting in bacteria. We thank Professor F. Tempia, Department of Neuroscience, University of Torino and Professor G. C. Panzica, Department of Anatomy, Pharmacology and Forensic Medicine, for helpful discussion during the preparation of the manuscript. This work has been supported by grants from Volkswagen foundation (Az: I/80 704) to R.S., and from Fondazione CRT, Torino and from the Health Ministry of Regione Piemonte (Progetti di Ricerca Scientifica Applicata 2004 and Progetti di Ricerca Sanitaria Finalizzata 2004) to C.E.
Abbreviations
-
- 3V
-
- third ventricle
-
- AH
-
- anterior hypothalamus
-
- AOM
-
- anterior olfactory nucleus, medial part
-
- Arc
-
- arcuate nucleus
-
- BAC
-
- bacterial artificial chromosome
-
- BLA
-
- basolateral amygdaloid nucleus, anterior part
-
- CPu
-
- caudate–putamen (striatum)
-
- DM
-
- dorsomedial hypothalamic nucleus
-
- Dox
-
- doxycycline
-
- Flp
-
- flippase recombinase
-
- Frt
-
- Flp recombinase target
-
- GrDG
-
- granule layer of the dentate gyrus
-
- itTA
-
- ‘improved’ Tet-dependent transcription factor
-
- LH
-
- lateral hypothalamic area
-
- Me
-
- medial amygdaloid nucleus
-
- MM
-
- mammillary nuclei
-
- NPY
-
- neuropeptide Y
-
- P
-
- postnatal day
-
- pA
-
- polyadenylation
-
- Pe
-
- periventricular hypothalamic nucleus
-
- PVN
-
- paraventricular nucleus
-
- SCh
-
- suprachiasmatic nucleus
-
- Tet
-
- Tetracycline
-
- tTA
-
- Tet-dependent transcription factor
-
- VMH
-
- ventromedial hypothalamic nucleus
-
- Y1R
-
- NPY Y1 receptor
-
- Y5R
-
- NPY Y5 receptor